Changes in Photosynthetic Pigments Content in Non-Transformed and AtCKX Transgenic Centaury (Centaurium erythraea Rafn) Shoots Grown under Salt Stress In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Media, and Culture Conditions
2.2. Quantification of Photosynthetic Pigments
2.3. Statistical Analysis
3. Results
3.1. Effect of NaCl on Photosynthetic Pigments Content of Centaury Shoots Grown In Vitro
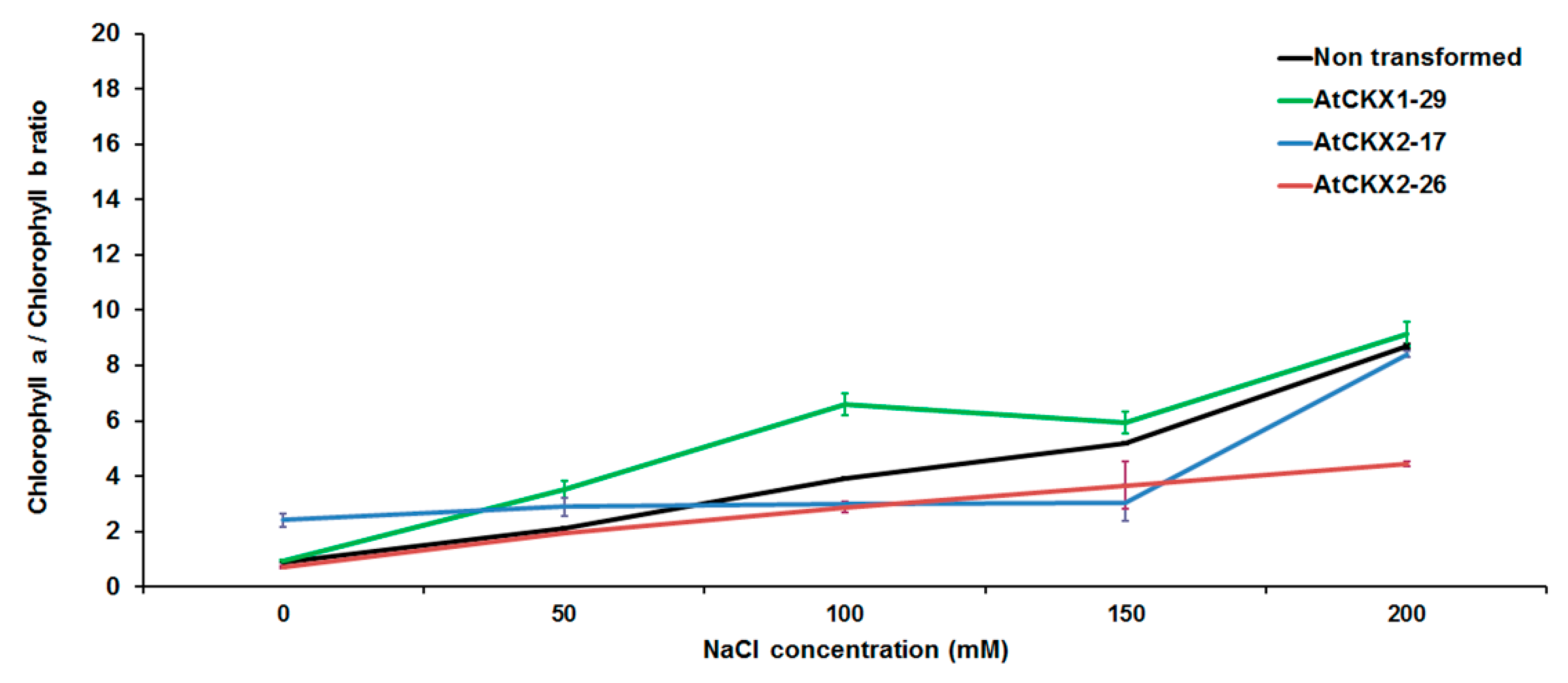
3.2. Effect of NaCl on Photosynthetic Pigments Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonović, A.D.; Trifunović-Momčilov, M.M.; Filipović, B.K.; Marković, M.P.; Bogdanović, M.D.; Subotić, A.R. Somatic embryogenesis in Centaurium erythraea Rafn—Current status and perspectives: A review. Plants 2021, 10, 70. [Google Scholar] [CrossRef]
- Majeed, A.; Nisar, M.F.; Hussain, K. Effect of saline culture on the concentration of Na+, K+ and Cl− in Agrostis tolonifera. Curr. Res. J. Biol. Sci. 2010, 2, 76–82. [Google Scholar]
- Li, W.; Li, Q. Effect of environmental salt stress on plants and the molecular mechanism of salt stress tolerance. Int. J. Environ. Sci. Nat. Res. 2017, 7, 555714. [Google Scholar] [CrossRef]
- Singhal, R.K.; Saha, D.; Skalicky, M.; Mishra, U.N.; Chauhan, J.; Behera, L.P.; Lenka, D.; Chand, S.; Kumar, V.; Dey, P.; et al. Crucial cell signaling compounds crosstalk and integrative multi-omics techniques for salinity stress tolerance in plants. Front. Plant Sci. 2021, 670369. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.K.; Mekapogu, M.; Kim, K.S. Effect of salinity stress on photosynthesis and related physiological responses in carnation (Dianthus caryophyllus). Hortic. Environ. Biotechnol. 2019, 60, 831–839. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Brestic, M.; Kalaji, H.M.; Skalicky, M.; Noedoost, F. Environmental pollution is reflected in the activity of the photosynthetic apparatus. Photosyhthetica 2020, 58, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Turan, S.; Tripathy, B.C. Salt-stress induced modulation of chlorophyll biosynthesisduring de-etiolation of rice seedlings. Physiol. Plant. 2015, 153, 477–491. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qi, C.; Maodong, Q.; Dong, X.X.; Ahmad, P.; Fathi, E.; Allah, A.; Zhang, L. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol. Bioch. 2019, 144, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Taha, R.S.; Mahdi, A.H. Proline enhances growth, productivity and anatomy of two varieties of Lupinus termis L. grown under salt stress. S. Afr. J. Bot. 2016, 102, 221–227. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.-L.; Liu, L.-N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef]
- Rao, G.G.; Rao, G.R. Pigment composition chlorophyllase activity in pigeon pea (Cajanus indicus Spreng) and Gingelley (Sesamum indicum L.) under NaCl salinity. Indian J. Exp. Biol. 1981, 19, 768–770. [Google Scholar]
- Ali, Y.; Aslam, Z.; Ashraf, M.Y.; Tahir, G.R. Effect of salinity on chlorophyll concentration, leaf area, yield and yield components of rice genotypes grown under saline environment. Int. J Environ. Sci. Technol. 2004, 1, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Langsdorf, G.; Lenk, S.; Bushmann, C. Chlorophyll fluorescence imaging of photosynthetic activity with the flesh lamp fluorescence imaging system. Phtosynthetica 2005, 43, 355–369. [Google Scholar] [CrossRef]
- Molazem, D.; Qurbanov, E.M.; Dunyamaliyev, S.A. Role of proline, Na and chlorophyll content in salt tolerance of corn (Zea mays L.). Amer. Eurasian J. Agric. Environ. Sci. 2010, 9, 319–324. [Google Scholar]
- Sevengor, S.; Yasar, F.; Kusvuran, S.; Ellialtıoglu, S. The effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidative enzymes of pumpkin seedling. Amer. J. Agri. Res. 2011, 6, 4920–4924. [Google Scholar] [CrossRef]
- Akca, Y.; Samsunlu, E. The effect of salt stress on growth, chlorophyll content, proline and nutrient accumulation, and K/Na ratio in in walnut. Pak. J. Bot. 2012, 44, 1513–1520. [Google Scholar]
- Zhani, K.; Mariem, B.F.; Fardaous, M.; Cherif, H. Impact of salt stress (NaCl) on growth, chlorophyll content and fluorescense of Tunusian cultivars of chili pepper (Capsicum frutescens L.). J. Stress Physiol. Biochem. 2012, 8, 236–252. [Google Scholar]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Yarsi, G.; Sivaci, A.; Dasgan, H.Y.; Altuntas, O.; Binzet, R.; Akhoundnejad, Y. Effects of salinity stress on chlorophyll and carotenoid contents and stomata size of grafted and ungrafted galia c8 melon cultivar. Pak. J. Bot. 2017, 49, 421–426. [Google Scholar]
- Fatehi, S.F.; Oraei, M.; Gohari, G.; Akbari, A.; Faramarzi, A. Proline-functionalized graphene oxide nanoparticles (GO–Pro NPs) mitigate salt-induced adverse effects on morpho-physiological traits and essential oils constituents in Moldavian Balm (Dracocephalum moldavica L.). J. Plant Growth Regul. 2021, 1–5. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Kubo, M.; Kakimoto, T. The cytokinin-hypersensitive genes of Arabidopsis nega-tively regulate the cytokinin-signaling pathway for cell division and chloroplast development. Plant J. 2000, 23, 385–394. [Google Scholar] [CrossRef]
- Kulaeva, O.N.; Burkhanova, E.A.; Karavaiko, N.N.; Selivankina, S.Y.; Porfirova, S.A.; Maslova, G.G.; Zemlyachenko, Y.V.; Börner, T. Chloroplasts affect the leaf response to cytokinin. J. Plant Physiol. 2002, 159, 1309–1316. [Google Scholar] [CrossRef]
- Cortleven, A.; Noben, J.P.; Valcke, R. Analysis of the photosynthetic apparatus in trans-genic tobacco plants with altered endogenous cytokinin content. Proteome. Sci. 2011, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortleven, A.; Schmülling, T. Regulation of chloroplast development and function by cytokinin. J. Exp. Bot. 2015, 66, 4999–5013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, S.; Amasino, R. Making sense of senescence (Molecular genetic regulation and manipulation of leaf senescence). Plant Physiol. 1997, 113, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [Green Version]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-defcient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, A.; Senning, M.; Hedden, P.; Sonnewald, U.; Sonnewald, S. Reactivation of meristem activity and sprout growth of potato tubers require both cytokinin and gibberellin. Plant Physiol. 2011, 155, 776–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspor, M.; Motyka, V.; Žižková, E.; Dobrev, P.I.; Trávníčková, A.; Zdravković-Korać, S.; Simonović, A.; Ninković, S.; Dragićević, I.Č. Cytokinin profles of AtCKX2-overexpressing potato plants and the impact of altered cytokinin homeostasis on tuberization in vitro. J. Plant. Growth Regul. 2012, 31, 460–470. [Google Scholar] [CrossRef]
- Raspor, M.; Motyka, V.; Ninković, S.; Malbeck, J.; Dobrev, P.I.; Zdravković-Korać, S.; Simonović, A.; Ćosić, T.; Cingel, A.; Savić, J.; et al. Overexpressing AtCKX1 in potato plants grown in vitro: The efects on cytokinin composition and tuberization. J. Plant. Growth Regul. 2021, 40, 37–47. [Google Scholar] [CrossRef]
- Trifunović, M.; Motyka, V.; Cingel, A.; Subotić, A.; Jevremovićm, S.; Petrić, M.; Holík, J.; Malbeck, J.; Dobrev, P.I.; Dragićević, I.Č. Changes in cytokinin content and altered cytokinin homeostasis in AtCKX1 and AtCKX2-overexpressing centaury (Centaurium erythraea Rafn.) plants grown in vitro. Plant. Cell Tiss. Org. 2015, 120, 767–777. [Google Scholar] [CrossRef]
- Nehnevajova, E.; Ramireddy, E.; Stolz, A.; Gerdemann-Knörck, M.; Novák, O.; Strnad, M.; Schmülling, T. Root enhancement in cytokinin-deficient oilseed rape causes leaf mineral enrichment, increases the chlorophyll concentration under nutrient limitation andenhances the phytoremediation capacity. BMC Plant. Biol. 2019, 19, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwack, P.J.; Rashotte, A.M. Cytokinin inhibition of leaf senescence. Plant. Signal. Behav. 2013, 8, e24737. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Halophytic herbs of the Mediterranean basin: An alternative approach to health. Food Chem. Toxicol. 2018, 114, 155–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šiler, B.; Mišić, D.; Filipović, B.; Popović, Z.; Cvetić, T.; Mijović, A. Effects of salinity on in vitro growth and photosynthesis of common centaury (Centaurium erythraea Rafn.). Arc. Biol. Sci. 2007, 59, 129–134. [Google Scholar] [CrossRef]
- Trifunović-Momčilov, M.; Paunović, D.; Milošević, S.; Marković, M.; Jevremović, S.; Dragićević, I.Č.; Subotić, A. Salinity stress response of non-transformed and AtCKX transgenic centaury (Centaurium erythraea Rafn.) shoots and roots grown in vitro. Ann. Appl. Biol. 2020, 177, 74–89. [Google Scholar] [CrossRef]
- Trifunović, M.; Cingel, A.; Simonović, A.; Jevremović, S.; Petrić, M.; Dragićević, I.Č.; Motyka, V.; Dobrev, P.I.; Zahajská, L.; Subotić, A. Overexpression of Arabidopsis cytokinin oxidase/dehydrogenase genes AtCKX1 and AtCKX2 in transgenic Centaurium erythraea Rafn. Plant. Cell Tiss. Org. 2013, 115, 139–150. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Wilhelmová, N.; Procházková, D.; Macháčková, I.; Vágner, M.; Srbová, M.; Wilhelm, J. The role of cytokinins and ethylene in bean cotyledon senescence. The effect of free radicals. Biol. Plant. 2004, 48, 523–529. [Google Scholar] [CrossRef]
- Jordi, W.; Schapendonk, A.; Davelaar, E.; Stoopen, G.M.; Pot, C.S.; De Visser, R.; Van Rhijn, J.A.; Gan, S.; Amasino, R.M. Increased cytokinin levels in transgenic PSAG12-IPT tobacco plants have large direct and indirect effects on leaf senescence, photosynthesis and N partitioning. Plant. Cell Environ. 2000, 23, 279–289. [Google Scholar] [CrossRef]
- Stessman, D.; Miller, A.; Spalding, M.; Rodermel, S. Regulation of photosynthesis during Arabidopsis leaf development in continuous light. Photosynth. Res. 2002, 72, 27–37. [Google Scholar] [CrossRef]
- Jiao, D.; Ji, B.; Li, X. Characteristics of chlorophyll fluorescence and membrane-lipid peroxidation during senescence of flag leaf in different cultivars of rice. Photosynthetica 2003, 41, 33–41. [Google Scholar] [CrossRef]
- Weng, X.Y.; Xu, H.X.; Jiang, D.A. Characteristics of gas exchange, chlorophyll fluorescence and expression of key enzymes in photosynthesis during leaf senescence in rice plants. J. Integr. Plant. Biol. 2005, 47, 560–566. [Google Scholar] [CrossRef]
- Heidari, A.; Bandehagh, A.; Toorchi, M. Effects of NaCl stress on chlorophyll content and chlorophyll fluorescence in sunflower (Helianthus annuus L.) lines. Yyu. J. Agr. Sci. 2014, 24, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Hniličková, H.; Hnilička, F.; Martinková, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant. Soil Environ. 2017, 63, 362–367. [Google Scholar]
- Najar, R.; Aydi, S.; Sassi-Aydi, S.; Zarai, A.; Abdelly, C. Effect of salt stress on photosynthesis and chlorophyll fluorescence in Medicago truncatula. Plant. Biosyst. 2018, 153, 88–97. [Google Scholar] [CrossRef]
- Mýtinová, Z.; Haisel, D.; Wilhelmová, N. Photosynthesis and protective mechanisms during ageing in transgenic tobacco leaves with over-expressed cytokinin oxidase/dehydrogenase and thus lowered cytokinin content. Photosynthetica 2006, 44, 599–605. [Google Scholar] [CrossRef]
- Conrad, K.; Motyka, V.; Schlüter, T. Increase in activity, glycosylation and expression of cytokinin oxidase/dehydrogenase during the senescence of barley leaf segments in the dark. Physiol. Plant. 2007, 130, 572–579. [Google Scholar] [CrossRef]
- Evans, T.; Song, J.; Jameson, P.E. Micro-scale chlorophyll analysis and developmental expression of a cytokinin oxidase/dehydrogenase gene during leaf development and senescence. Plant. Growth Regul. 2012, 66, 95–99. [Google Scholar] [CrossRef]
- Ciobanu, I.; Sumalan, R. The effects of the salinity stress on the growing rates and physiological characteristics to the Lycopersicum esculentum Specie. B. Uasvm. Horti. 2009, 66, 616–620. [Google Scholar]
- Li, P.; Zhu, Y.; Song, X.; Song, F. Negative effects of long-term moderate salinity and short-term drought stress on the photosynthetic performance of Hybrid Pennisetum. Plant. Physiol. Bioch. 2020, 155, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; de Sousa, S.; da Silva, J.M.; Fevereiro, M.P.; da Silva, A.B. Physiological responses of the legume model Medicago truncatula cv. Jemalong to water deficit. Environ. Exp. Bot. 2008, 63, 289–296. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Turan, M.A.; Kalkat, V.; Taban, S. Salinity-induced stomatal resistance, proline, chlorophyll and ion concentrations of bean. Int. J. Agric. Res. 2007, 2, 483–488. [Google Scholar] [CrossRef]
- Cheruth, J.; Ragupathi, G.; Ashot, K.; Paramasivam, M.; Beemarao, S.; Rajaram, P. Interactive effects of triadimefon and salt stress on antioxidative status and ajmalicine accumulation in Catharanthus roseus. Acta Physiol. Plant. 2008, 30, 287–292. [Google Scholar] [CrossRef]
- Taffouo, V.D.; Kouamou, J.K.; Ngalangue, L.M.T.; Ndjeudji, B.A.N.; Akoa, A. Effects of salinity stress on growth, ions partitioning and yield of some cowpea (Vigna ungiuculata L., walp.) cultivars. Int. J. Bot. 2009, 5, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Taffouo, V.D.; Wamba, O.F.; Yombi, E.; Nono, G.V.; Akoe, A. Growth, yield, water status and ionic distribution response of three bambara groundnut (Vigna subterranean (L.) verdc.) landraces grown under saline conditions. Int. J. Bot. 2010, 6, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Heidari, M. Effects of salinity stress on growth, chlorophyll content and osmotic components of two basil (Ocimum basilicum L.) genotypes. Afr. J. Biotechnol. 2012, 11, 379–384. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Cho, M.C.; Lee, J.G. Evaluation of chlorophyll fluorescence parameters and proline content in tomato seedlings grown under different salt stress conditions. Hortic. Environ. Biote. 2020, 61, 433–443. [Google Scholar] [CrossRef]
- Rastogi, A.; Kovar, M.; He, X.; Zivcak, M.; Kataria, S.; Kalaji, H.M.; Skalicky, M.; Ibrahimova, U.F.; Hussain, S.; Mbarki, S.; et al. JIP-test as a tool to identify salinity tolerance in sweet sorghum genotypes. Photosynthetica 2020, 58, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Wei, Y.; Wang, J.; Liang, M.; Zhao, G. Salinity-induced alterations in physiological and biochemical processes of blessed thistle and peppermint. J. Soil Sci. Plant. Nut. 2021, 1–14. [Google Scholar] [CrossRef]
- Misra, A.N.; Sahu, S.M.; Misra, M.; Singh, P.; Meera, I.; Das, N.; Kar, M.; Sahu, P. Sodium chloride induced changes in leaf growth, and pigment and protein contents in two rice cultivars. Biol. Plant. 1997, 39, 257–262. [Google Scholar] [CrossRef]
- Biswal, A.K.; Dilnawaz, F.; Ramaswamy, N.K.; David, K.A.V.; Misra, A.N. Thermoluminescence characteristics of sodium chloride salt-stressed Indian mustard seedlings. Luminescence 2002, 17, 135–140. [Google Scholar] [CrossRef]
- Youssef, T.; Awad, M. Mechanisms of enhancing photosynthetic gas exchange in date palm seedlings (Phoenix dactylifera L.) under salinity stress by a 5-aminolevulinic acid-based fertilizer. J. Plant. Growth Regul. 2008, 27, 1–9. [Google Scholar] [CrossRef]
- Khan, N.A. NaCl-inhibited chlorophyll synthesis and associated changes in ethylene evolution and antioxidative enzyme activities in wheat. Biol. Plant. 2003, 47, 437–440. [Google Scholar] [CrossRef]
- Tort, N.; Turkyilmaz, B. A physiological investigation on the mechanisms of salinity tolerance in some barley culture forms. J. Forensic Sci. 2004, 27, 1–16. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Panda, S.K. Salt tolerance of two aquatic macrophytes. Pistia stratiotes and Salvinia molesta. Biol. Plant. 2005, 49, 157–159. [Google Scholar] [CrossRef]
- Mustard, J.; Renault, S. Response of red-osier dogwood (Cornus sericea) seedling to NaCl during the onset of bud break. Can. J. Bot. 2006, 84, 844–851. [Google Scholar] [CrossRef]
- Sharma, P.K.; Hall, D.O. Interaction of salt stress and photoinhibition on photosynthesis in barley and sorghum. J. Plant. Physiol. 1991, 138, 614–619. [Google Scholar] [CrossRef]
- Park, S.; Fischer, A.L.; Steen, C.J.; Iwai, M.; Morris, J.M.; Walla, P.J.; Niyogi, K.K.; Fleming, G.R. Chlorophyll-carotenoid excitation energy transfer in high-light-exposed thylakoid membranes investigated by snapshot transient absorption spectroscopy. J. Am. Chem. Soc. 2018, 140, 11965–11973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajihashemi, S.; Mbarki, S.; Skalicky, M.; Noedoost, F.; Raeisi, M.; Brestic, M. Effect of wastewater irrigation on photosynthesis, growth, and anatomical features of two wheat cultivars (Triticum aestivum L.). Water 2020, 12, 607. [Google Scholar] [CrossRef] [Green Version]
- Caffarri, S.; Tibiletti, T.; Jennings, R.C.; Santabarbara, S. A comparison between plant photosystem I and photosystem II architecture and functioning. Curr. Protein Pept. Sci. 2014, 15, 296–331. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Buschmann, C.; Döll, M.; Fietz, H.J.; Bach, T.; Kozel, U.; Meier, D.; Rahmsdorf, U. Photosynthetic activity, chloroplast ultrastructure and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 1981, 2, 115–141. [Google Scholar] [CrossRef]
- Bavani, M.R.Z.; Peyvast, G.; Ghasemnezhad, M.; Forghani, A. Assessment of genotypic variation in salt tolerance of pepper (Capsicum annuum L.) cultivars using gas exchange characteristic, growth parameters and chlorophyll content. Southwest J. Hort. Biol. Environ. 2015, 6, 71–90. [Google Scholar]
- Fang, Z.; Bouwkamp, J.; Solomos, T. Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J. Exp. Bot. 1998, 49, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Franck, N.; Winkler, S.; Pastenes, C.; Infante, R. Acclimation to sun and shade of three accessions of the Chilean native berry-crop murta. Agroforest. Syst. 2007, 69, 215–229. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; Harbinson, J.; van Ieperen, W. Photosynthetic acclimation in relation to nitrogen allocation in cucumber leaves in response to changes in irradiance. Physiol. Plant. 2011, 142, 157–169. [Google Scholar] [CrossRef]
- Miersch, I.; Heise, J.; Zelmer, I.; Humbeck, K. Differential degradation of the photosynthetic apparatus during leaf senescence in barley (Hordeum vulgare L.). Plant. Biol. 2000, 2, 618–623. [Google Scholar] [CrossRef]
- Mishev, K.; Stefanov, D.; Ananieva, K.; Slavov, C.; Ananiev, E.D. Different effects of dark treatment on pigment composition and photosystem I and II activities in intact cotyledons and primary leaves of Cucurbita pepo (zucchini). Plant. Growth Regul. 2009, 58, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Weaver, L.M.; Amasino, R.M. Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants. Plant. Physiol. 2001, 127, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Ananieva, K.; Ananiev, E.D.; Doncheva, S.; Georgieva, K.; Tzvetkova, N.; Kamínek, M.; Motyka, V.; Dobrev, P.; Gajdošová, S.; Malbeck, J. Senescence progression in a single darkened cotyledon depends on the light status of the other cotyledon in Cucurbita pepo (zucchini) seedlings: Potential involvement of cytokinins and cytokinin oxidase/dehydrogenase activity. Physiol. Plant. 2008, 134, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Prot. F. Anal. Chem. 2001, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F.; Langsdorf, G. Chlorophyll fluorescence imaging of photosynthetic activity in sun and shade leaves of trees. Photosynth. Res. 2007, 93, 235–244. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifunović-Momčilov, M.; Milošević, S.; Marković, M.; Đurić, M.; Jevremović, S.; Dragićević, I.Č.; Subotić, A.R. Changes in Photosynthetic Pigments Content in Non-Transformed and AtCKX Transgenic Centaury (Centaurium erythraea Rafn) Shoots Grown under Salt Stress In Vitro. Agronomy 2021, 11, 2056. https://doi.org/10.3390/agronomy11102056
Trifunović-Momčilov M, Milošević S, Marković M, Đurić M, Jevremović S, Dragićević IČ, Subotić AR. Changes in Photosynthetic Pigments Content in Non-Transformed and AtCKX Transgenic Centaury (Centaurium erythraea Rafn) Shoots Grown under Salt Stress In Vitro. Agronomy. 2021; 11(10):2056. https://doi.org/10.3390/agronomy11102056
Chicago/Turabian StyleTrifunović-Momčilov, Milana, Snežana Milošević, Marija Marković, Marija Đurić, Slađana Jevremović, Ivana Č. Dragićević, and Angelina R. Subotić. 2021. "Changes in Photosynthetic Pigments Content in Non-Transformed and AtCKX Transgenic Centaury (Centaurium erythraea Rafn) Shoots Grown under Salt Stress In Vitro" Agronomy 11, no. 10: 2056. https://doi.org/10.3390/agronomy11102056









