Chemical Traits of Fermented Alfalfa Brown Juice: Its Implications on Physiological, Biochemical, Anatomical, and Growth Parameters of Celosia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Brown Juice Production and Its Characteristics
2.1.1. Source of Alfalfa Biomass
2.1.2. Extraction of Brown Juice
2.1.3. Fermentation of Brown Juice
2.1.4. Determination of Lactic Acid Bacteria
2.1.5. Chemical Properties of Brown Juice
2.2. Celosia Experiment
2.2.1. Experimental Design
2.2.2. Determination of Water-Soluble Protein and Antioxidant Enzymes
2.2.3. Malondialdehyde Measurement
2.2.4. Photosynthetic Pigment
2.2.5. Histology
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Brown Juice
3.1.1. Chemical Traits of Brown Juice
3.1.2. Contents of Sugars and Organic Acids in the Brown Juice
3.1.3. Macro- and Microelements Content of Brown Juice
3.2. Fermented Brown Juice as A Growth Stimulator
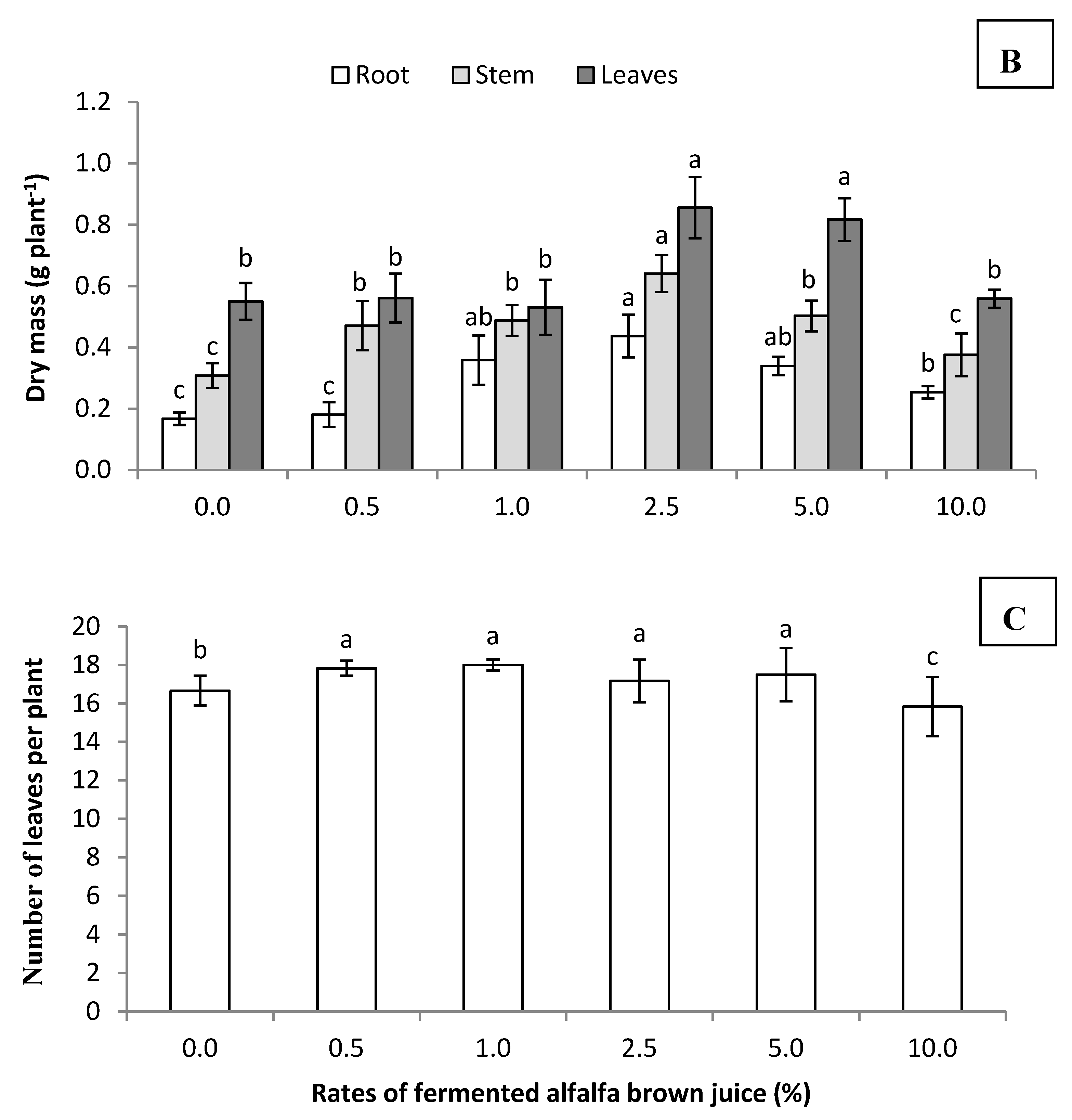
3.2.1. Growth Dynamic of Celosia
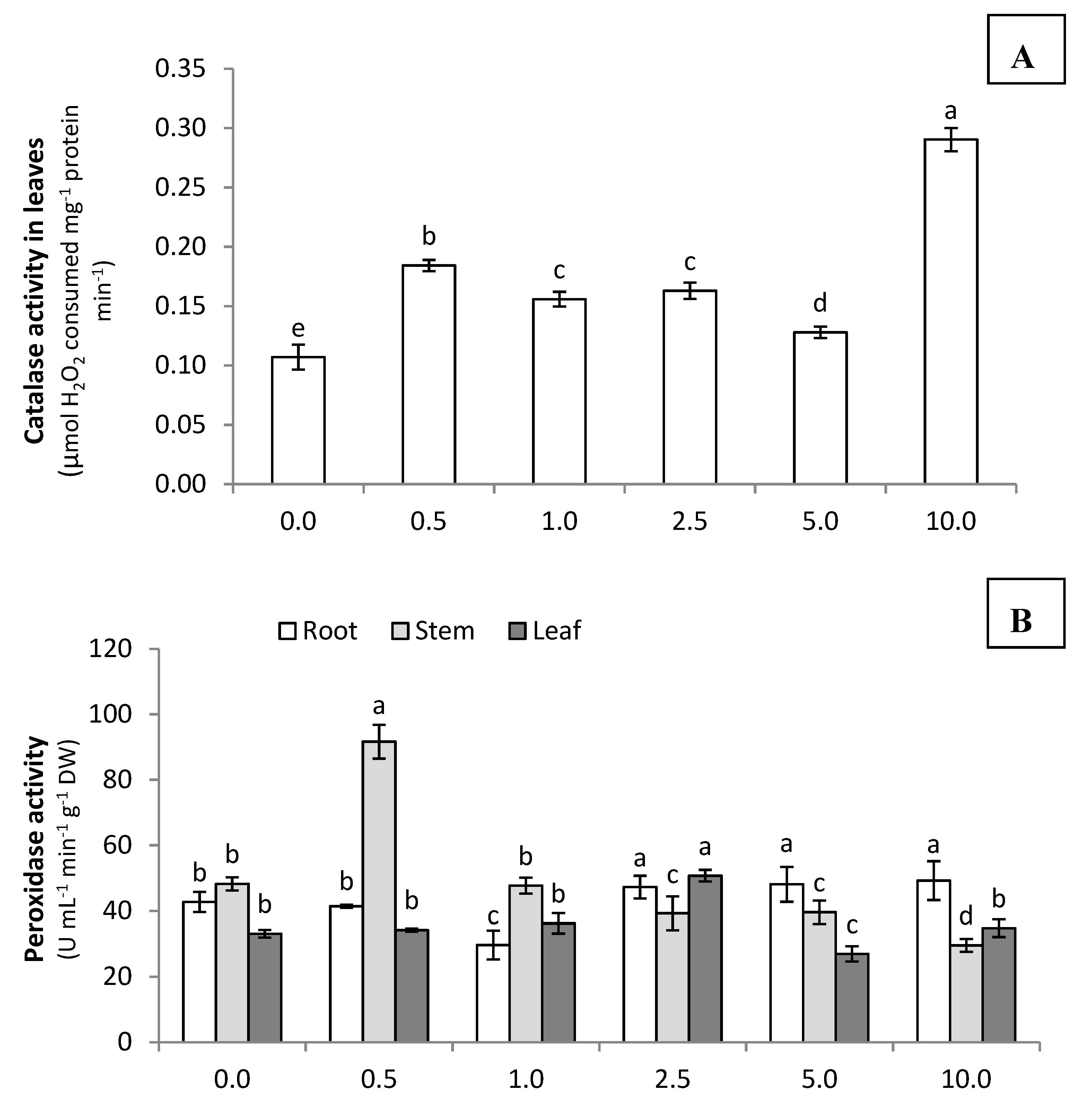
3.2.2. Antioxidant Capacity of Celosia Plants Treated with Fermented Brown Juice
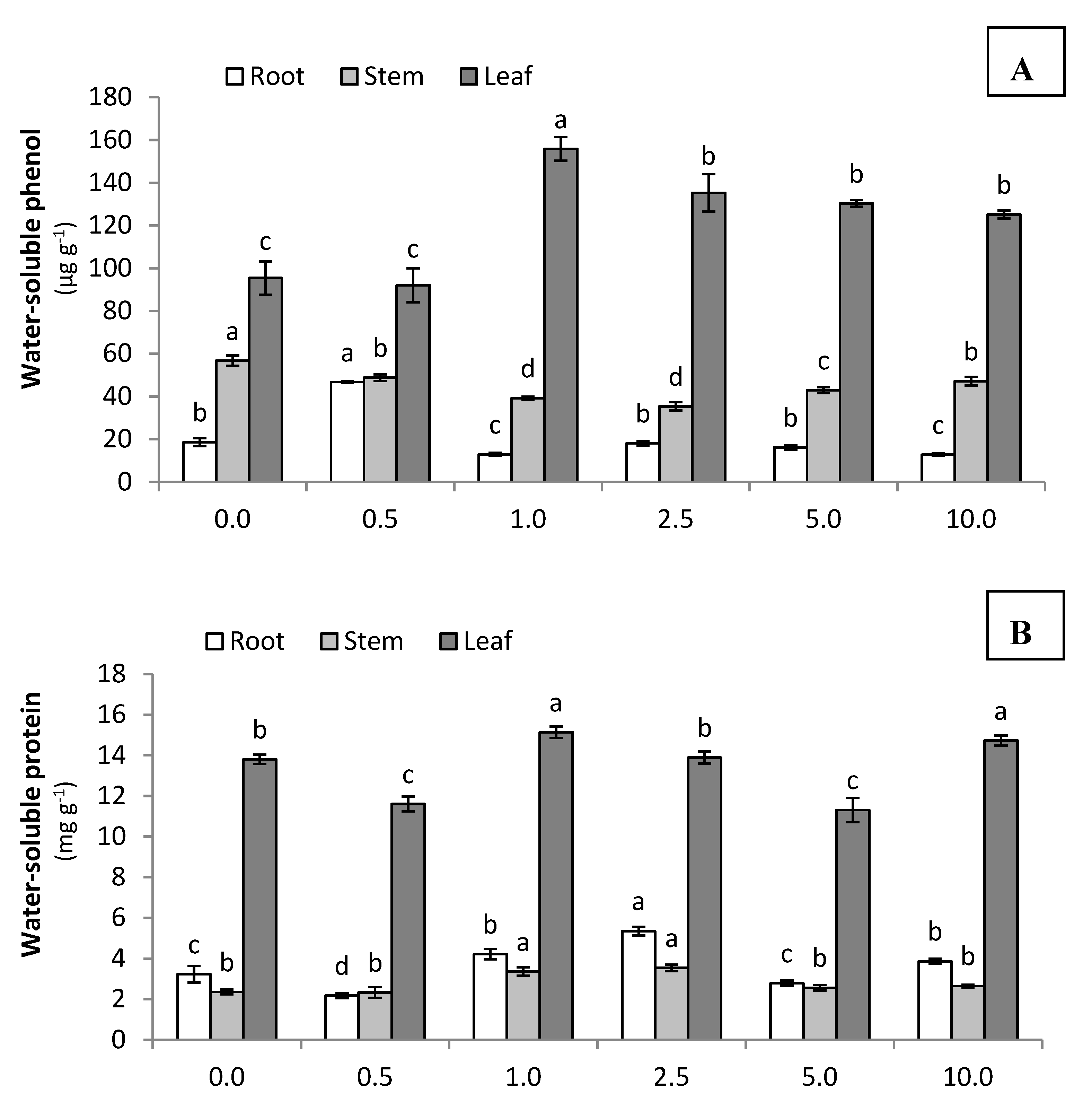
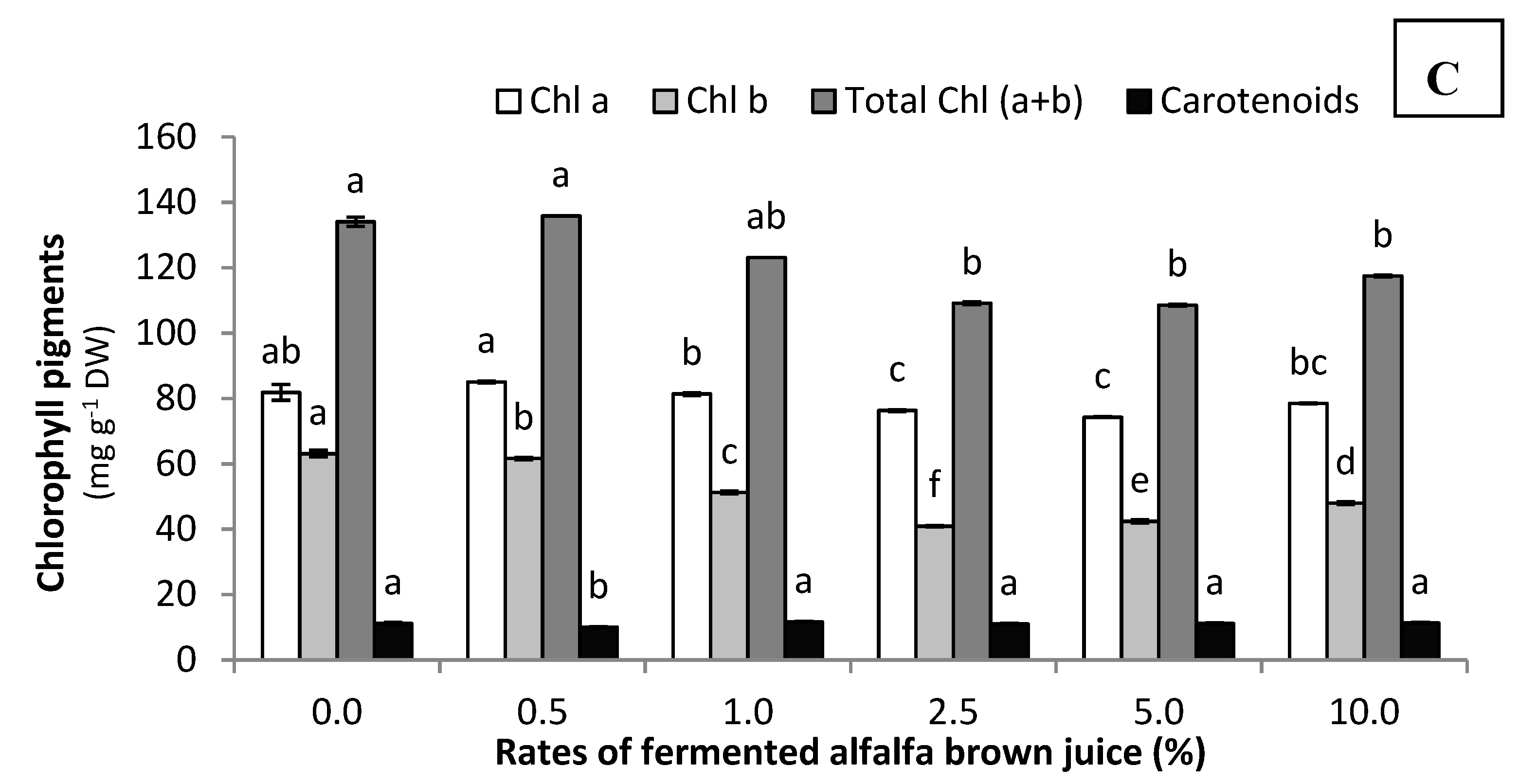
3.2.3. Phenolic, Protein, and Photosynthetic Pigments Contents
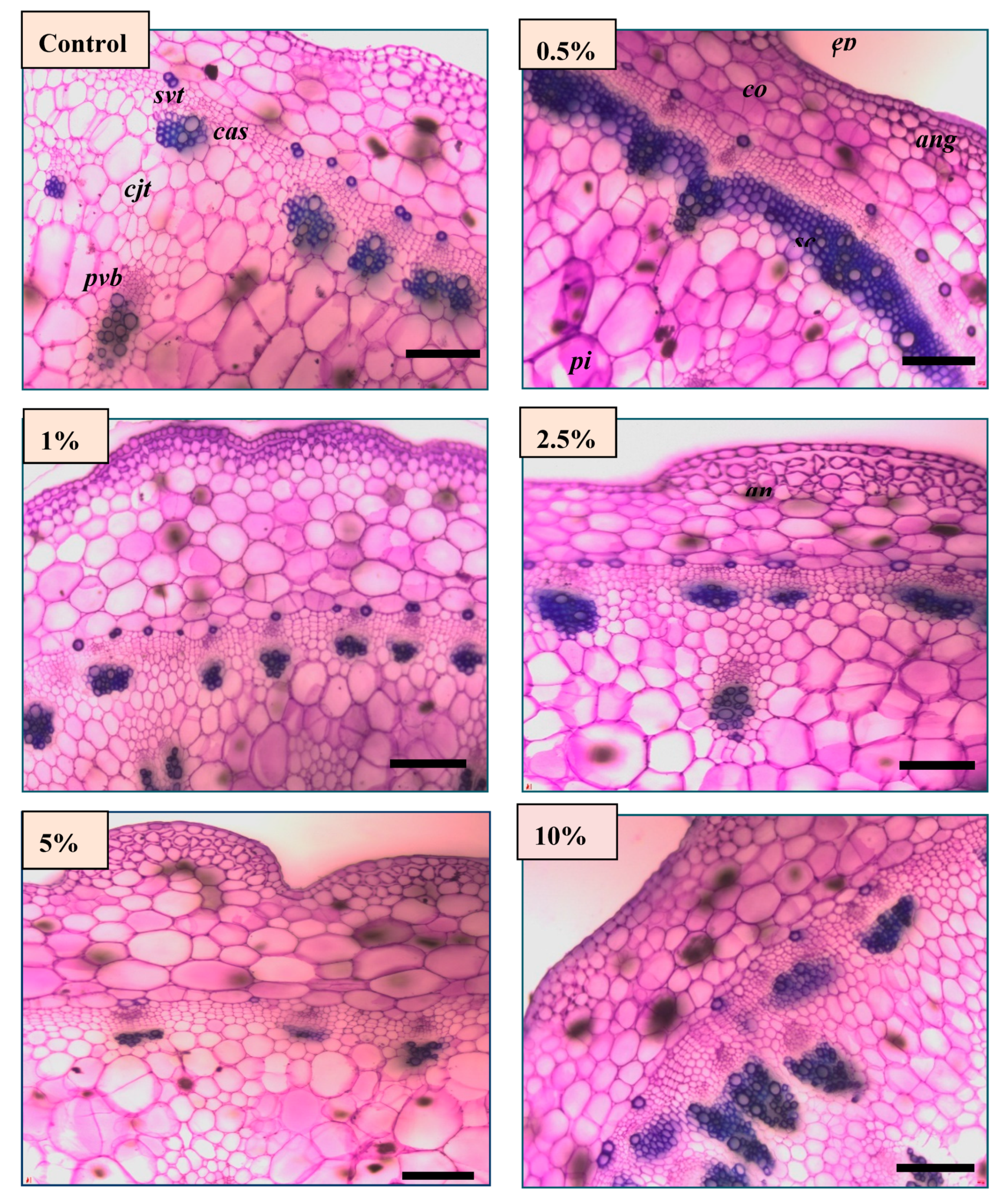
3.2.4. Anatomical Features of Celosia Stem after Brown Juice Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripathi, A.D.; Mishra, R.; Maurya, K.K.; Singh, R.B.; Wilson, D.W. Estimates for world population and global food availability for global health. In The Role of Functional Food Security in Global Health; Academic Press: Cambridge, MA, USA.
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Paper No. 12-03; FAO: Rome, Italy, 2012. [Google Scholar]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boer, J.; Aiking, H. Strategies towards healthy and sustainable protein consumption: A transition framework at the levels of diets, dishes, and dish ingredients. Food Qual. Prefer. 2019, 73, 171–181. [Google Scholar] [CrossRef]
- Tenorio, A.T.; Kyriakopoulou, K.E.; Suarez-Garcia, E.; van den Berg, C.; van der Goot, A.J. Understanding differences in protein fractionation from conventional crops, and herbaceous and aquatic biomass—Consequences for industrial use. Trends Food Sci. Technol. 2018, 71, 235–245. [Google Scholar] [CrossRef]
- Kromus, S.; Wachter, B.; Koschuh, W.; Mandl, M.; Krotscheck, C.; Narodoslawsky, M. The green biorefinery Austria-development of an integrated system for green biomass utilization. Chem. Biochem. Eng. Q. 2004, 18, 7–12. [Google Scholar]
- Zhang, W.; Grimi, N.; Jaffrin, M.Y.; Ding, L.; Tang, B. A short review on the research progress in alfalfa leaf protein separation technology. J. Chem. Technol. Biotechnol. 2017, 2, 2894–2900. [Google Scholar]
- Tenorio, A.T.; Gieteling, J.; de Jong, G.A.H.; Boom, R.M.; van der Goot, A.J. Recovery of protein from green leaves: Overview of crucial steps for utilisation. Food Chem. 2016, 203, 402–408. [Google Scholar] [CrossRef]
- Manwatkar, V.G.; Gogle, D.P. The Effect of Deproteinised Juice (DPJ) on Seed Germination and Seedling Growth of Different Plants (by Paper Towel Method). Int. J. Life Sci. 2014, 2, 65–68. [Google Scholar]
- Cheyniera, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant. Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Salah, E.H. Sustainability of Agricultural and Rural Waste Management, 1st ed.; Cradle-to-Cradle for Sustainable Development eBook; Academic Press: New York, NY, USA, 2007; 424p, ISBN 9780080550145. [Google Scholar]
- Popp, J.; Lakner, Z.; Harangi-Rakos, M.; Fari, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sustain. Energy Rev. 2014, 32, 559–578. [Google Scholar] [CrossRef] [Green Version]
- Obi, F.O.; Ugwuishiwu, B.O.; Nwakaire, J.N. Agricultural waste concept, generation, utilization and management. Niger. J. Technol. 2016, 35, 957–964. [Google Scholar] [CrossRef]
- Grela, E.R.; Pietrzak, K. Production technology, chemical composition and use of alfalfa protein-xanthophyll concentrate as dietary supplement. J. Food Process. Technol. 2014, 5, 10. [Google Scholar]
- Pirie, N.W. Leaf Protein and its By-products in Human and Animal Nutrition; Cambridge University Press: Cambridge, UK, 1987; p. 209. [Google Scholar]
- Iliyas, S. Effect of different flours on α -amylase production. Int. J. Bioassays 2014, 3, 3011–3015. [Google Scholar]
- Jadhav, R.K.; Chhaya, G. Influence of deproteinised foliage fluid liquid biofertilizer on nitrate reductase activity of Eleusine coracana plants. Curr. Bot. 2018, 9, 33–36. [Google Scholar] [CrossRef]
- Zanin, V. A New Nutritional Idea for Man: Lucerne Leaf Concentrate. APEF, Association Pour la Promotion des Extraits Foliaires en Nutrition. 1998. Available online: http://www.nutrition-luzerne.org/anglais/pdf/EtudeZaninenglish.pdf (accessed on 29 October 2019).
- Thomsen, M.H.; Bech, D.; Kiel, P. Manufacturing of Stabilised Brown Juice for L-lysine production—From University Lab Scale over Pilot Scale to Industrial Production. Chem. Biochem. Eng. Q. 2003, 18, 37–46. [Google Scholar]
- Shende, G.C.; Gogle, D.P. To study the effect of various concentration of deproteinized leaf juice (DPJ) of selected plants on growth of Aspergillus niger. Int. J. Life Sci. 2016, 6, 186–188. [Google Scholar]
- Jadhav, R.K.; Kadam, R.; Joshi, D. Physiology of Gramineae Crops by Deproteinised Foliage Extract and its Influence on Photosynthetic Chemistry. J. Phytochem. 2019, 110, 149–163. [Google Scholar]
- Hegyi, F. Examination of Lactobacilli by Advanced Colorimetric Method. In Hungarian. Laktobacilluszok Vizsgálata továbbfejlesztett Kolorimetriás Módszerrel. Ph.D. Thesis, Corvinus University, Budapest, Hungary, 2014; pp. 8–93. [Google Scholar]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegard, M.; Smith, D.L. From yogurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Smith, D.L.; Subramanian, S.; Lamont, J.R.; Bywater-Ekeg€ard, M. Signaling in the phytomicrobiome: Breadth and potential. Front. Plant Sci. 2015, 6, 709. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.L.; Praslickova, D.; Ilangumaran, G. Inter-organismal signaling and management of the phytomicrobiome. Front. Plant. Sci. 2015, 6, 722. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Xin, H.-L.; Guo, M.-L. Review on research of the phytochemistry and pharmacological activities of Celosia argentea. Rev. Bras. Farmacogn. 2016, 26, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Lakshmi, A.J.; Manjunath, M.N.; Prakash, J. Analysis of nutrient and antinutrient content of underutilized green leafy vegetables. LWT Food Sci. Technol. 2005, 38, 339–345. [Google Scholar] [CrossRef]
- Fári, M.G.; Domokos-Szabolcsy, É. Method for Producing Plant Protein Coagulum. Hun Patent WO/2019/150144, 8 August 2019. [Google Scholar]
- Gibbs, P.A. Novel uses for lactic acid fermentation in food preservation. J. Appl. Bacteriol. 1987, 63, 51S–58S. [Google Scholar] [CrossRef]
- Kovács, B.; Győri, Z.; Prokisch, J.; Loch, J.; Dániel, P. A study of plant sample preparation and inductively coupled plasma emission spectrometry parameters. Commun. Soil Sci. Plant Anal. 1996, 27, 1177–1198. [Google Scholar] [CrossRef]
- Boór, A.; Bélafiné Bakó, K. Determination of antioxidant content in sloe (Prunus spinosa L.) and dogwood (Cornus mas L.) fruits. In Proceedings of the Conference of Chemical Engineering ’2010, Singapore, 26–28 February 2010; pp. 55–58. (in Hungarian). [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Summner, M.E. Methods of Soil Analysis: Chemical Methods; Part 3; ASA and SSSA: Madison, WI, USA, 1996. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1979, 72, 248–254. [Google Scholar] [CrossRef]
- Roxas, V.P.; Smith, R.K.; Allen, E.R.; Allen, R.D. Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat. Biotechnol. 1997, 15, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, W.; Spencer, A.K.; Stahmann, M.A. An improved procedure using ferricyanide for detecting catalase isozymes. Anal. Biochem. 1971, 44, 301–305. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, R. Analysis of Malondialdehyde, Chlorophyll Proline, Soluble Sugar, and Glutathione Content in Arabidopsis seedling. Bio-Protocol 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F-tests. Biometric 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Balfour, E. Anomalous secondary thickening in Chenopodiaceae, Nyctaginaceae and Amaranthaceae. Phytomorphology 1965, 15, 111–122. [Google Scholar]
- Myśkow, E.; Gola, E.; Tulik, M. Continuity of procambium and anomalous cambium during formation of successive cambia in Celosia argentea. J. Plant. Growth Regul. 2019, 38, 1458–1466. [Google Scholar] [CrossRef] [Green Version]
- Carlquist, S. Successive cambia revisited: Ontogeny, histology, diversity, and functional significance. J. Torrey Bot. Soc. 2007, 134, 301–332. [Google Scholar] [CrossRef]
- Reddy, G.U.; Deshmukh, V.R.; Joshi, R.N.; Kayama, R. Utilization of alfalfa (Mediacago sativa L.) whey as a fertilizer in irrigation. J. Jpn. Grassl. Sci. 1987, 33, 32–37. [Google Scholar]
- Monteros, J.M.; Bouton, H.J. The future of alfalfa and forage crops. In Proceedings of the Western Alfalfa & Forage Conference, Reno, Nevada, 2–4 December 2009. [Google Scholar]
- Martin, N.P. Alfalfa: Forage crop of the future. In Proceedings of the 28th Kentucky Alfalfa Conference; Lacefield, G., Forsythe, C., Eds.; CaveCity Convention Center: Cave, KY, USA, 2008; pp. 17–26. [Google Scholar]
- Ream, H.W.; Smith, D.; Walgenbach, R. Effects of deproteinized alfalfa juice applied to alfalfa-bromegrass, bromegrass, and corn. Agron. J. 1977, 69, 685–689. [Google Scholar] [CrossRef]
- Novik, G.; Olga Meerovskaya, O.; Savich, V. Waste Degradation and Utilization by Lactic Acid Bacteria: Use of Lactic Acid Bacteria in Production of Food Additives, Bioenergy and Biogas. In Food Additives; Karunaratne, D.N., Pamunuwa, G., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Zubaidah, E.; Akhadiana, W. Comparative study of inulin extracts from dahlia, yam, and gembili tubers as prebiotic. Food Nutr. Sci. 2013, 4, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Bautista-Trujillo, G.U.; Cobos, M.A.; Ventura-Canseco, L.M.C.; Ayora-Talavera, T.; Abud-Archila, M.; Oliva-Llaven, M.A.; Dendooven, L.; Gutierrez-Miceli, F.A. Effect of Sugarcane Molasses and Whey on Silage Quality of Maize. Asian J. Crop Sci. 2009, 1, 34–39. [Google Scholar]
- Cheng, X.; Dong, Y.; Su, P.; Xiao, X. Improvement of the fermentative activity of lactic acid bacteria starter culture by the addition of Mn. Appl. Biochem. Biotechnol. 2014, 174, 1752–1760. [Google Scholar] [CrossRef]
- Dimitrovski, D.; Velickova, E.; Dimitrovska, M.; Langerholc, T.; Winkelhausen, E. Synbiotic functional drink from Jerusalem artichoke juice fermented by probiotic Lactobacillus plantarum PCS26. J. Food Sci. Technol. 2016, 53, 766–774. [Google Scholar] [CrossRef] [Green Version]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid fermented mulberry juice. Food Chem. 2018, 1, 148–154. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of fermentation with different lactic acid bacteria and in vitro digestion on the biotransformation of phenolic compounds in fermented pomegranate Juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef]
- Kim, S.Y. Production of fermented kale juices with Lactobacillus strains and nutritional composition. Prev. Nutr. Food Sci. 2017, 22, 231–236. [Google Scholar] [PubMed]
- Pirie, N.W. Leaf Protein and Other Aspects of Fodder Fractionation; Cambridge University Press: London, UK, 1978; pp. 118–123. [Google Scholar]
- Anugoolprasert, O.; Kinoshita, S.; Naito, H.; Shimizu, M.; Ehara, H. Effect of low pH on the growth, physiological characteristics and nutrient absorption of sago palm in a hydroponic system. Plant Prod. Sci. 2012, 15, 125–131. [Google Scholar] [CrossRef]
- Long, A.; Zhang, J.; Yang, L.T.; Ye, X.; Lai, N.W.; Tan, L.L.; Lin, D.; Chen, L.S. Effects of low pH on photosynthesis, related physiological parameters, and nutrient profiles of citrus. Front. Plant Sci. 2017, 8, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, N.A. Book review: Oxidative damage to plants-antioxidant networks and signaling. Front. Plant. Sci. 2015, 6, 452. [Google Scholar] [CrossRef] [Green Version]
- Szollosi, R. Superoxide dismutase (SOD) and abiotic stress tolerance in plants: An overview. In Oxidative Damage to Plants; Academic Press: New York, NY, USA, 2014; pp. 89–129. [Google Scholar]
- Santamaría-Fernández, M.; Karkov Ytting, N.; Lübeck, M. Influence of the development stage of perennial forage crops for the recovery yields of extractable proteins using lactic acid fermentation. J. Clean. Prod. 2019, 218, 1055–1064. [Google Scholar] [CrossRef]
- Solati, Z.; Jørgensen, U.; Eriksen, J.; Søegaard, K. Dry matter yield, chemical composition and estimated extractable protein of legume and grass species during the spring growth. J. Sci. Food Agric. 2017, 97, 3958–3966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovács, Z.; Fári, M.G.; Kaszás, L.; Koroknai, J.Á.; Domokosné-Szabolcsy, É. Obtention of functional leaf protein concentrate (F-LPC) based on Ereky-process: Historical survey and preliminary results. New Biotechnol. 2018, 44, s100–s101. [Google Scholar] [CrossRef]
- Kamm, B.; Hille, C.; Schönicke, P. Green biorefinery demonstration plant in Havelland (Germany). Biofuels Bioprod. Biorefin. 2010, 6, 253–262. [Google Scholar] [CrossRef]



| Parameter | Before | After |
|---|---|---|
| pH | 4.54 ± 0.03 | 3.91 ± 0.05 |
| Brix † (%) | 7.03 ± 0.02 | 7.20 ± 0.01 |
| Total phenolic content (µg mL−1) | 36.5 ± 1.19 | 24.26 ± 0.55 |
| Electrical conductivity (dS m−1) | 11.13 ± 0.11 | 8.47 ± 0.06 |
| Color-absorbance (at 430 nm) | 0.594 ± 0.006 | 0.381 ± 0.004 |
| Lactic acid bacteria (CFU × 108 per mL) | 11.33 ± 4.04 | 8.00 ± 4.36 |
| Sugars content (g L−1) | ||
| Glucose monomer H | 21.19 ± 0.64 | 1.33 ± 0.03 |
| Glucose oligomer H | 2.80 ± 0.58 | Nd ‡ |
| Xylose monomer Pb | 12.0 ± 0.06 | nd |
| Xylose oligomer Pb | 1.90 ± 0.03 | 0.60 ± 0.02 |
| Arabinose monomer Pb | nd | 0.10 ± 0.01 |
| Arabinose oligomer Pb | 1.50 ± 1.16 | 0.64 ± 0.01 |
| Fructose monomer Pb | 3.70 ± 0.02 | 0.89 ± 0.01 |
| Fructose oligomer Pb | nd | nd |
| Acids content (g L−1) | ||
| Acetic acid H | 1.5 ± 0.02 | 10.4 ± 0.03 |
| Lactic acid H | 5.0 ± 0.25 | 50.1 ± 0.68 |
| Propionic acid H | nd | 1.2 ± 0.02 |
| Elements | Before | After |
|---|---|---|
| N | 18.24 ± 0.66 † | 16.19 ± 0.01 |
| P | 286 ± 30 | 238 ± 5.98 |
| K | 6090 ± 571 | 5276 ± 153 |
| Ca | 1270 ± 70 | 2326 ± 66.58 |
| Mg | 379 ± 16 | 739 ± 24.83 |
| Na | 31.03 ± 8.70 | 452 ± 15.02 |
| S | 352 ± 22 | 425 ± 6.57 |
| Mn | 1.61 ± 0.13 | 4.66 ± 0.09 |
| Mo | 0.29 ± 0.12 | 0.45 ± 0.01 |
| Fe | 2.04 ± 0.45 | 32.20 ± 0.86 |
| Cu | 0.06 ± 0.05 | nd ‡ |
| Zn | 2.03 ± 0.19 | 8.59 ± 0.21 |
| Sr | 5.46 ± 0.24 | 8.80 ± 0.23 |
| B | 3.69 ± 0.33 | 11.61 ± 0.33 |
| Al | 0.24 ± 0.27 | 1.67 ± 0.13 |
| Ba | 0.39 ± 0.02 | 0.94 ± 0.02 |
| Epidermis | Cortex | Pith | Primary Vascular Bundle | Secondary Vascular Tissue | |
|---|---|---|---|---|---|
| Cont. | 31.44 ± 5.28 a | 356.14 ± 57.69 ab | 1873.30 ± 295.29 b | 236.47 ± 79.43 b | 221.47 ± 51.79 b |
| 0.5% | 30.81 ± 6.72 a | 374.10 ± 99.50 ab | 1903.79 ± 187.39 b | 271.65 ± 73.59 ab | 295.59 ±76.55 a |
| 1% | 25.83 ± 3.59 b | 322.42 ± 61.76 bc | 2033.30 ± 205.45 b | 242.23 ± 41.22 b | 212.56 ± 51.56 b |
| 2.5% | 28.50 ± 4.42 ab | 261.69 ± 19.64 d | 2011.11 ± 198.88 b | 303.98 ± 88.94 a | 230.18 ± 73.69 b |
| 5% | 31.43 ± 3.81 a | 339.46 ± 68.14 b | 2227.77 ± 310.33 a | 348.30 ±122.47 a | 256.93 ± 66.78 a |
| 10% | 28.69 ± 3.49 ab | 392.07 ± 91.05 a | 1915.50 ± 209.11 b | 310.12 ± 102.47 a | 274.80 ± 89.62 a |
| Rates of Brown Juice (%) | pH | EC | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| 0.5 | 4.65 ± 0.02 † | 4.21 ± 0.07 | 0.15 ± 0.01 | 0.12 ± 0.03 |
| 1.0 | 4.67 ± 0.02 | 4.16 ± 0.00 | 0.28 ± 0.01 | 0.54 ± 0.58 |
| 2.5 | 4.68 ± 0.01 | 4.16 ± 0.01 | 0.67 ± 0.07 | 0.46 ± 0.02 |
| 5.0 | 4.72 ± 0.01 | 4.18 ± 0.00 | 1.20 ± 0.04 | 0.81 ± 0.05 |
| 10.0 | 4.72 ± 0.01 | 4.19 ± 0.00 | 2.26 ± 0.04 | 1.44 ± 0.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bákonyi, N.; Kisvarga, S.; Barna, D.; O. Tóth, I.; El-Ramady, H.; Abdalla, N.; Kovács, S.; Rozbach, M.; Fehér, C.; Elhawat, N.; et al. Chemical Traits of Fermented Alfalfa Brown Juice: Its Implications on Physiological, Biochemical, Anatomical, and Growth Parameters of Celosia. Agronomy 2020, 10, 247. https://doi.org/10.3390/agronomy10020247
Bákonyi N, Kisvarga S, Barna D, O. Tóth I, El-Ramady H, Abdalla N, Kovács S, Rozbach M, Fehér C, Elhawat N, et al. Chemical Traits of Fermented Alfalfa Brown Juice: Its Implications on Physiological, Biochemical, Anatomical, and Growth Parameters of Celosia. Agronomy. 2020; 10(2):247. https://doi.org/10.3390/agronomy10020247
Chicago/Turabian StyleBákonyi, Nóra, Szilvia Kisvarga, Döme Barna, Ibolya O. Tóth, Hassan El-Ramady, Neama Abdalla, Szilvia Kovács, Margaréta Rozbach, Csaba Fehér, Nevien Elhawat, and et al. 2020. "Chemical Traits of Fermented Alfalfa Brown Juice: Its Implications on Physiological, Biochemical, Anatomical, and Growth Parameters of Celosia" Agronomy 10, no. 2: 247. https://doi.org/10.3390/agronomy10020247








