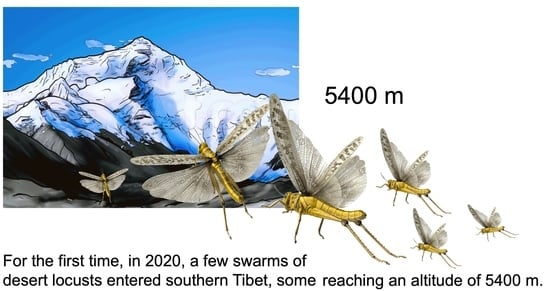
Desert Locust Stopped by Tibetan Highlands during the 2020 Upsurge
Abstract
:1. Introduction
2. Material and Methods
3. Results
3.1. Desert Locust Penetration in the Tibetan Highlands
3.2. Population Dynamics of the Desert Locust in Tibet in 2020
3.2.1. Field Survey
3.2.2. Field Caging Experiments
3.2.3. Food Preference Tests
4. Discussion
4.1. Desert Locust Routes in Tibet in July 2020
4.2. High Altitude Climate as a Major Obstacle for Desert Locusts
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Bennett, L.V. The development and termination of the 1968 plague of the desert locust, Schistocerca gregaria (Forskål) (Orthoptera, Acrididae). Bull. Entomol. Res. 1976, 66, 511–552. [Google Scholar] [CrossRef]
- Steedman, A. Locust Handbook, 3rd ed.; Overseas Development Natural Resource Institute: London, UK, 1990. [Google Scholar]
- Despland, E. Diet breadth and anti-predator strategies in desert locusts and other Orthopterans. J. Orthoptera Res. 2005, 14, 227–233. [Google Scholar] [CrossRef]
- Roffey, J.; Popov, G. Environmental and behavioural processes in a desert locust outbreak. Nature 1968, 219, 446–450. [Google Scholar] [CrossRef]
- Magor, J.I.; Lecoq, M.; Hunter, D.M. Preventive control and Desert Locust plagues. Crop Prot. 2008, 27, 1527–1533. [Google Scholar] [CrossRef]
- Kennedy, J.S. The migration of the desert locust (Schistocerca gregaria Forsk). I. The Behaviour of Swarms. II. A theory of long-range migrations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1951, 235, 163–290. [Google Scholar]
- Lorenz, M.W. Migration and trans-Atlantic flight of locusts. ScienceDirect 2009, 196, 4–12. [Google Scholar] [CrossRef]
- Uvarov, B.P. A revision of the genus Locusta, L. (= Pachytylus, Fieb.), with a new theory as to the periodicity and migrations of locusts. Bull. Entomol. Res. 1921, 12, 135–163. [Google Scholar] [CrossRef] [Green Version]
- Uvarov, B.P. Locust as a world problem. In Proceedings of the Première Conférence Internationale Pour la Protection Contre les Calamités Naturelles, Paris, France, 13–17 September 1937; Commission Française d’études des Calamités with the Support of Union Internationale de Secours: Paris, France, 1938; pp. 376–382. [Google Scholar]
- Uvarov, B.P. Grasshoppers and Locusts: A Handbook of General Acridology. II: Behaviour, Ecology, Biogeography, Population Dynamics; Centre for Overseas Pest Research: London, UK, 1977. [Google Scholar]
- Magor, J.I.; Ceccato, P.N.; Dobson, H.M.; Pender, J.; Ritchie, L. Preparedness to Prevent Desert Locust Plagues in the Central Region, an History Review, No. AGP/DL/TS/35; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007; Available online: http://www.fao.org/ag/locusts/common/ecg/1288/en/TS35ePart1text.pdf (accessed on 17 June 2021).
- Symmons, P.M.; Cressman, K. Desert Locust Guidelines 1. Biology and Behavior; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001; Available online: http://www.fao.org/ag/LOCUSTS/common/ecg/347_en_DLG1e.pdf (accessed on 17 June 2021).
- Cressman, K. Desert Locust. In Biological and Environmental Hazards, Risks, and Disasters; Shroder, J.F., Sivanpillai, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 87–105. [Google Scholar]
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and Grasshopper Management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef]
- FAO. Desert Locust Bulletin. No. 486, General Situation during March 2019; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; Available online: http://www.fao.org/ag/locusts/common/ecg/2471/en/DL486e.pdf (accessed on 17 June 2021).
- FAO. Desert Locust Bulletin. No. 488, General Situation during May 2019; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; Available online: http://www.fao.org/ag/locusts/common/ecg/2475/en/DL488e.pdf (accessed on 17 June 2021).
- FAO. Desert Locust Bulletin. No. 501, General Situation during June 2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; Available online: http://www.fao.org/ag/locusts/common/ecg/2556/en/DL501e.pdf (accessed on 17 June 2021).
- FAO. Desert Locust Bulletin. No. 502, General Situation during July 2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; Available online: http://www.fao.org/ag/locusts/common/ecg/2559/en/DL502e.pdf (accessed on 17 June 2021).
- Waloff, Z. The upsurges and recessions of the Desert Locust plague: An historical survey. Anti-Locust Mem. 1966, 8, 1–111. [Google Scholar]
- Waloff, Z. Some temporal characteristics of Desert Locust plagues. Anti-Locust Mem. 1976, 13, 1–36. [Google Scholar]
- COPR. The Locust and Grasshopper Agricultural Manual; Centre for Overseas Pest Research: London, UK, 1982. [Google Scholar]
- Chen, Y.L. A new record of the desert locust, Schistocerca gregaria (Forskål) in Tibet, China. Acta Entomol. Sin. 1982, 25, 67. [Google Scholar]
- Chen, Y.L. Watch out for the outbreaks of desert locusts. Entomol. Knowl. 2002, 39, 335–339. [Google Scholar]
- Cai, B.H. Taxonomy of Insects; Financial and Economic Publishing House: Beijing, China, 1956; Volume 1. [Google Scholar]
- Zhang, W. Will Desert Locust Invade Tibet? It’s not just the Himalayas that Stand in Front of It. Available online: https://www.tellerreport.com/life/2020-03-13---will-desert-locust-invade-tibet---it-’s-not-just-the-himalayas-that-stand-in-front-of-it-.BJDLn1YH8.html (accessed on 1 September 2021).
- Rainey, R.C. Biometeorology and insect flight: Some aspects of energy exchange. Annu. Rev. Entomol. 1974, 19, 407–439. [Google Scholar] [CrossRef]
- Cressman, K.; Stefanski, R. Weather and Desert Locusts; WMO-No. 1175; WMO: Geneva, Switzerland; FAO: Rome, Italy, 2016; Available online: https://library.wmo.int/doc_num.php?explnum_id=3213 (accessed on 17 June 2021).
- Schaefer, G.W. Radar detection of individual locusts and swarms. In Proceedings of the International Study Conference on the Current and Future Problems of Acridology, London, UK, 6–16 July 1970; Hemming, C.F., Taylor, T.H.C., Eds.; Centre for Overseas Pest Research: London, UK, 1972; pp. 379–380. [Google Scholar]
- Alexander, G. Occurrence of grasshoppers as accidentals in the Rocky Mountains of northern Colorado. Ecology 1964, 45, 77–86. [Google Scholar] [CrossRef]
- Rainey, R.C. Meteorology and the migration of desert locusts. In Applications of a Synoptic Meteorology in Locust Control; Technical Notes, No. 54; World Meteorological Organization: Geneva, Switzerland, 1963. [Google Scholar] [CrossRef]
- Shrestha, S.; Thakur, G.; Gautam, J.; Acharya, N.; Shrestha, J. Desert locust and its management in Nepal: A review. J. Agric. Nat. Resour. 2021, 4, 1–28. [Google Scholar]
- Karki, S. Locust: Winged-Terror Declines Gradually in Nepal; Khabarhub: Kathmandu, Nepal, 2020; Available online: https://english.khabarhub.com/2020/07/110094/ (accessed on 1 September 2021).
- Jia, S.L.; Wang, Q.; Li, L.; Fang, X.K.; Shi, Y.H.; Xu, W.G.; Hu, J.H. Comparative study on PCDD/F pollution in soil from the Antarctic, Arctic and Tibetan Plateau. Sci. Total Environ. 2014, 497, 353–359. [Google Scholar] [CrossRef]
- Liu, D.X.; Tong, B.; Yang, Y.S.; Yao, X.Y.; Ding, Y.; Xia, Z.Y. Basic Characteristics and Proneness Evaluation about Debris Flow in Zhangmu Gully of Tibet. Electron. J. Geotech. Eng. 2016, 21, 7433–7446. [Google Scholar]
- Liu, X.D.; Chen, B.D. Climatic warming in the Tibetan Plateau during recent decades. Int. J. Climatol. 2000, 20, 1729–1742. [Google Scholar] [CrossRef]
- Wu, G.X.; Duan, A.; Liu, Y.; Mao, J.; Ren, R.; Bao, Q.; He, B.; Liu, B.; Hu, W. Tibetan Plateau climate dynamics: Recent research progress and outlook. Natl. Sci. Rev. 2015, 2, 100–116. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.H.; Yin, Y.H.; Zheng, D.; Yang, Q.Y. Climate changes in the Tibetan Plateau during the last three decades. Acta Geogr. Sin. 2005, 60, 3–11. [Google Scholar]
- Neville, A.C.; Weis-Fogh, T. The effect of temperature on locust flight muscle. J. Exp. Biol. 1963, 40, 111–121. [Google Scholar] [CrossRef]
- Roffey, J. Observations on gliding in the desert locust. Anim. Behav. 1963, 11, 359–366. [Google Scholar] [CrossRef]
- Weis-Fogh, T. Biology and physics of locust flight II. Flight performance of the desert locust (Schistocerca gregaria). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1956, 239, 459–510. [Google Scholar]
- Harrison, J.F.; Kaiser, A.; VandenBrooks, J.M. Atmospheric oxygen level and the evolution of insect body size. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 277, 1937–1946. [Google Scholar] [CrossRef] [Green Version]
- Hoback, W.W.; Stanley, D.W. Insects in hypoxia. J. Insect Physiol. 2001, 47, 533–542. [Google Scholar] [CrossRef]
- Rascón, B.; Harrison, J.F. Oxygen partial pressure effects on metabolic rate and behavior of tethered flying locusts. J. Insect Physiol. 2005, 51, 1193–1199. [Google Scholar] [CrossRef]
- Dahlhoff, E.P.; Dahlhoff, V.C.; Grainger, C.A.; Zavala, N.A.; Otepola-Bello, D.; Sargent, B.A.; Roberts, K.T.; Heidl, S.J.; Smiley, J.T.; Rank, N.E. Getting chased up the mountain: High elevation may limit performance and fitness characters in a montane insect. Funct. Ecol. 2019, 33, 809–818. [Google Scholar] [CrossRef]
- Duranton, J.F.; Lecoq, M. Le Criquet pèlerin au Sahel; Collection Acridologie Opérationnelle n°6; Comité Inter-Etats de Lutte contre la Sécheresse dans le Sahel, Département de Formation en Protection des Végétaux: Niamey, Niger, 1990; Available online: http://locust.cirad.fr/ouvrages_pratiques/pdf/DFPV6.pdf (accessed on 2 June 2021).
- Norris, M.J. Reproduction in the desert locust Schistocerca gregaria (Forsk.) in relation to density and phase. Anti-Locust Bull. 1952, 13, 1–49. [Google Scholar]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, C.; Taratiel, D.; Casanova, J.L. Desert locust detection using Earth observation satellite data in Mauritania. J. Arid Environ. 2019, 164, 29–37. [Google Scholar] [CrossRef]
- Huque, H.; Jaleel, M.A. Temperature-Induced Quiescence in the Eggs of the Desert Locust. J. Econ. Entomol. 1970, 63, 1398–1400. [Google Scholar] [CrossRef]





| Sites | Altitude (m) | Locust Density | Latitude | Longitude |
|---|---|---|---|---|
| Saga (Jilong valley) | 5400 | Light | 29°19′48″ | 85°13′56″ |
| Zhongba (Zhongba County) | 4800 | Light | 29°46′12″ | 84°01′53″ |
| Jialong Lake | 4750 | None | 28°03′54″ | 86°03′43″ |
| Kuimai lake | 4725 | None | 28°40′52″ | 85°56′27″ |
| Langqiang Lake | 4670 | Very light | 28°43′26″ | 85°53′31″ |
| Puluo pasture | 4200 | Very light | 28°10′39″ | 85°56′47″ |
| Jimuxiong | 3750 | Very light | 28°08′33″ | 85°58′17″ |
| Pulan (Pulan valley) | 3600 | Light | 30°16′05″ | 81°10′06″ |
| Jilong (Jilong valley) | 3530 | Middle | 28°45′01″ | 85°21′47″ |
| 4.1 | 3380 | Light | 28°06′27″ | 85°59′55″ |
| Qu Town | 3365 | Middle | 28°05′49″ | 85°59′46″ |
| 3.2 | 3350 | Middle | 28°05′59″ | 85°59′50″ |
| 3.1 | 3340 | Middle | 28°05′45″ | 85°59′46″ |
| Chentang (Chentang valley) | 3300 | Light | 27°51′39″ | 87°25′13″ |
| Dingri (Gama valley) | 3200 | Light | 28°34′00″ | 86°38′00″ |
| Cuoxiamu | 3150 | Middle | 28°04′37″ | 85°59′54″ |
| 2.2 | 2500 | Heavy | 27°59′37″ | 85°59′8″ |
| 2.1 | 2350 | Heavy | 27°59′34″ | 85°58′57″ |
| 1.3 | 2330 | Very heavy | 27°59′50″ | 85°58′47″ |
| 1.2 | 2320 | Very heavy | 27°59′50″ | 85°58′47″ |
| 1.1 | 1950 | Very heavy | 27°58′09″ | 85°57′54″ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Lecoq, M.; Zhang, L. Desert Locust Stopped by Tibetan Highlands during the 2020 Upsurge. Agronomy 2021, 11, 2287. https://doi.org/10.3390/agronomy11112287
Liu J, Lecoq M, Zhang L. Desert Locust Stopped by Tibetan Highlands during the 2020 Upsurge. Agronomy. 2021; 11(11):2287. https://doi.org/10.3390/agronomy11112287
Chicago/Turabian StyleLiu, Jun, Michel Lecoq, and Long Zhang. 2021. "Desert Locust Stopped by Tibetan Highlands during the 2020 Upsurge" Agronomy 11, no. 11: 2287. https://doi.org/10.3390/agronomy11112287