Bio-Inspired Polymer Membrane Surface Cleaning
Abstract
:1. Introduction
2. Experimental Section
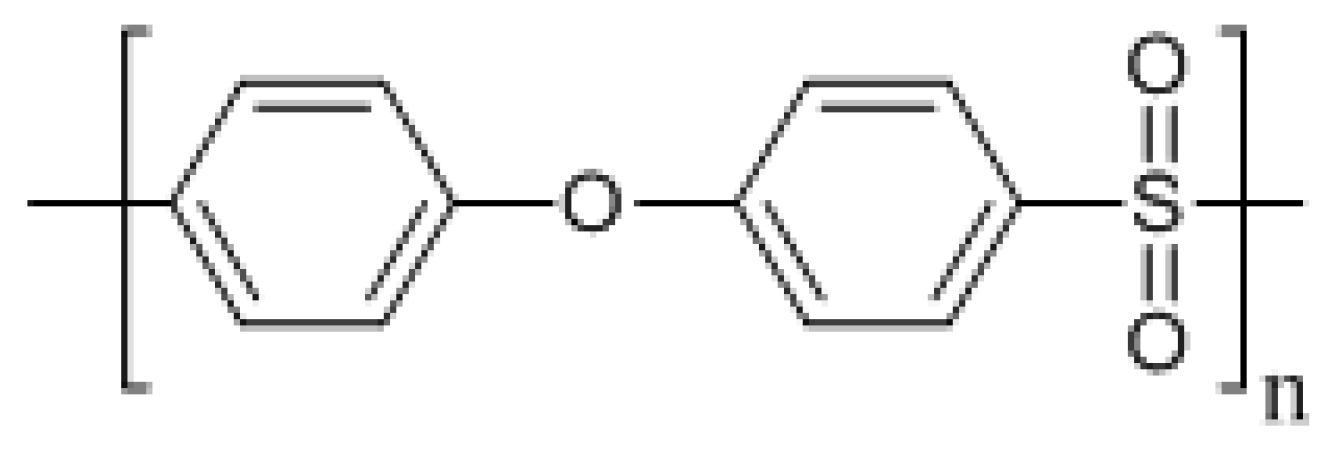
2.1. Chemicals and Materials
2.2. Pancreatin Immobilization
2.3. Membrane Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. X-ray Photoelectron Spectroscopy (XPS)
2.3.3. Mercury Porosimetry
2.3.4. Contact Angle Measurement
2.3.5. Pancreatin Concentration
2.3.6. Protease Activity
2.3.7. Lipase Activity
2.3.8. Fouling and Self-Cleaning Experiments
3. Results and Discussion
3.1. Biocatalyst Immobilization on Membrane Surface
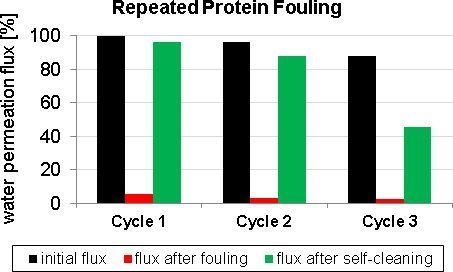
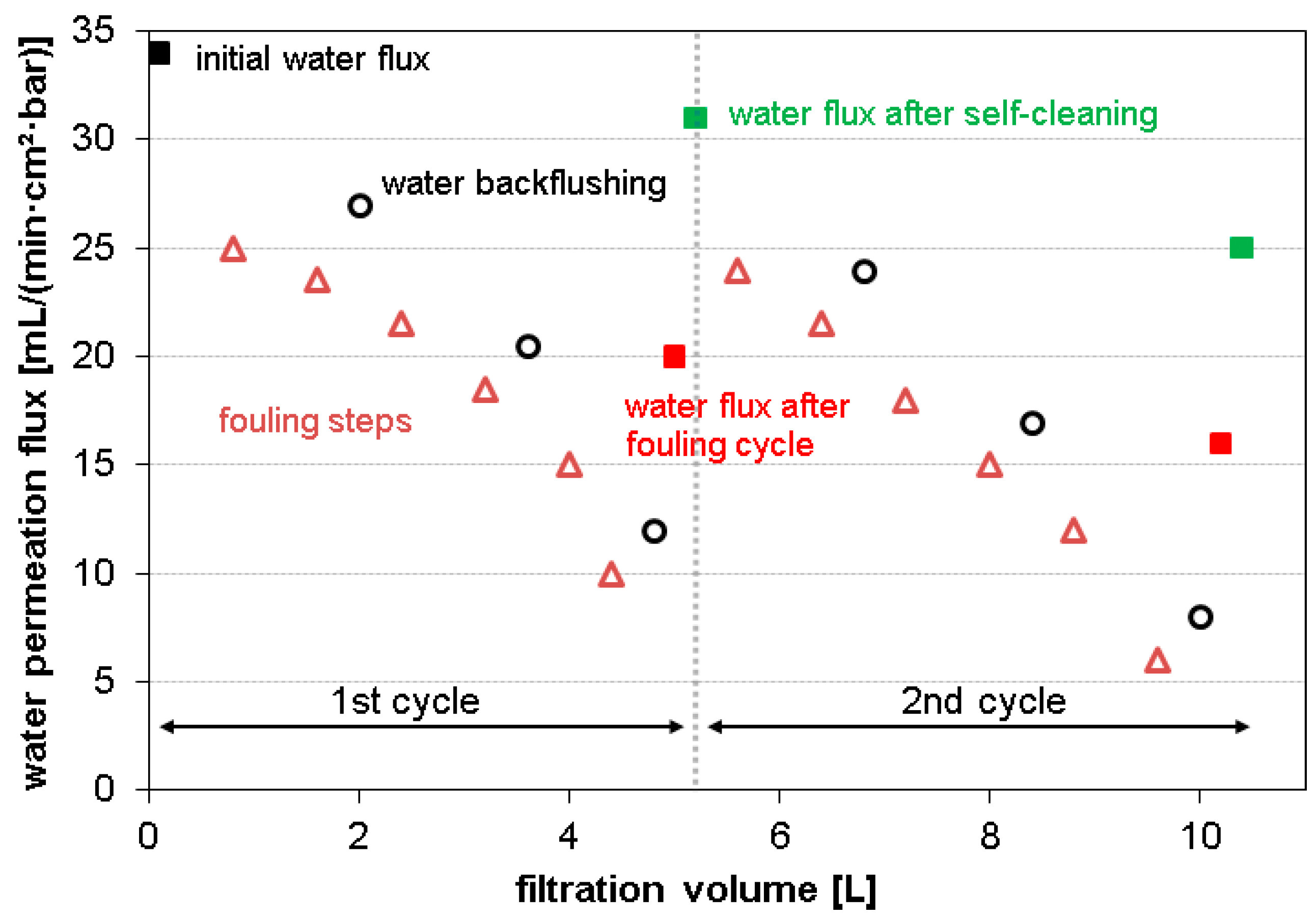
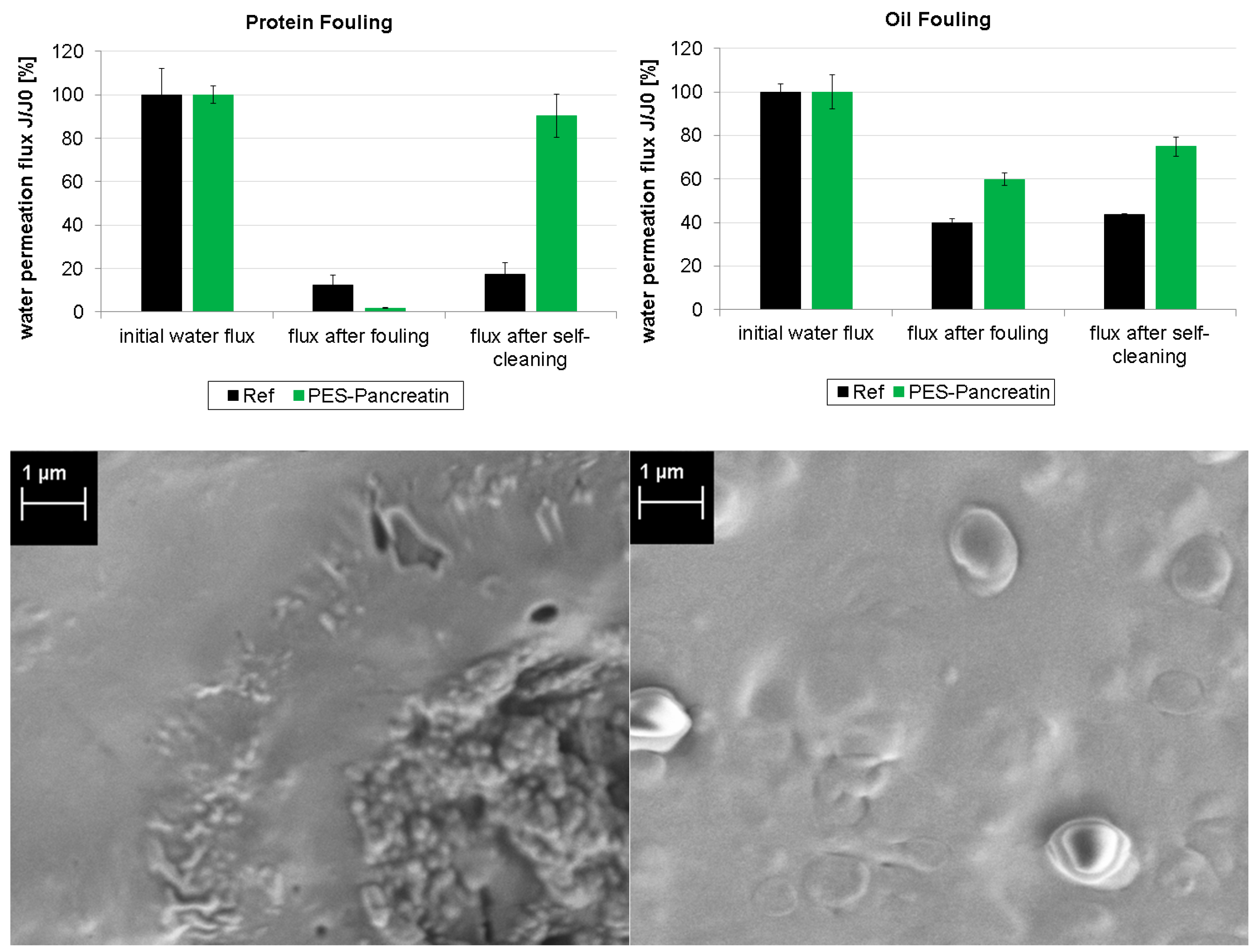
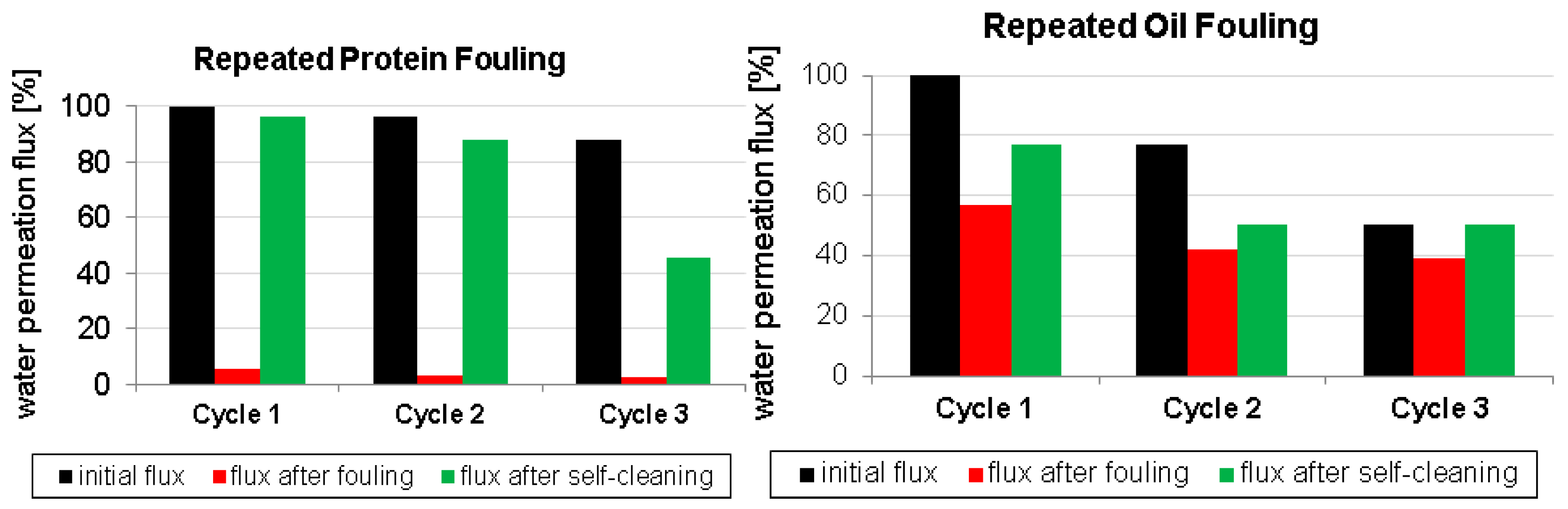
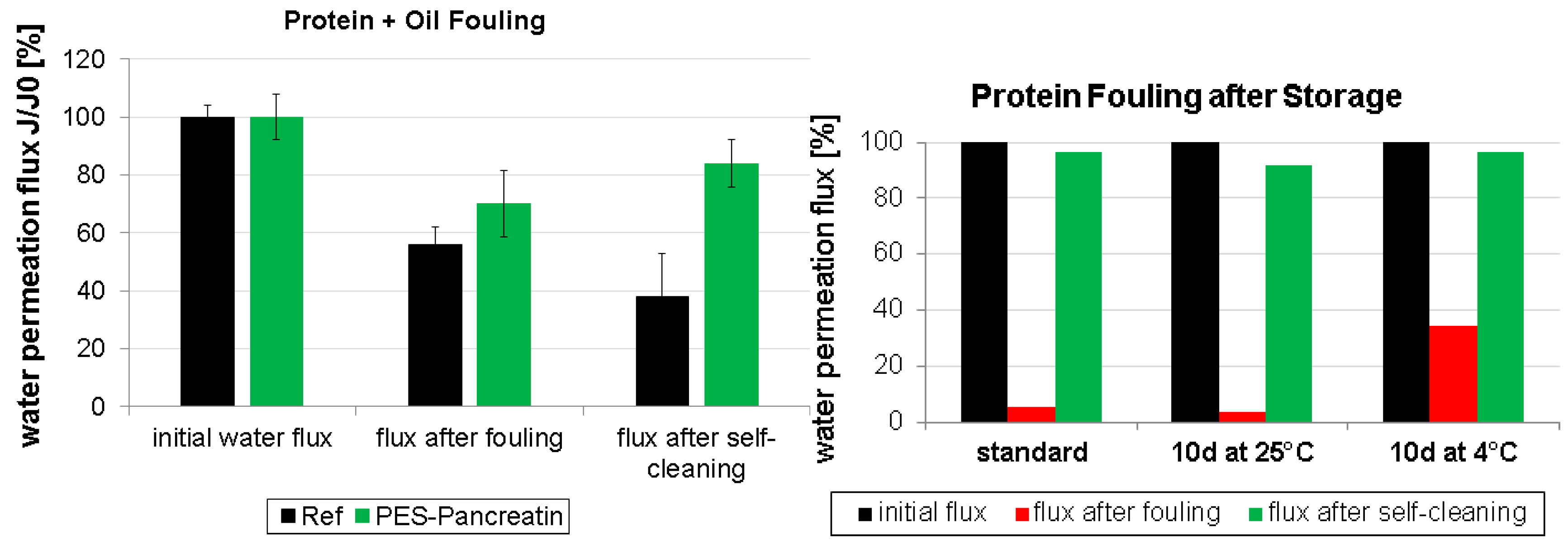
3.2. Fouling and Self-Cleaning
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marchand-Brynaert, J. Polymer membranes, 1st ed.; CRC Press: Boka Raton, FL, USA, 2012; pp. 4854–4873. [Google Scholar]
- Nunes, S.P.; Peinemann, K.V. Membrane Technology in the Chemical Industry, 2nd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2006; pp. 328–358. [Google Scholar]
- Hilal, N.; Ogunbiyi, O.O.; Miles, N.J.; Nigmatullin, R. Methods employed for control of fouling in MF and UF membranes: A comprehensive review. Sep. Sci. Technol. 2005, 40, 1957–2005. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, Z.K.; Wu, J.; Hoek, E.M.V.; Tarabara, V.V. Surface modification of membranes. In Encyclopedia of Membrane Science and Technology, 1st ed.; Hoek, E.M.V., Tarabara, V.V., Eds.; Wiley: Hoboken, NJ, USA, 2013; Volume 1, pp. 1–10. [Google Scholar]
- Kochkodan, V.; Johnson, D.J.; Hilal, N. Polymeric membranes: Surface modification for minimizing (bio)colloidal fouling. Adv. Colloid Interface Sci. 2014, 206, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, X.; Wu, G.; Ren, L.; Wang, Y. Comparative degradation study of surface-modified polyacrylamide/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) membranes. Polym. Sci. Ser. B 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Wang, J.; Wang, S. Improving the water flux and bio-fouling resistance of reverse osmosis (RO) membrane through surface modification by zwitterionic polymer. J. Membr. Sci. 2015, 493, 188–199. [Google Scholar] [CrossRef]
- Nazri, N.; Lau, W.; Ismail, A. Improving water permeability and anti-fouling property of polyacrylonitrile-based hollow fiber ultrafiltration membranes by surface modification with polyacrylonitrile-g-poly(vinyl alcohol) graft copolymer. Korean J. Chem. Eng. 2015, 9, 1–11. [Google Scholar] [CrossRef]
- Ren, P.-F.; Fang, Y.; Wan, L.-S.; Ye, X.-Y.; Xu, Z.-K. Surface modification of polypropylene microfiltration membrane by grafting poly(sulfobetaine methacrylate) and poly(ethylene glycol): Oxidative stability and antifouling capability. J. Membr. Sci. 2015, 492, 249–256. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Yang, L.; Deng, C.; Tian, Q.; Yang, B. Surface modification of ultrafiltration membranes by grafting glycine-functionalized PVAva based on polydopamine coatings. Appl. Surf. Sci. 2015, 345, 301–309. [Google Scholar] [CrossRef]
- Cheng, Q.; Zheng, Y.; Yu, S.; Zhu, H.; Peng, X.; Liu, J.; Liu, J.; Liu, M.; Gao, C. Surface modification of a commercial thin-film composite polyamide reverse osmosis membrane through graft polymerization of N-isopropylacrylamide followed by acrylic acid. J. Membr. Sci. 2013, 447, 236–245. [Google Scholar] [CrossRef]
- Chung, Y.T.; Ng, L.Y.; Mohammad, A.W. Sulfonated-polysulfone membrane surface modification by employing methacrylic acid through UV-grafting: Optimization through response surface methodology approach. J. Ind. Eng. Chem. 2014, 20, 1549–1557. [Google Scholar] [CrossRef]
- Kim, Y.; Rana, D.; Matsuura, T.; Chung, W.-J. Towards antibiofouling ultrafiltration membranes by blending silver containing surface modifying macromolecules. Chem. Commun. 2012, 48, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Scheier, B.; Narbaitz, R.M.; Matsuura, T.; Tabe, S.; Jasim, S.Y.; Khulbe, K.C. Comparison of cellulose acetate (CA) membrane and novel CA membranes containing surface modifying macromolecules to remove pharmaceutical and personal care product micropollutants from drinking water. J. Membr. Sci. 2012, 409, 346–354. [Google Scholar] [CrossRef]
- Rana, D.; Kim, Y.; Matsuura, T.; Arafat, H.A. Development of antifouling thin-film-composite membranes for seawater desalination. J. Membr. Sci. 2011, 367, 110–118. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Dadhich, P.; Dhara, S.; De, S. In vitro cytocompatibility and blood compatibility of polysulfone blend, surface-modified polysulfone and polyacrylonitrile membranes for hemodialysis. RSC Adv. 2015, 5, 7023–7034. [Google Scholar] [CrossRef]
- Rana, D.; Narbaitz, R.M.; Garand-Sheridan, A.-M.; Westgate, A.; Matsuura, T.; Tabe, S.; Jasim, S.Y. Development of novel charged surface modifying macromolecule blended PES membranes to remove EDCs and PPCPs from drinking water sources. J. Mat. Chem. A 2014, 2, 10059–10072. [Google Scholar] [CrossRef]
- Ouradi, A.; Nguyen, Q.T.; Benaboura, A. Polysulfone–AN69 blend membranes and its surface modification by polyelectrolyte-layer deposit—Preparation and characterization. J. Membr. Sci. 2014, 454, 20–35. [Google Scholar] [CrossRef]
- Mehrparvar, A.; Rahimpour, A.; Jahanshahi, M. Modified ultrafiltration membranes for humic acid removal. J. Taiwan Inst. Chem. Eng. 2014, 45, 275–282. [Google Scholar] [CrossRef]
- Mahlicli, F.; Altinkaya, S. Surface modification of polysulfone based hemodialysis membranes with layer by layer self assembly of polyethyleneimine/alginate-heparin: A simple polyelectrolyte blend approach for heparin immobilization. J Mater Sci 2013, 24, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-M.; Wang, L.-L.; Wang, Y.; Gu, J.-S.; Yu, H.-Y. Surface modification of polypropylene macroporous membrane by marrying raft polymerization with click chemistry. J. Membr. Sci. 2012, 421, 60–68. [Google Scholar] [CrossRef]
- Ulbricht, M. Membranen: Ggrundlagen, verfahren und industrielle anwendungen, 1st ed.; Ohlrogge, K., Ebert, K., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2006; pp. 47–75. [Google Scholar]
- Sun, S.; Yue, Y.; Huang, X.; Meng, D. Protein adsorption on blood-contact membranes. J. Membr. Sci. 2003, 222, 3–18. [Google Scholar] [CrossRef]
- Schulze, A.; Marquardt, B.; Kaczmarek, S.; Schubert, R.; Prager, A.; Buchmeiser, M.R. Electron beam-based functionalization of poly(ethersulfone) membranes. Macromol. Rapid Commun. 2010, 31, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Marquardt, B.; Went, M.; Prager, A.; Buchmeiser, M.R. Electron beam-based functionalization of polymer membranes. Water Sci. Technol. 2012, 65, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Maitz, M.F.; Zimmermann, R.; Marquardt, B.; Fischer, M.; Werner, C.; Went, M.; Thomas, I. Permanent surface modification by electron-beam-induced grafting of hydrophilic polymers to PVDF membranes. RSC Adv. 2013, 3, 22518–22526. [Google Scholar] [CrossRef]
- Schulze, A.; Went, M.; Prager, A. Membrane functionalization with hyperbranched polymers. Materials 2016, 9, 706. [Google Scholar] [CrossRef]
- Starke, S.; Went, M.; Prager, A.; Schulze, A. A novel electron beam-based method for the immobilization of trypsin on poly(ethersulfone) and poly(vinylidene fluoride) membranes. React. Funct. Polym. 2013, 73, 698–702. [Google Scholar] [CrossRef]
- Jahangiri, E.; Reichelt, S.; Thomas, I.; Hausmann, K.; Schlosser, D.; Schulze, A. Electron beam-induced immobilization of laccase on porous supports for waste water treatment applications. Molecules 2014, 19, 11860–11882. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Stoelzer, A.; Striegler, K.; Starke, S.; Prager, A. Biocatalytic self-cleaning polymer membranes. Polymers 2015, 7, 1837–1849. [Google Scholar] [CrossRef]
- Bykov, V.A.; Demina, N.B.; Kataeva, N.N.; Kemenova, V.A.; Bagirova, V.L. Enzyme preparations used for the treatment of digestion insufficiency: A review. J. Pharm. Chem. 2000, 34, 105–109. [Google Scholar] [CrossRef]
- Hammer, H.F. Pancreatic exocrine insufficiency: Diagnostic evaluation and replacement therapy with pancreatic enzymes. Dig. Dis. 2010, 28, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Sarner, M. Treatment of pancreatic exocrine deficiency. World J. Surg. 2003, 27, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Peter, L.; Jutta, K.; Lankisch, P.G. Pancreatic enzyme replacement therapy. Curr. Gastroenterol. Rep. 2001, 3, 101–108. [Google Scholar] [CrossRef]
- Ferrone, M.; Raimondo, M.; Scolapio, J.S. Pancreatic enzyme pharmacotherapy. Pharmacotherapy 2007, 27, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Friess, H.; Böhm, J.; Ebert, M.; Büchler, M. Enzyme treatment after gastrointestinal surgery. Digestion 1993, 54, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Layer, P.; Gröger, G. Fate of pancreatic enzymes in the human intestinal lumen in health and pancreatic insufficiency. Digestion 1993, 54, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Terra, G.D.P.; Farias, M.V.D.; Trevisan, M.G.; Garcia, J.S. Evaluation of pancreatin stability through enzyme activity determination. Acta Pharm. 2016, 66, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.M. Study to compare the enzyme activity, acid resistance and dissolution characteristics of currently available pancreatic enzyme preparations. Pharma. Weekbl. Sci. 1988, 10, 12–16. [Google Scholar] [CrossRef]
- Baines, M.G.; Cai, F.; Backman, H.A. Adsorption and removal of protein bound to hydrogel contact lenses. Optom. Vis. Sci. 1990, 67, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A.; Gallun, A.F. Pancreatin as an unhairing agent. Ind. Eng. Chem. 1923, 15, 267–269. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. Tailoring membrane surface charges: A novel study on electrostatic interactions during membrane fouling. Polymers 2015, 7, 2017–2030. [Google Scholar] [CrossRef]
- Fang, B.; Ling, Q.; Zhao, W.; Ma, Y.; Bai, P.; Wei, Q.; Li, H.; Zhao, C. Modification of polyethersulfone membrane by grafting bovine serum albumin on the surface of polyethersulfone/poly(acrylonitrile-co-acrylic acid) blended membrane. J. Membr. Sci. 2009, 329, 46–55. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fukimotot, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Oliveira, G.B.; Lima Filho, J.L.; Cavalcante Chaves, M.E.; Azevedo, W.M.; Carvalho, L.B., Jr. Enzyme immobilization on anodic aluminum oxide/polyethyleneimine or polyaniline composites. React. Funct. Polym. 2008, 68, 27–32. [Google Scholar] [CrossRef]
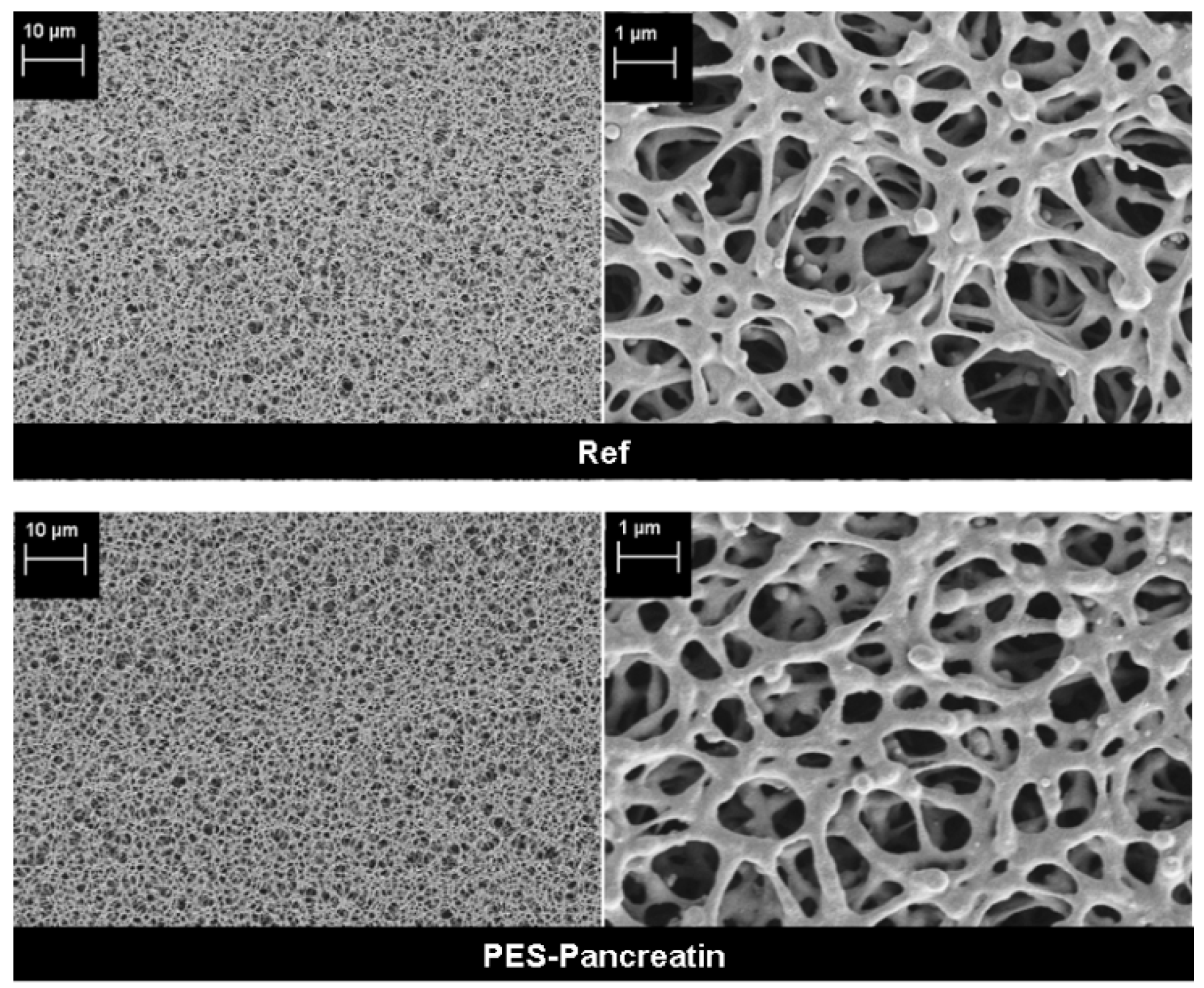
| Sample | Elemental ratio (rel. atom %) | Protein conc. | Contact angle | Average pore size | Porosity | |||
|---|---|---|---|---|---|---|---|---|
| C 1s | O 1s | N 1s | S 2p | (µg/cm²) | (°) | (µm) | (%) | |
| Ref | 68.13 | 27.98 | - | 3.88 | 0.3 ± 0.5 | 58 ± 2 | 0.88 ± 0.02 | 85 ± 2 |
| PES-Pancreatin | 67.45 | 22.32 | 8.72 | 1.51 | 107.4 ± 4.7 | 55 ± 4 | 0.89 ± 0.02 | 86 ± 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze, A.; Breite, D.; Kim, Y.; Schmidt, M.; Thomas, I.; Went, M.; Fischer, K.; Prager, A. Bio-Inspired Polymer Membrane Surface Cleaning. Polymers 2017, 9, 97. https://doi.org/10.3390/polym9030097
Schulze A, Breite D, Kim Y, Schmidt M, Thomas I, Went M, Fischer K, Prager A. Bio-Inspired Polymer Membrane Surface Cleaning. Polymers. 2017; 9(3):97. https://doi.org/10.3390/polym9030097
Chicago/Turabian StyleSchulze, Agnes, Daniel Breite, Yongkyum Kim, Martin Schmidt, Isabell Thomas, Marco Went, Kristina Fischer, and Andrea Prager. 2017. "Bio-Inspired Polymer Membrane Surface Cleaning" Polymers 9, no. 3: 97. https://doi.org/10.3390/polym9030097