Members of the Trichoderma harzianum Species Complex with Mushroom Pathogenic Potential
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Species Identification of Green Mould Agents
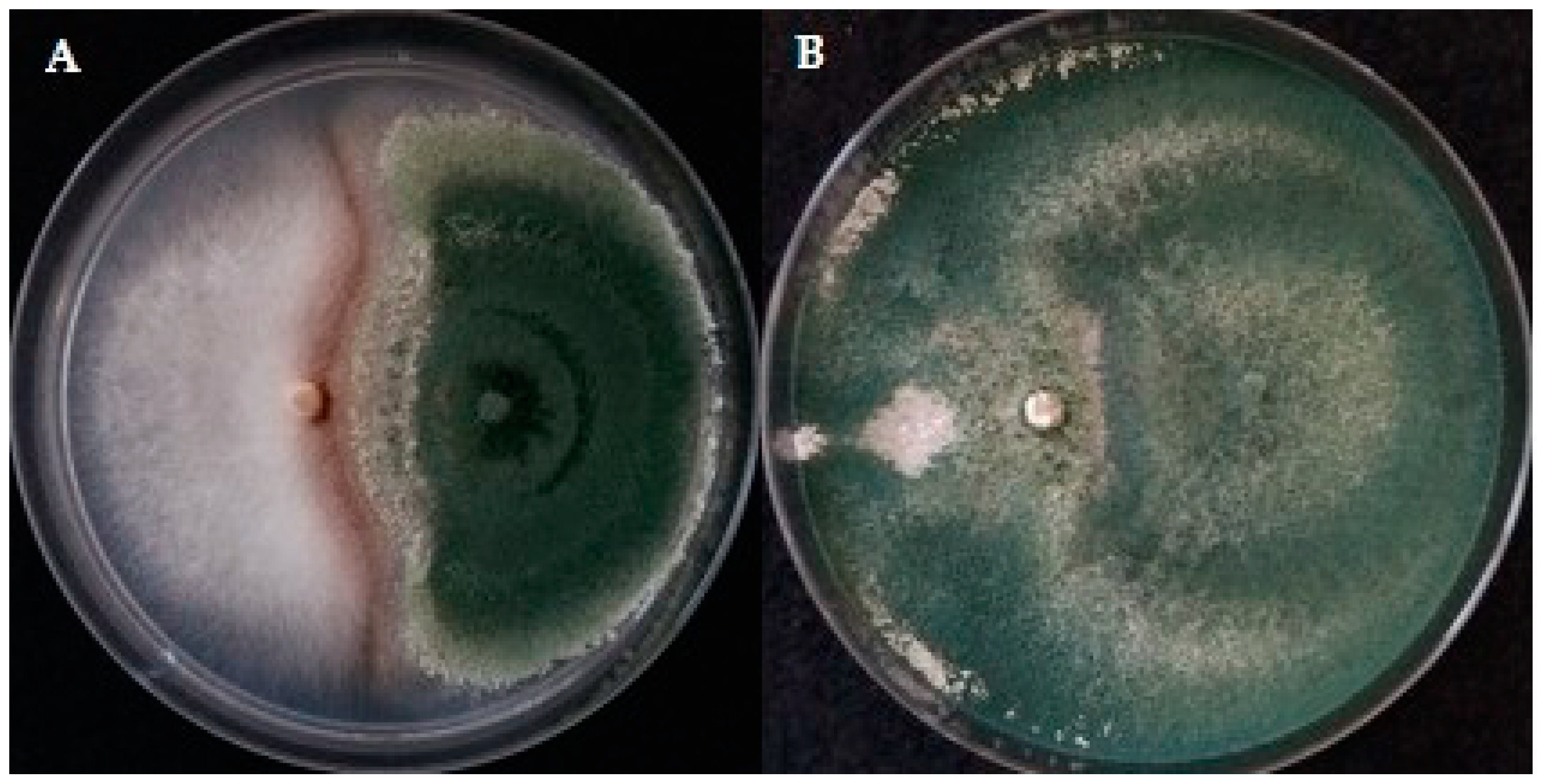
3.2. Antagonistic Potential of Trichoderma Isolates against Cultivated Mushrooms In Vitro
3.3. Influence of Commercial Fungicides on Mycelial Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons: Chichester, UK, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- da Eira, A.F. Cultivo do Cogumelo Medicinal Agaricus blazei (Murrill) SS Heinemann ou Agaricus brasiliensis; Aprenda Fácil: Viçosa, Brazil, 2003; 398p. [Google Scholar]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Grogan, H.M. Fungicide resistance among Cladobotryum spp.—Causal agents of cobweb disease of the edible mushroom Agaricus bisporus. Mycol. Res. 2000, 104, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Adie, B.; Grogan, H.; Archer, S.; Mills, P. Temporal and spatial dispersal of Cladobotryum conidia in the controlled environment of a mushroom growing room. Appl. Environ. Microbiol. 2006, 72, 7212–7217. [Google Scholar] [CrossRef] [Green Version]
- McKay, G.J.; Egan, D.; Morris, E.; Scott, C.; Brown, A.E. Genetic and morphological characterization of Cladobotryum species causing cobweb disease of mushrooms. Appl. Environ. Microbiol. 1999, 65, 606–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, R.L.; Baars, J.J.P.; Kalkhove, S.I.C.; Lugones, L.G.; Wösten, H.A.N.A.B.; Bakker, P.A.H.M. Lecanicillium fungicola: Causal agent of dry bubble disease in white-button mushroom. Mol. Plant Pathol. 2010, 11, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Thao, L.D.; Binh, V.T.P.; Khanh, T.N.; Thanh, H.M.; Hien, L.T.; Anh, P.T.; Trang, T.T.T.; Dung, N.V.; Hoai, H.T. First report of Lecanicillium fungicola var. aleophilum infecting white-button mushroom in Vietnam. New Dis. Rep. 2020, 41, 23. [Google Scholar] [CrossRef]
- Sharma, S.R.; Kumar, S. Studies on wet bubble disease of white button mushrooms (Agaricus bisporus) caused by Mycogone perniciosa. In Science and Cultivation of Edible Fungi, Proceedings of the 15th International Congress on the Science and Cultivation of Edible Fungi, Maastricht, The Netherlands, 15–19 May 2000; A.A. Balkema: Rotterdam, The Netherlands, 2000; pp. 569–575. [Google Scholar]
- Bora, T.; Özaktan, H. Biological control of some important mushroom diseases in Turkey by fluorescent Pseudomonads. Mushroom Sci. 2000, 15, 30. [Google Scholar]
- Sharma, V.P.; Singh, C. Biology and control of Mycogone perniciosa Magn. causing wet bubble disease of white button mushroom. J. Mycol. Plant Pathol. 2003, 33, 257–264. [Google Scholar]
- Zhang, C.; Kakishima, M.; Xu, J.; Wang, Q.; Li, Y. The effect of Hypomyces perniciosus on the mycelia and basidiomes of Agaricus bisporus. Microbiology 2017, 163, 1273–1282. [Google Scholar] [CrossRef]
- Hatvani, L.; Kredics, L.; Allaga, H.; Manczinger, L.; Vágvölgyi, C.; Kuti, K.; Geösel, A. First report of Trichoderma aggressivum f. aggressivum green mold on Agaricus bisporus in Europe. Plant Dis. 2017, 101, 1052. [Google Scholar] [CrossRef] [Green Version]
- Kredics, L.; Naeimi, S.; Hatvani, L.; Vágvölgyi, C.; Cai, F.; Druzhinina, I.S.; Manczinger, L. ‘The Good, the Bad and the Ugly’ in the shades of green: The genus Trichoderma in the spotlight. Indian Phytopathol. 2021, 74, 403–411. [Google Scholar] [CrossRef]
- Luković, J.; Milijašević-Marčić, S.; Hatvani, L.; Kredics, L.; Szűcs, A.; Vágvölgyi, C.; Duduk, N.; Vico, I.; Potočnik, I. Sensitivity of Trichoderma strains from edible mushrooms to the fungicides prochloraz and metrafenone. J. Environ. Sci. Health B 2021, 56, 54–63. [Google Scholar] [CrossRef]
- Castle, A.; Speranzini, D.; Rghei, N.; Alm, G.; Rinker, D.; Bissett, J. Morphological and molecular identification of Trichoderma isolates on North American mushroom farms. Appl. Environ. Microbiol. 1998, 64, 133–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, O.; Morris, E.; Clancy, K. Trichoderma green mould update. Irish Mushroom Rev. 1991, 3, 13–17. [Google Scholar]
- Seaby, D.A. Infection of mushroom compost by Trichoderma species. Mushroom J. 1987, 179, 355–361. [Google Scholar]
- Seaby, D.A. Further observations on Trichoderma. Mushroom J. 1989, 197, 147–151. [Google Scholar]
- Samuels, G.J.; Dodd, S.L.; Gams, W.; Castlebury, L.A.; Petrini, O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 2002, 94, 146–170. [Google Scholar] [CrossRef]
- Hatvani, L.; Sabolić, P.; Kocsubé, S.; Kredics, L.; Czifra, D.; Vágvölgyi, C.; Kaliterna, J.; Ivić, D.; Đermić, E.; Kosalec, I. The first report on mushroom green mould disease in Croatia. Arh. Hig. Rada Toksikol. 2012, 63, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Bae, K.S.; Yu, S.H. Two new species of Trichoderma associated with green mold of oyster mushroom cultivation in Korea. Mycobiology 2006, 34, 111. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.; Di Benedetto, P.; Senatore, M.; Abadi, K.; Gigante, S.; Soriente, I.; Ferraioli, S.; Scala, F.; Lorito, M. Identification and characterization of Trichoderma species aggressive to Pleurotus in Italy. Zhejiang Univ. J. Agric. Life Sci. 2004, 30, 469–470. [Google Scholar]
- Hatvani, L.; Antal, Z.; Manczinger, L.; Szekeres, A.; Druzhinina, I.S.; Kubicek, C.P.; Nagy, A.; Nagy, E.; Vágvölgyi, C.; Kredics, L. Green mold diseases of Agaricus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathology 2007, 97, 532–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komoń-Zelazowska, M.; Bissett, J.; Zafari, D.; Hatvani, L.; Manczinger, L.; Woo, S.; Lorito, M.; Kredics, L.; Kubicek, C.P.; Druzhinina, I.S. Genetically closely related but phenotypically divergent Trichoderma species cause green mold disease in oyster mushroom farms worldwide. Appl. Environ. Microbiol. 2007, 73, 7415–7426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Frużyńska-Jóźwiak, D. Diversity of Trichoderma spp. causing Pleurotus green mould diseases in Central Europe. Folia Microbiol. 2013, 58, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.L.; Kubicek, C.P.; Druzhinina, I.S.; Vinale, F.; Cavallo, P.; Lorito, M. Characterization of Trichoderma species associated with the production of Pleurotus ostreatus in Italy. J. Plant Pathol. 2009, 91, 94. [Google Scholar]
- Miyazaki, K.; Tsuchiya, Y.; Okuda, T. Specific PCR assays for the detection of Trichoderma harzianum causing green mold disease during mushroom cultivation. Mycoscience 2009, 50, 94–99. [Google Scholar] [CrossRef]
- Kim, C.S.; Yu, S.H.; Nakagiri, A.; Shirouzu, T.; Sotome, K.; Kim, S.C.; Maekawa, N. Re-evaluation of Hypocrea pseudogelatinosa and H. pseudostraminea isolated from shiitake mushroom (Lentinula edodes) cultivation in Korea and Japan. Plant Pathol. J. 2012, 28, 341–356. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.S.; Shirouzu, T.; Nakagiri, A.; Sotome, K.; Maekawa, N. Trichoderma eijii and T. pseudolacteum, two new species from Japan. Mycol. Progr. 2013, 12, 739–753. [Google Scholar] [CrossRef]
- Kim, C.S.; Shirouzu, T.; Nakagiri, A.; Sotome, K.; Nagasawa, E.; Maekawa, N. Trichoderma mienum sp. nov., isolated from mushroom farms in Japan. Antonie Van Leeuwenhoek 2012, 102, 629–641. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yun, Y.H.; Hyun, M.W.; Kim, M.H.; Kim, S.H. Identification and characterization of Gliocladium viride isolated from mushroom fly infested oak log beds used for shiitake cultivation. Mycobiology 2010, 38, 7. [Google Scholar] [CrossRef] [Green Version]
- Jaklitsch, W.M. European species of Hypocrea part II: Species with hyaline ascospores. Fungal Divers. 2011, 48, 1–250. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Cao, X.; Ma, X.; Guo, M.; Liu, C.; Yan, L.; Bian, Y. Diversity and effect of Trichoderma spp. associated with green mold disease on Lentinula edodes in China. Microbiol. Open 2016, 5, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.T.; Bian, Y.B.; Xu, Z.Y. First report of Trichoderma oblongisporum causing green mold disease on Lentinula edodes (shiitake) in China. Plant Dis. 2014, 98, 1440. [Google Scholar] [CrossRef] [PubMed]
- Rifai, M.A. A Revision of the Genus Trichoderma; Mycological Papers; Commonwealth Mycological Institute: Richmond Keew, UK, 1969; Volume 116, pp. 1–56. [Google Scholar]
- Chaverri, P.; Castlebury, L.A.; Samuels, G.J.; Geiser, D.M. Multilocus phylogenetic structure within the Trichoderma harzianum/Hypocrea lixii complex. Mol. Phylogenet. Evol. 2003, 27, 302–313. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, M.; Du, X.; Zhang, Z.; Xu, J.; Yu, Z. Three new species of soil-inhabiting Trichoderma from southwest China. MycoKeys 2018, 44, 63–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.-X.; Xu, H.-X.; Zhuang, W.-Y.; Li, Y. Two new green-spored species of Trichoderma (Sordariomycetes, Ascomycota) and their phylogenetic positions. MycoKeys 2017, 26, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Wang, R.; Sun, Q.; Wu, B.; Sun, J.-Z. Four new species of Trichoderma in the Harzianum clade from northern China. MycoKeys 2020, 73, 109–132. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K.; et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef] [Green Version]
- Chaverri, P.; Samuels, G.J. Evolution of habitat preference and nutrition mode in a cosmopolitan fungal genus with evidence of interkingdom host jumps and major shifts in ecology. Evolution 2013, 67, 2823–2837. [Google Scholar] [CrossRef] [PubMed]
- Oskiera, M.; Szczech, M.; Bartoszewski, G. Molecular identification of Trichoderma strains collected to develop plant growth-promoting and biocontrol agents. J. Hortic. Res. 2015, 23, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Innocenti, G.; Montanari, M.; Righini, H.; Roberti, R. Trichoderma species associated with green mould disease of Pleurotus ostreatus and their sensitivity to prochloraz. Plant Pathol. 2019, 68, 392–398. [Google Scholar] [CrossRef]
- Grujić, M.; Dojnov, B.; Potočnik, I.; Atanasova, L.; Duduk, B.; Srebotnik, E.; Druzhinina, I.S.; Kubicek, C.P.; Vujčić, Z. Superior cellulolytic activity of Trichoderma guizhouense on raw wheat straw. World J. Microbiol. Biotechnol. 2019, 35, 194. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Kubicek, C.P.; Komoń-Zelazowska, M.; Mulaw, T.B.; Bissett, J. The Trichoderma harzianum demon: Complex speciation history resulting in coexistence of hypothetical biological species, recent agamospecies and numerous relict lineages. BMC Evol. Biol. 2010, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Atanasova, L.; Jaklitsch, W.M.; Komon-Zelazowska, M.; Kubicek, C.P.; Druzhinina, I.S. Clonal species Trichoderma parareesei sp. nov. likely resembles the ancestor of the cellulase producer Hypocrea jecorina/T. reesei. Appl. Environ. Microbiol. 2010, 76, 7259–7267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druzhinina, I.S.; Komoń-Zelazowska, M.; Kredics, L.; Hatvani, L.; Antal, Z.; Belayneh, T.; Kubicek, C.P. Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiology 2008, 154, 3447–3459. [Google Scholar] [CrossRef] [Green Version]
- Hatvani, L.; Homa, M.; Chenthamara, K.; Cai, F.; Kocsubé, S.; Atanasova, L.; Mlinaric-Missoni, E.; Manikandan, P.; Revathi, R.; Dóczi, I. Agricultural systems as potential sources of emerging human mycoses caused by Trichoderma: A successful, common phylotype of Trichoderma longibrachiatum in the frontline. FEMS Microbiol. Lett. 2019, 366, fnz246. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.Z.; Zhang, Z.F.; Cai, L.; Peng, W.J.; Liu, F. Four new filamentous fungal species from newly-collected and hive-stored bee pollen. Mycosphere 2018, 9, 1089–1116. [Google Scholar] [CrossRef]
- Szekeres, A.; Leitgeb, B.; Kredics, L.; Manczinger, L.; Vágvölgyi, C. A novel, image analysis-based method for the evaluation of in vitro antagonism. J. Microbiol. Meth. 2006, 65, 619–622. [Google Scholar] [CrossRef]
- Körmöczi, P.; Leitgeb, B.; Cseh, T.; Hatvani, L.; Manczinger, L.; Vágvölgyi, C.; Kredics, L. An image analysis-based method for the evaluation of the aggressivity of Trichoderma strains towards Pleurotus ostreatus. Acta Microbiol. Immunol. Hung. 2009, 56, 188–189. [Google Scholar]
- R (Version 4.0.3). Available online: https://www.r-project.org (accessed on 12 October 2020).
- RStudio Desktop (Version 1.3.1093). Available online: http://www.rstudio.com (accessed on 23 September 2020).
- Choi, I.Y.; Joung, G.T.; Ryu, J.; Choi, J.S.; Choi, Y.G. Physiological characteristics of green mold (Trichoderma spp.) isolated from oyster mushroom (Pleurotus spp.). Mycobiology 2003, 31, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Park, M.S.; Seo, G.S.; Bae, K.S.; Yu, S.H. Characterization of Trichoderma spp. associated with green mold of oyster mushroom by PCR-RFLP and sequence analysis of ITS regions of rDNA. Plant Pathol. J. 2005, 21, 229–236. [Google Scholar] [CrossRef]
- Singh, S.K.; Sharma, V.P.; Sharma, S.R.; Kumar, S.; Tiwari, M. Molecular characterization of Trichoderma taxa causing green mould disease in edible mushrooms. Curr. Sci. 2006, 90, 427–431. [Google Scholar]
- Kim, W.G.; Weon, H.Y.; Seok, S.J.; Lee, K.H. In vitro antagonistic characteristics of bacilli isolates against Trichoderma spp. and three species of mushrooms. Mycobiology 2008, 36, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Błaszczyk, L.; Popiel, D.; Chełkowski, J.; Koczyk, G.; Samuels, G.J.; Sobieralski, K.; Siwulski, M. Species diversity of Trichoderma in Poland. J. Appl. Genet. 2011, 52, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Kosanović, D.; Potočnik, I.; Duduk, B.; Vukojević, J.; Stajić, M.; Rekanović, E.; Milijašević-Marčić, S. Trichoderma species on Agaricus bisporus farms in Serbia and their biocontrol. Ann. Appl. Biol. 2013, 163, 218–230. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwon, H.W.; Yun, Y.H.; Kim, S.H. Identification and characterization of Trichoderma species damaging shiitake mushroom bed-logs infested by Camptomyia pest. J. Microbiol. Biotechnol. 2016, 26, 909–917. [Google Scholar] [CrossRef]
- Aydoğdu, M.; Kurbetli, İ.; Kitapçı, A.; Sülü, G. Aggressiveness of green mould on cultivated mushroom (Agaricus bisporus) in Turkey. J. Plant Dis. Prot. 2020, 127, 695–708. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, M.S.; Kim, S.C.; Maekawa, N.; Yu, S.H. Identification of Trichoderma, a competitor of shiitake mushroom (Lentinula edodes), and competition between Lentinula edodes and Trichoderma species in Korea. Plant Pathol. J. 2012, 28, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Sun, J. Fifteen fungicolous Ascomycetes on edible and medicinal mushrooms in China and Thailand. Asian J. Mycol. 2019, 2, 129–169. [Google Scholar] [CrossRef]
- Goltapeh, M.E.; Danesh, Y.R. Pathogenic interactions between Trichoderma species and Agaricus bisporus. J. Agric. Technol. 2006, 2, 29–37. [Google Scholar]
- Milijašević-Marčić, S.; Stepanović, M.; Todorović, B.; Duduk, B.; Stepanović, J.; Rekanović, E.; Potočnik, I. Biological control of green mould on Agaricus bisporus by a native Bacillus subtilis strain from mushroom compost. Eur. J. Plant Pathol. 2017, 148, 509–519. [Google Scholar] [CrossRef]
- Šantrić, L.; Potočnik, I.; Radivojević, L.; Umiljendić, J.G.; Rekanović, E.; Duduk, B.; Milijašević-Marčić, S. Impact of a native Streptomyces flavovirens from mushroom compost on green mold control and yield of Agaricus bisporus. J. Environ. Sci. Health B 2018, 53, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, O.; Berić, T.; Potočnik, I.; Rekanović, E.; Stanković, S.; Milijašević-Marčić, S. Biological control of green mould and dry bubble diseases of cultivated mushroom (Agaricus bisporus L.) by Bacillus spp. Crop Prot. 2019, 126, 104944. [Google Scholar] [CrossRef]
- Gea, F.J.; Navarro, M.J.; Santos, M.; Diánez, F.; Carrasco, J. Control of fungal diseases in mushroom crops while dealing with fungicide resistance: A review. Microorganisms 2021, 9, 585. [Google Scholar] [CrossRef]
- Peil, R.M.; Rossetto, E.A.; Pierobom, C.R.; Rocha, M.T. Desinfestação de composto para cultivo de cogumelo Agaricus bisporus (Lange) Imbach. Rev. Bras. Agrociência 1996, 2, 159–164. [Google Scholar]
- Catlin, N.J.; Wuest, P.J.; Beyer, D.M. Green mold harbored by wood: Post-crop steaming and preservatives. Mushroom Sci. 2004, 16, 449–458. [Google Scholar]
- Morris, E.; Harrington, O.; Doyle, O.P.E.; Clancy, K.J.C. Green mould disease—The study of survival and dispersal characteristics of the weed mould Trichoderma, in the Irish mushroom industry. In Science and Cultivation of Edible Fungi, Proceedings of the 15th International Congress on the Science and Cultivation of Edible Fungi, Maastricht, The Netherlands, 15–19 May 2000; A.A. Balkema: Rotterdam, The Netherlands, 2000; pp. 645–651. [Google Scholar]
- Velázquez-Cedeño, M.; Mata, G.; Farnet, A.M.; Savoie, J.M. Estudio preliminar de la microflora bacteriana termotolerante de la pulpa de café y la paja de trigo con potencial de inhibición contra Trichoderma viride en el cultivo de Pleurotus spp. Rev. Mex. Micol. 2006, 22, 33–39. [Google Scholar]
- Colavolpe, M.B.; Mejía, S.J.; Albertó, E. Efficiency of treatments for controlling Trichoderma spp. during spawning in cultivation of lignicolous mushrooms. Braz. J. Microbiol. 2014, 45, 1263–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinker, D.L.; Alm, G. Management of casing Trichoderma using fungicides. In Science and Cultivation of Edible and Medicinal Fungi: Mushroom Science XVII, Proceeding of the 17th Congress of the International Society for Mushroom Science, Cape Town, South Africa, 20–24 May 2008; South African Mushroom Farmers Association: Pretoria, South Africa, 2008; pp. 496–509. [Google Scholar]
- Abosriwil, S.O.; Clancy, K.J. A protocol for evaluation of the role of disinfectants in limiting pathogens and weed moulds in commercial mushroom production. Pest Manag. Sci. 2002, 58, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Chittihunsa, T.; Bangeekhan, E.; Wongsamitkul, N.; Subsomboon, T. Screening of Bacillus spp. suppressing the infection of Trichoderma sp. in mushroom cultivation. Curr. Appl. Sci. Technol. 2007, 7, 19–27. [Google Scholar]
- Aydoğdu, M.; Sülü, S.M.; Kurbetli, İ.; Sülü, G. In vitro and in vivo biological control of the green mold using different bacteria in button mushroom cultivation. Egypt. J. Biol. Pest Control 2021, 31, 70. [Google Scholar] [CrossRef]
- NCBI GenBank. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 25 November 2021).
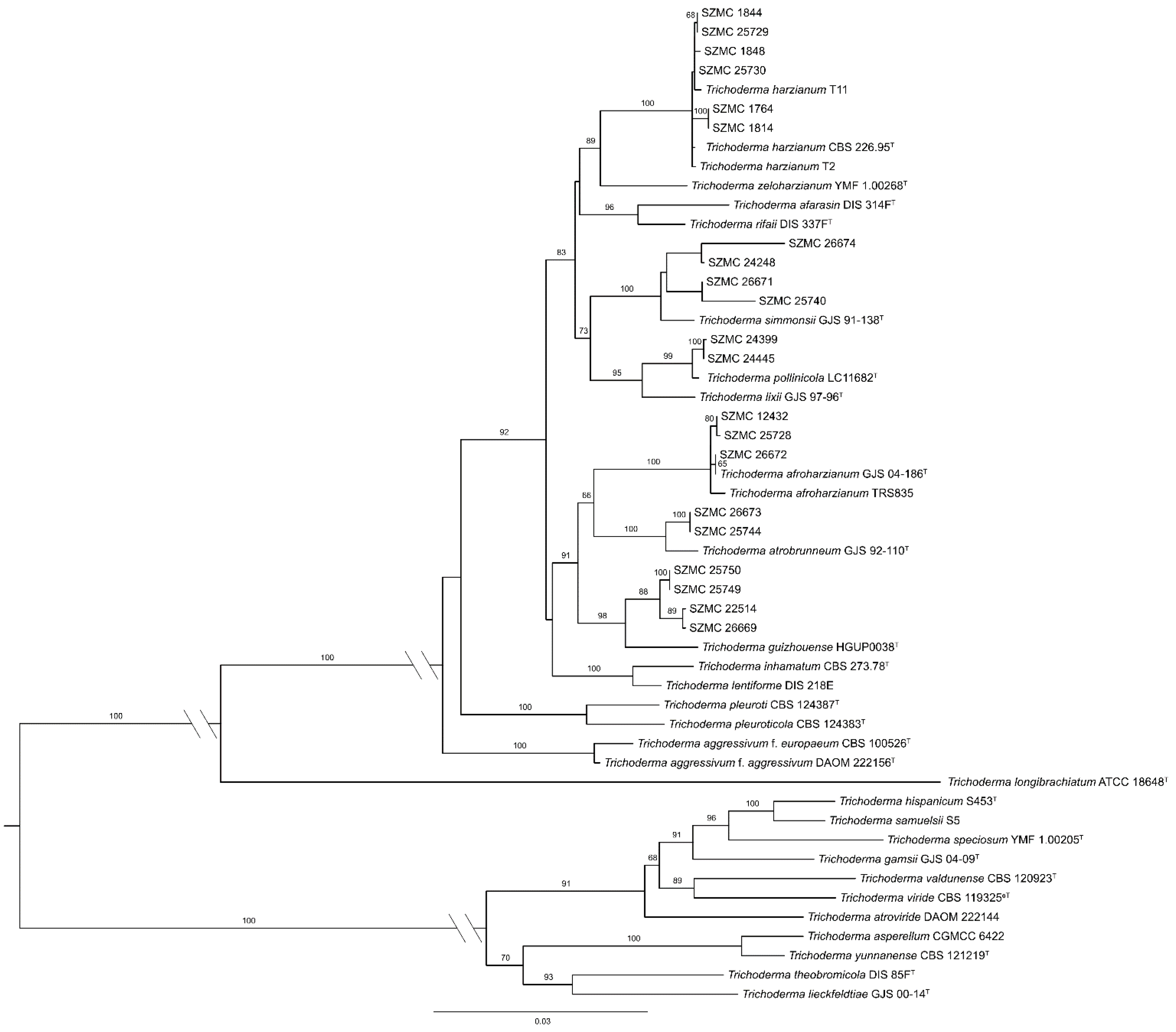
| Species | Strain Number | Source | GenBank Accession Numbers | Reference | |||
|---|---|---|---|---|---|---|---|
| ITS | tef1 | cal1 | rpb2 | ||||
| T. afroharzianum | SZMC 12432 | Pleurotus ostreatus, Spain | MZ754498 | MZ773449 | MZ773454 | MZ773428 | Present study |
| T. afroharzianum | SZMC 25728 | P. ostreatus, North Macedonia | MZ754499 | MZ773444 | MZ773455 | MZ773423 | Present study |
| T. afroharzianum | SZMC 26672, KG10 | P. ostreatus, Serbia | MT876582 | MZ773435 | MZ773456 | MZ773414 | [15] |
| T. atrobrunneum | SZMC 25744 | Lentinula edodes, Serbia | MZ754500 | MZ773440 | MZ773457 | MZ773419 | Present study |
| T. atrobrunneum | SZMC 26673 | P. ostreatus, North Macedonia | MZ754501 | MZ773434 | MZ773458 | MZ773413 | Present study |
| T. guizhouense | SZMC 22514 | P. ostreatus, Croatia | MZ754502 | MZ773448 | MZ773459 | MZ773427 | Present study |
| T. guizhouense | SZMC 25749, T58 | L. edodes, Serbia | MT876594 | MZ773439 | MZ773460 | MZ773418 | [15] |
| T. guizhouense | SZMC 25750, T59 | L. edodes, Serbia | MT876595 | MZ773438 | MZ773461 | MZ773417 | [15] |
| T. guizhouense | SZMC 26669 | P. ostreatus, Serbia | MZ754503 | MZ773437 | MZ773462 | MZ773416 | Present study |
| T. harzianum | SZMC 1764, C08 | Agaricus bisporus, Hungary | MZ754504 | MZ773453 | MZ773463 | MZ773432 | [15] |
| T. harzianum | SZMC 1814, C22 | A. bisporus, Hungary | MZ754505 | MZ773452 | MZ773464 | MZ773431 | [24] |
| T. harzianum | SZMC 1844 | A. bisporus, Croatia | MZ754506 | MZ773451 | MZ773465 | MZ773430 | Present study |
| T. harzianum | SZMC 1848 | A. bisporus, Croatia | MZ754507 | MZ773450 | MZ773466 | MZ773429 | Present study |
| T. harzianum | SZMC 25729 | P. ostreatus, North Macedonia | MZ754508 | MZ773443 | MZ773467 | MZ773422 | Present study |
| T. harzianum | SZMC 25730 | P. ostreatus, North Macedonia | MZ754509 | MZ773442 | MZ773468 | MZ773421 | Present study |
| T. pollinicola | SZMC 24399 | L. edodes, Hungary | MZ754510 | MZ773446 | MZ773469 | MZ773425 | Present study |
| T. pollinicola | SZMC 24445 | L. edodes, Hungary | MZ754511 | MZ773445 | MZ773470 | MZ773424 | Present study |
| T. simmonsii | SZMC 24248 | L. edodes, Hungary | MZ754512 | MZ773447 | MZ773471 | MZ773426 | Present study |
| T. simmonsii | SZMC 25740 | L. edodes, Serbia | MZ754513 | MZ773441 | MZ773472 | MZ773420 | Present study |
| T. simmonsii | SZMC 26671 | P. ostreatus, Serbia | MZ754514 | MZ773436 | MZ773473 | MZ773415 | Present study |
| T. simmonsii | SZMC 26674 | P. ostreatus, North Macedonia | MZ754515 | MZ773433 | MZ773474 | MZ773412 | Present study |
| T. aggressivum f. aggressivum | SZMC 23834 | A. bisporus, Hungary | nr | nr | nr | nr | [13] |
| T. pleuroticola | SZMC 23033, TUCIM 2104 | P. ostreatus, Hungary | nr | nr | nr | nr | [25] |
| T. reesei | SZMC 22614, TUCIM 917 | nr | nr | nr | nr | nr | [48] |
| Genomic Region | Selected Model |
|---|---|
| cal1 intron | TPM1uf + G4 |
| cal1 exon | TIM1 + G4 |
| ribosomal region | TPM1uf + G4 |
| internal transcribed spacer | TPM2uf + G4 |
| rpb2 | GTR + G4 |
| tef1 intron | TIM2 + G4 |
| tef1 exon | TIM1 + G4 |
| Trichoderma Strains | AI (%) | ||
|---|---|---|---|
| Button Mushroom | Oyster Mushroom | Shiitake | |
| T. afroharzianum SZMC 12432 | - | 80.90 ± 1.22 | - |
| T. afroharzianum SZMC 25728 | - | 83.84 ± 0.98 | - |
| T. afroharzianum SZMC 26672 | - | 80.97 ± 2.81 | - |
| T. atrobrunneum SZMC 25744 | - | - | 81.99 ± 1.87 |
| T. atrobrunneum SZMC 26673 | - | 81.90 ± 0.00 | - |
| T. guizhouense SZMC 22514 | - | 87.33 ± 2.45 | - |
| T. guizhouense SZMC 25749 | - | - | 57.58 ± 1.92 |
| T. guizhouense SZMC 26669 | - | 85.67 ± 1.37 | - |
| T. harzianum SZMC 1764 | 98.67 ± 1.15 | - | |
| T. harzianum SZMC 1844 | 100.00 ± 0.00 | - | - |
| T. harzianum SZMC 25730 | - | 86.44 ± 0.78 | - |
| T. pollinicola SZMC 24399 | - | - | 100.00 ± 0.00 |
| T. simmonsii SZMC 24248 | - | - | 100.00 ± 0.00 |
| T. simmonsii SZMC 25740 | - | 100.00 ± 0.00 | |
| T. simmonsii SZMC 26671 | - | 88.70 ± 1.67 | |
| T. aggressivum f. aggressivum SZMC 23834 | 100.00 ± 0.00 | - | - |
| T. pleuroticola SZMC 23033 | - | 87.83 ± 1.41 | 73.15 ± 1.61 |
| T. reesei SZMC 22614 | 100.00 ± 0.00 | 82.66 ± 0.00 | 80.80 ± 2.11 |
| Trichoderma Strains | Prochloraz (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | |
| T. afroharzianum SZMC 12432 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 97.31 ± 2.33 | 94.63 ± 1.16 | 91.27 ± 2.33 | 75.17 ± 5.07 |
| T. afroharzianum SZMC 25728 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 96.55 ± 3.16 | 95.86 ± 0.00 | 78.62 ± 1.20 |
| T. afroharzianum SZMC 26672 | 100.00 ± 0.00 | 96.72 ± 5.68 | 93.44 ± 5.68 | 96.72 ± 5.68 | 88.52 ± 7.51 | 96.72 ± 5.68 | 62.30 ± 12.38 |
| T. atrobrunneum SZMC 25744 | 100.00 ± 0.00 | 89.91 ± 4.37 | 92.44 ± 4.37 | 84.03 ± 2.91 | 65.55 ± 5.25 | 71.43 ± 2.91 | 15.12 ± 5.83 |
| T. atrobrunneum SZMC 26673 | 100.00 ± 0.00 | 93.14 ± 1.70 | 87.25 ± 3.40 | 88.24 ± 5.10 | 77.45 ± 6.12 | 78.43 ± 1.70 | 19.61 ± 7.40 |
| T. guizhouense SZMC 22514 | 95.24 ± 3.72 | 96.43 ± 4.72 | 93.75 ± 3.79 | 94.64 ± 3.09 | 80.36 ± 3.09 | 80.35 ± 6.44 | 33.93 ± 0.00 |
| T. guizhouense SZMC 25749 | 95.56 ± 7.70 | 88.89 ± 3.85 | 86.67 ± 0.00 | 91.11 ± 7.70 | 80.00 ± 11.55 | 84.45 ± 16.78 | 57.78 ± 10.18 |
| T. guizhouense SZMC 26669 | 95.58 ± 0.96 | 91.16 ± 0.95 | 90.61 ± 0.95 | 85.08 ± 1.66 | 75.14 ± 8.77 | 79.56 ± 4.17 | 37.57 ± 4.17 |
| T. harzianum SZMC 1764 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 96.53 ± 6.02 | 100.00 ± 0.00 | 84.03 ± 1.21 |
| T. harzianum SZMC 1844 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 95.58 ± 1.54 | 97.35 ± 2.66 | 78.76 ± 0.00 |
| T. harzianum SZMC 25730 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 99.28 ± 1.25 | 92.09 ± 4.49 | 95.68 ± 5.71 | 81.29 ± 4.49 |
| T. pollinicola SZMC2 4399 | 100.00 ± 0.00 | 97.87 ± 3.68 | 96.45 ± 3.25 | 88.65 ± 1.23 | 83.69 ± 2.46 | 82.27 ± 1.23 | 40.43 ± 5.63 |
| T. simmonsii SZMC 24248 | 89.63 ± 3.39 | 88.89 ± 0.00 | 88.15 ± 1.28 | 82.22 ± 4.45 | 75.56 ± 2.23 | 71.11 ± 3.85 | 28.15 ± 5.59 |
| T. simmonsii SZMC 25740 | 89.70 ± 2.10 | 83.63 ± 6.30 | 71.82 ± 1.29 | 64.24 ± 4.20 | 41.82 ± 3.64 | 50.30 ± 11.69 | 12.73 ± 3.15 |
| T. simmonsii SZMC 26671 | 82.68 ± 3.49 | 82.12 ± 1.93 | 77.09 ± 3.49 | 59.22 ± 10.10 | 41.90 ± 4.22 | 51.40 ± 6.71 | 18.99 ± 4.84 |
| T. simmonsii SZMC 26674 | 95.42 ± 0.00 | 95.42 ± 2.29 | 91.60 ± 2.64 | 87.02 ± 1.32 | 79.39 ± 2.29 | 82.44 ± 4.77 | 35.12 ± 17.49 |
| Trichoderma Strains | Metrafenone (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | |
| T. afroharzianum SZMC 12432 | 24.32 ± 2.34 | 31.09 ± 5.73 | 27.03 ± 8.11 | 18.92 ± 7.02 | 20.27 ± 2.34 | 22.98 ± 5.73 | 22.97 ± 7.02 |
| T. afroharzianum SZMC 25728 | 27.63 ± 2.28 | 25.00 ± 5.59 | 22.37 ± 6.03 | 32.90 ± 6.84 | 30.26 ± 2.27 | 30.26 ± 6.03 | 32.89 ± 3.95 |
| T. afroharzianum SZMC 26672 | 32.79 ± 17.27 | 38.53 ± 10.43 | 37.71 ± 12.38 | 37.71 ± 19.88 | 47.54 ± 17.27 | 45.90 ± 9.84 | 52.46 ± 11.36 |
| T. atrobrunneum SZMC 25744 | 26.67 ± 7.64 | 25.00 ± 8.66 | 13.33 ± 2.89 | 20.00 ± 5.00 | 26.67 ± 10.41 | 13.33 ± 10.41 | 20.00 ± 5.00 |
| T. atrobrunneum SZMC 26673 | 73.68 ± 0.00 | 45.61 ± 8.04 | 57.90 ± 22.32 | 59.65 ± 13.24 | 63.16 ± 13.92 | 43.86 ± 3.04 | 61.40 ± 8.04 |
| T. guizhouense SZMC 22514 | 33.33 ± 3.71 | 34.57 ± 2.14 | 25.93 ± 0.00 | 30.87 ± 5.66 | 23.46 ± 13.01 | 28.40 ± 4.27 | 27.16 ± 9.32 |
| T. guizhouense SZMC 25749 | 59.26 ± 12.83 | 62.96 ± 23.13 | 61.11 ± 23.57 | 59.26 ± 12.83 | 51.85 ± 12.83 | 44.44 ± 0.00 | 70.37 ± 6.41 |
| T. guizhouense SZMC 26669 | 17.78 ± 1.92 | 15.56 ± 1.93 | 18.89 ± 3.85 | 15.56 ± 1.93 | 20.00 ± 5.77 | 24.45 ± 3.85 | 12.22 ± 5.09 |
| T. harzianum SZMC 1764 | 21.82 ± 11.36 | 21.82 ± 15.74 | 15.46 ± 19.28 | 10.91 ± 31.01 | 5.45 ± 3.15 | 12.73 ± 0.00 | 5.45 ± 3.15 |
| T. harzianum SZMC 1844 | 11.43 ± 4.95 | 27.14 ± 4.29 | 18.58 ± 6.06 | 11.43 ± 2.48 | 24.28 ± 4.95 | 21.43 ± 8.92 | 22.86 ± 4.29 |
| T. harzianum SZMC 25730 | 27.85 ± 3.80 | 31.65 ± 7.60 | 24.05 ± 0.00 | 24.05 ± 0.00 | 29.12 ± 4.39 | 26.58 ± 5.80 | 22.79 ± 5.80 |
| T. pollinicola SZMC 24399 | 26.15 ± 4.62 | 44.62 ± 13.06 | 23.08 ± 2.66 | 26.15 ± 0.00 | 27.69 ± 5.33 | 29.23 ± 2.67 | 26.15 ± 4.62 |
| T. simmonsii SZMC 24248 | 31.51 ± 6.28 | 35.62 ± 4.75 | 35.62 ± 4.75 | 38.36 ± 4.11 | 39.73 ± 6.28 | 36.99 ± 12.55 | 36.99 ± 2.37 |
| T. simmonsii SZMC 25740 | 52.78 ± 6.37 | 44.44 ± 2.40 | 50.00 ± 5.90 | 40.28 ± 10.48 | 38.89 ± 6.36 | 55.56 ± 2.40 | 36.11 ± 18.79 |
| T. simmonsii SZMC 26671 | 10.12 ± 8.77 | 20.26 ± 6.58 | 7.59 ± 4.39 | 15.19 ± 2.19 | 6.33 ± 5.80 | 11.39 ± 2.19 | 10.13 ± 5.80 |
| T. simmonsii SZMC 26674 | 50.00 ± 10.20 | 40.54 ± 6.19 | 40.54 ± 11.70 | 27.03 ± 7.03 | 25.68 ± 6.19 | 44.60 ± 13.03 | 33.79 ± 11.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaga, H.; Zhumakayev, A.; Büchner, R.; Kocsubé, S.; Szűcs, A.; Vágvölgyi, C.; Kredics, L.; Hatvani, L. Members of the Trichoderma harzianum Species Complex with Mushroom Pathogenic Potential. Agronomy 2021, 11, 2434. https://doi.org/10.3390/agronomy11122434
Allaga H, Zhumakayev A, Büchner R, Kocsubé S, Szűcs A, Vágvölgyi C, Kredics L, Hatvani L. Members of the Trichoderma harzianum Species Complex with Mushroom Pathogenic Potential. Agronomy. 2021; 11(12):2434. https://doi.org/10.3390/agronomy11122434
Chicago/Turabian StyleAllaga, Henrietta, Anuar Zhumakayev, Rita Büchner, Sándor Kocsubé, Attila Szűcs, Csaba Vágvölgyi, László Kredics, and Lóránt Hatvani. 2021. "Members of the Trichoderma harzianum Species Complex with Mushroom Pathogenic Potential" Agronomy 11, no. 12: 2434. https://doi.org/10.3390/agronomy11122434







