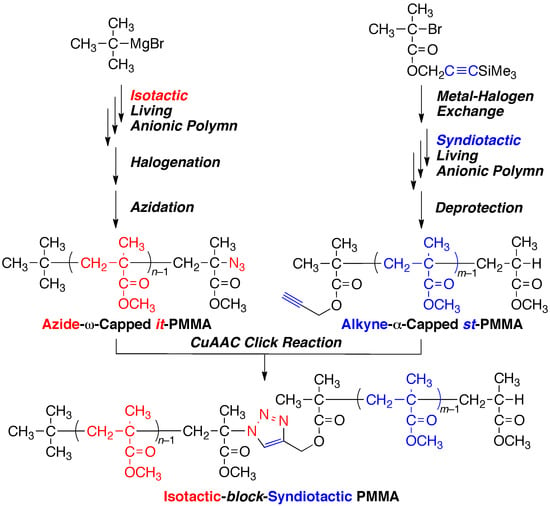
Synthesis of Isotactic-block-Syndiotactic Poly(methyl Methacrylate) via Stereospecific Living Anionic Polymerizations in Combination with Metal-Halogen Exchange, Halogenation, and Click Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
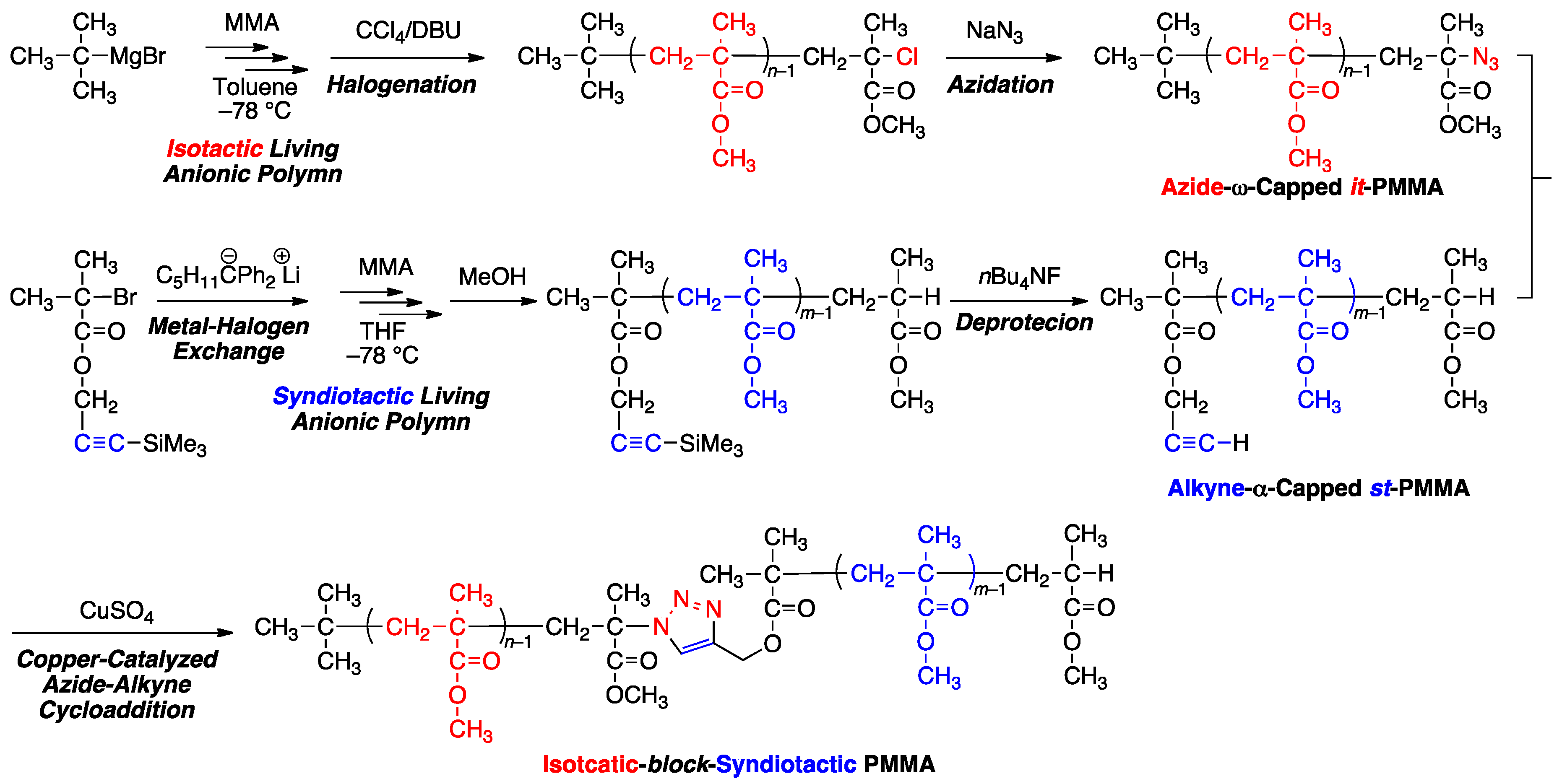
2.2. Synthesis of Azide-ω-Capped it-PMMA via Isotactic Living Anionic Polymerization of MMA Followed by Termination with Halogenation and Subsequent Azidation
2.3. Synthesis of Alkyne-α-Capped st-PMMA via Syndiotactic Living Anionic Polymerization of MMA Initiated via a Metal-Halogen Exchange Reaction
2.4. Synthesis of it-b-st-Stereoblock PMMA via Copper-Catalyzed Azide-Alkyne Cycloaddition
2.5. Measurements
3. Results and Discussion
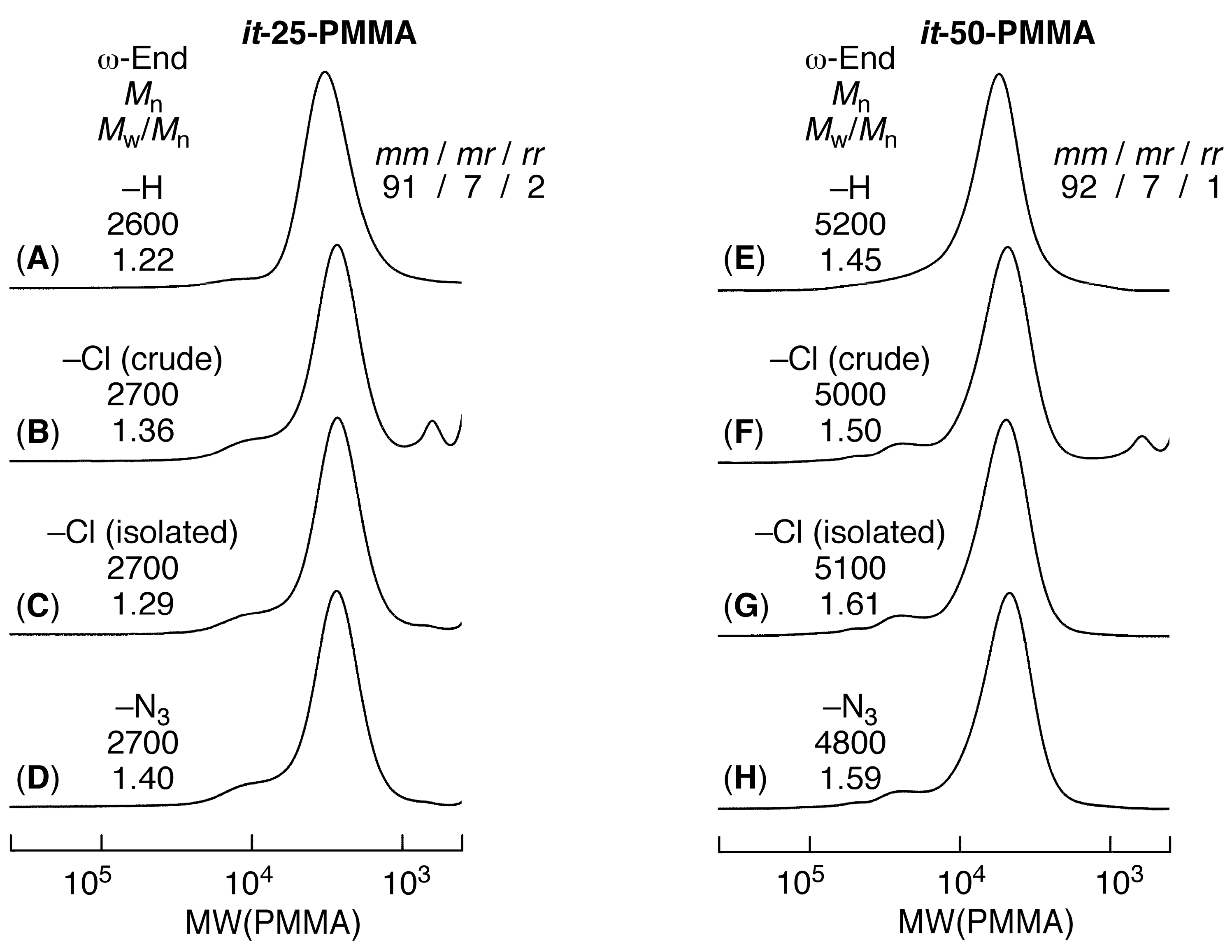
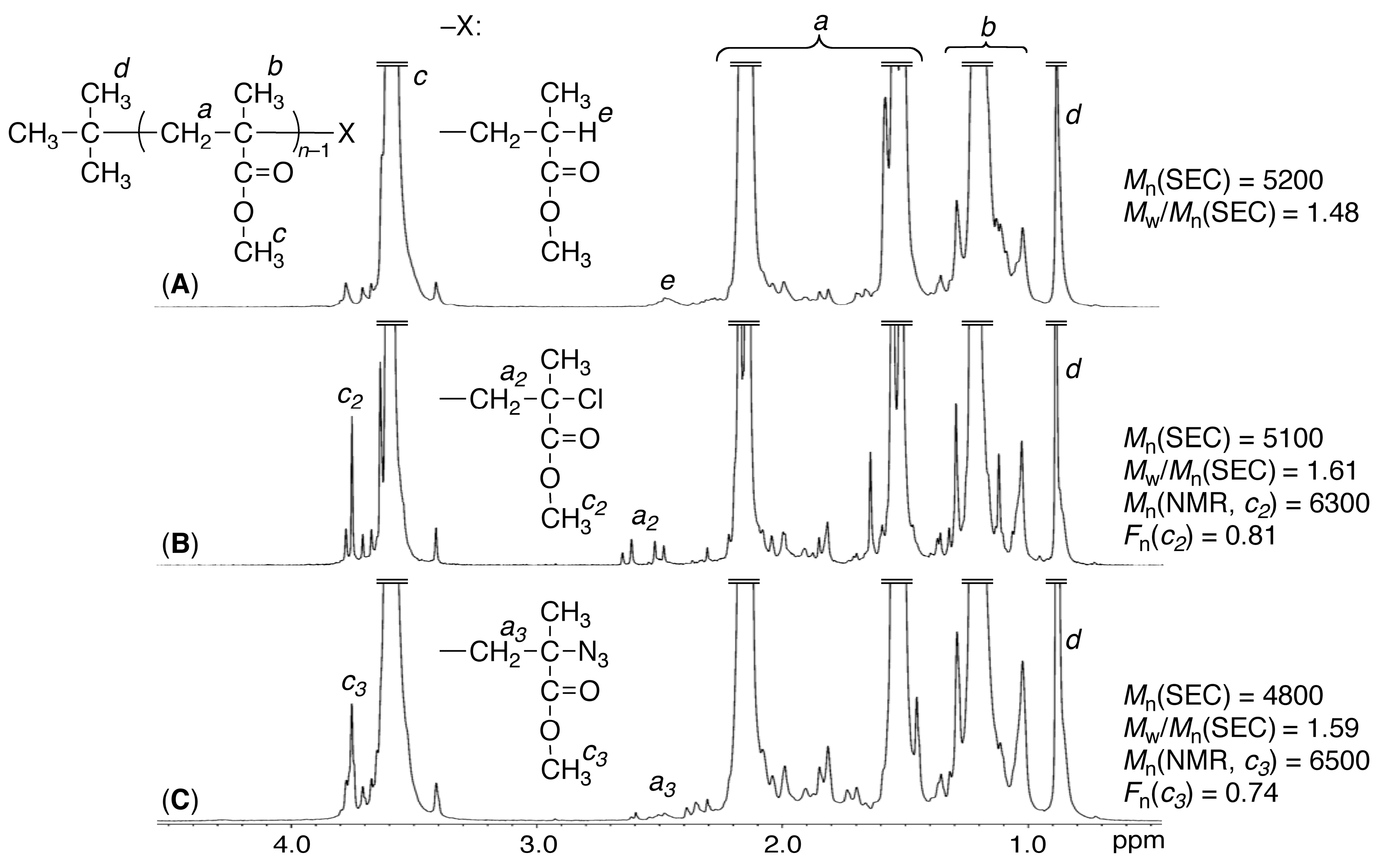
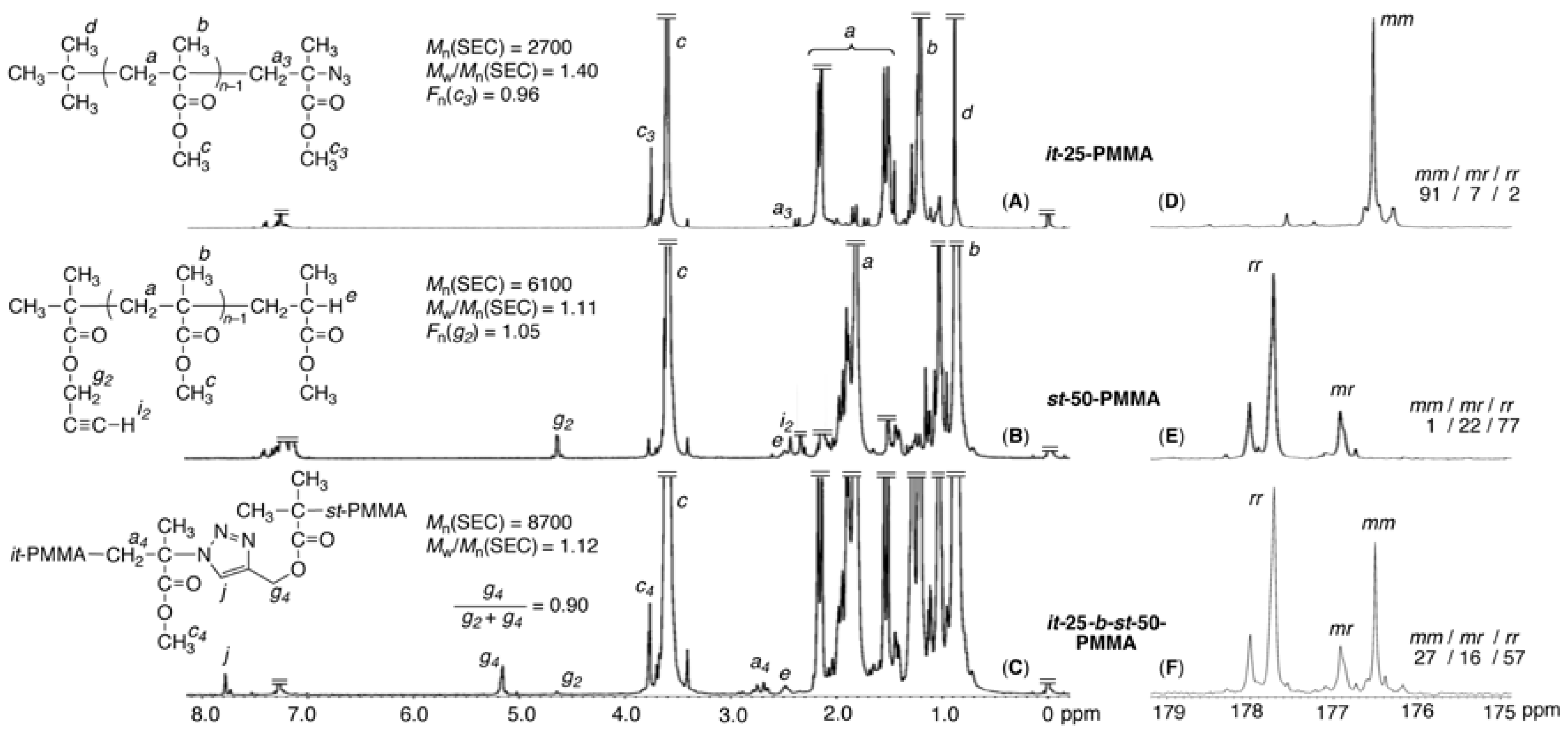
3.1. Synthesis of Azide-ω-Capped it-PMMA via Isotactic Living Anionic Polymerization of MMA Followed by Termination with Halogenation and Subsequent Azidation
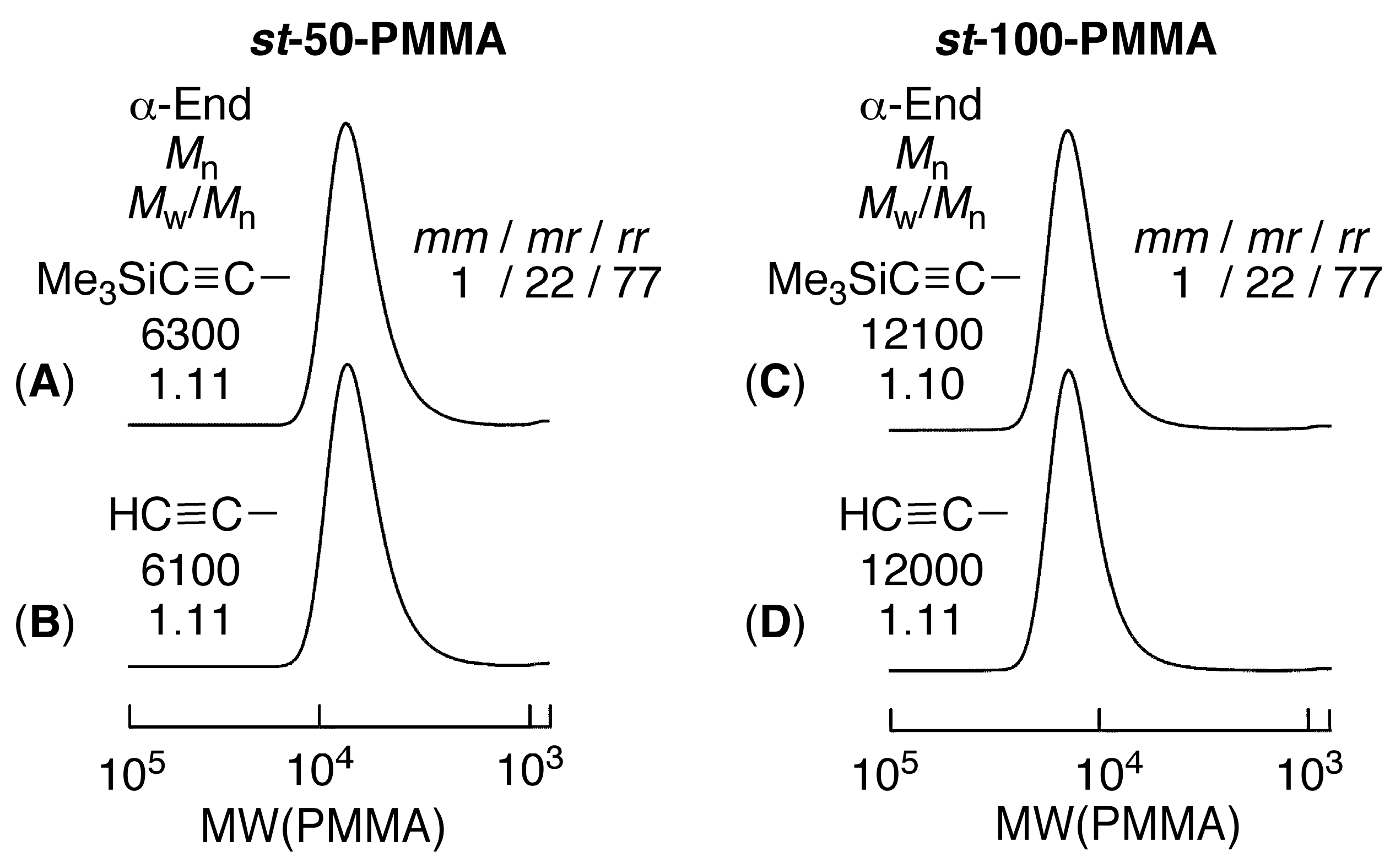
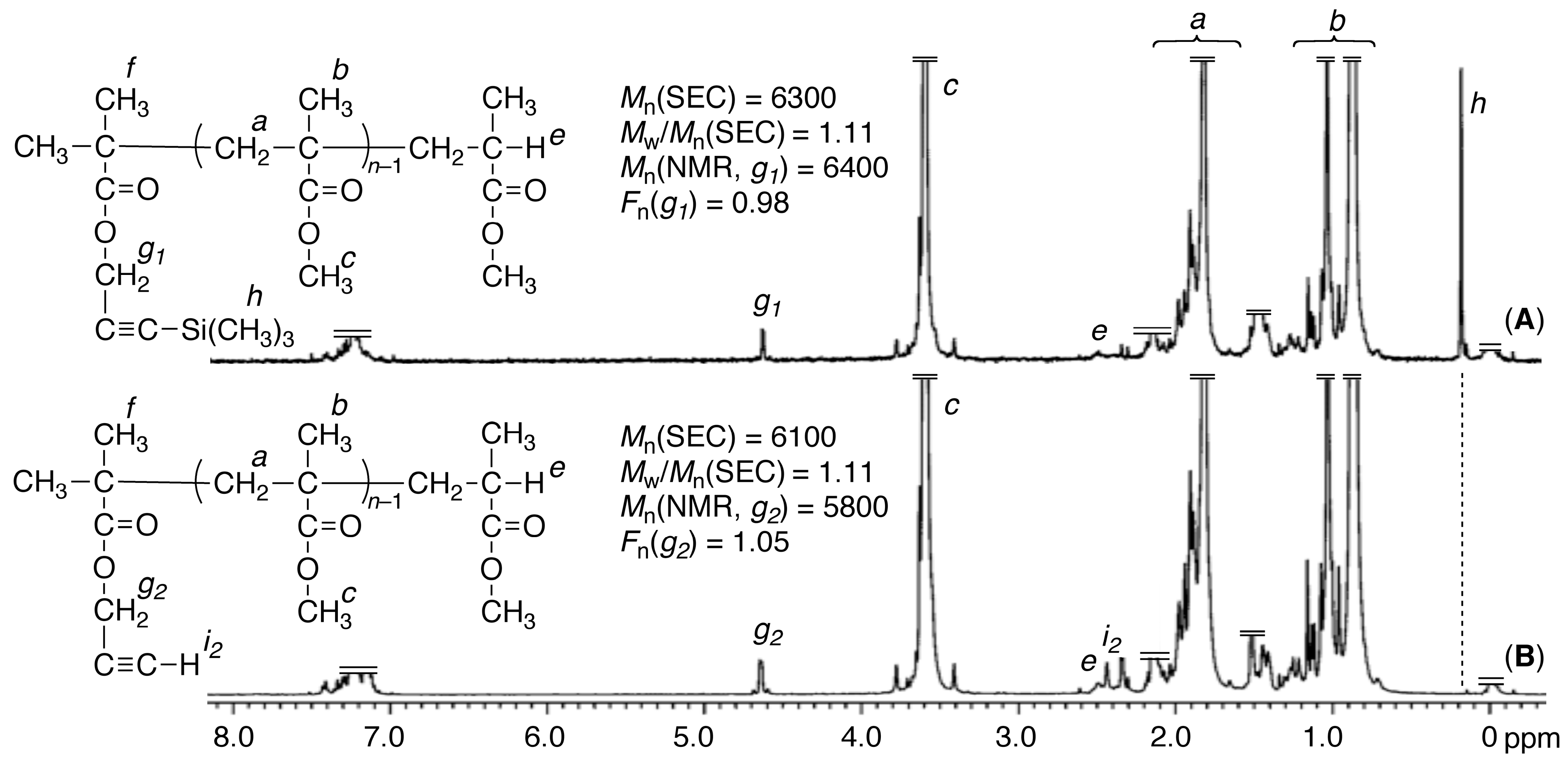
3.2. Synthesis of Alkyne-α-Capped st-PMMA via Syndiotactic Living Anionic Polymerization of MMA Initiated via a Metal-Halogen Exchange Reaction
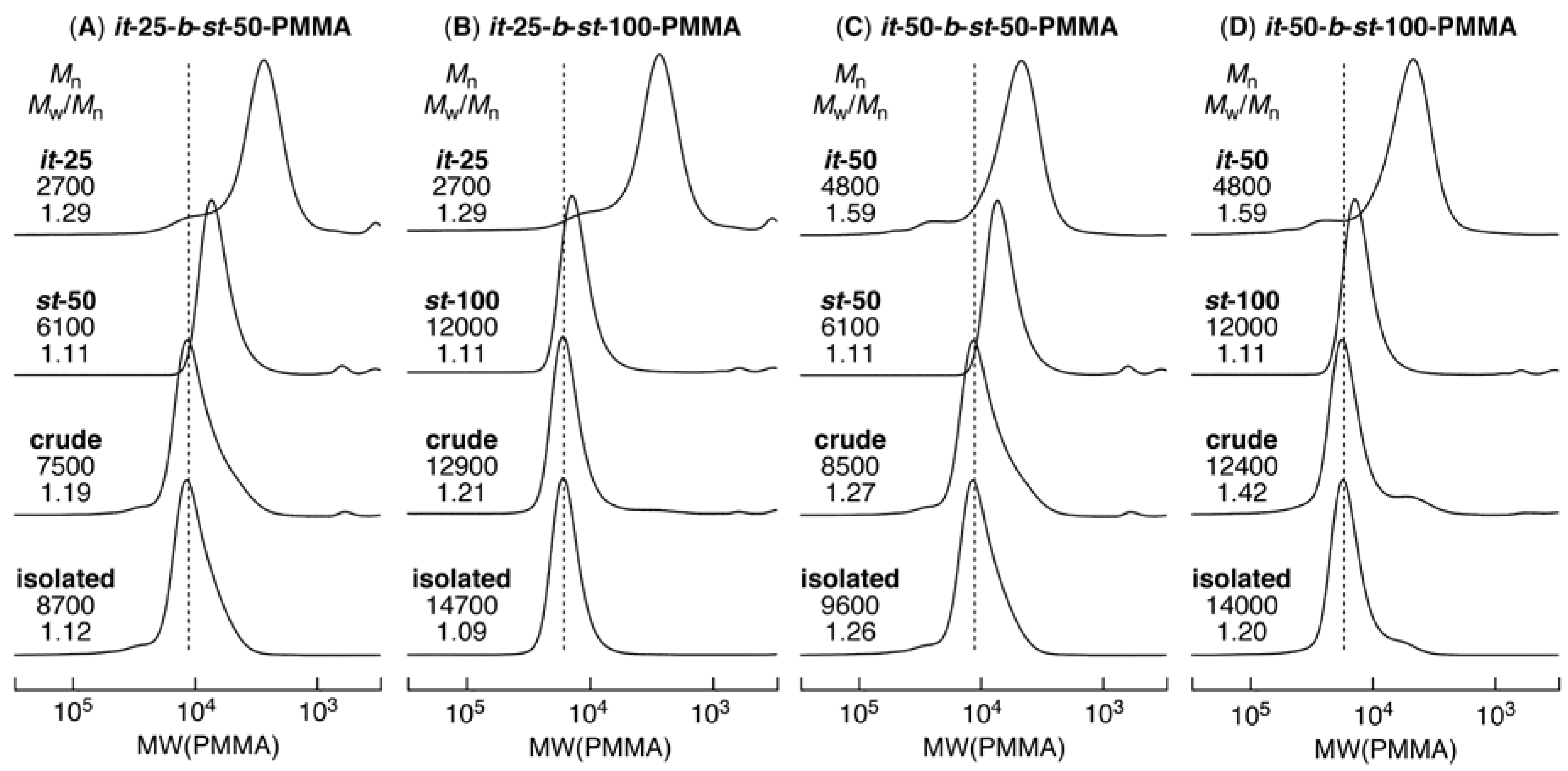
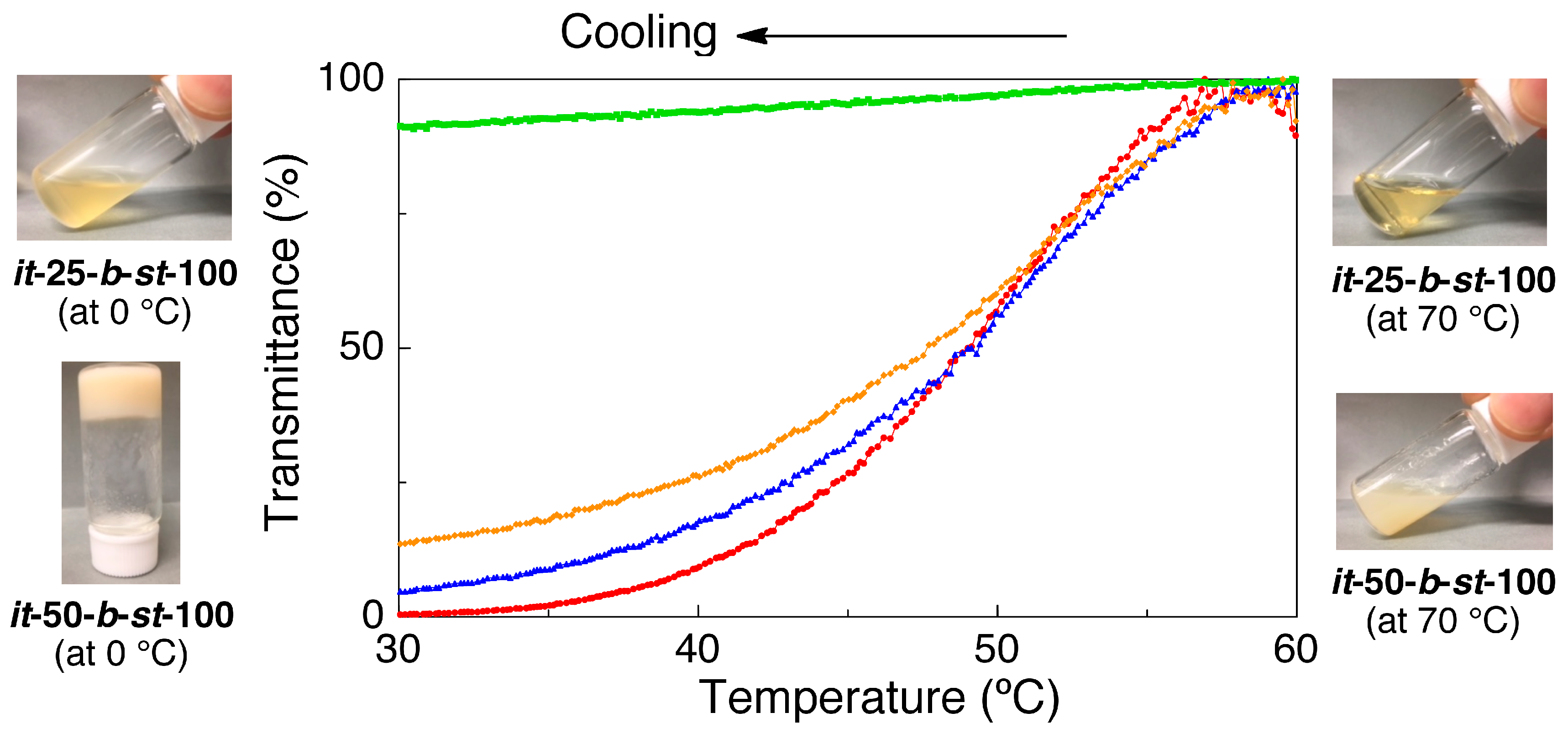
3.3. Synthesis of it-b-st-Stereoblock PMMA via Copper-Catalyzed Azide-Alkyne Cycloaddition and Stereocomplexation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lutz, J.-F.; Lehn, J.-M.; Meijer, E.W.; Matyjaszewski, K. From precision polymers to complex materials and systems. Nat. Rev. Mater. 2016, 1, 16024. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supremolecular helical systems: Helical assembles of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.G.; Garrett, B.S.; Goode, W.E.; Gratch, S.; Kincaid, J.F.; Spell, A.; Stroupe, J.D. Crystalline polymers of methyl methacryalte. J. Am. Chem. Soc. 1958, 80, 1768–1769. [Google Scholar] [CrossRef]
- Liquori, A.M.; Anzuino, G.; Coiro, V.M.; D’Alagni, M.; De Santis, P.; Savino, M. Complementary stereospecific interaction between isotactic and syndiotactic polymer molecules. Nature 1965, 206, 358–362. [Google Scholar] [CrossRef]
- Schomaker, E.; Challa, G. Complexation of stereoregular poly(methyl methacryalte). 14. The basic structure of the stereocomplex of isotactic and syndiotactic poly(methyl methacryalte). Macromolecules 1989, 22, 3337–3341. [Google Scholar] [CrossRef]
- Spěváček, J.; Schneider, B. Aggregation of stereoregular poly(methyl methacryalte). Adv. Colloid Interface Sci. 1987, 27, 81–150. [Google Scholar] [CrossRef]
- Kumaki, J.; Kawauchi, T.; Okoshi, K.; Kusanagi, H.; Yashima, E. Supramolecular helical structure of the stereocomplex composed of complementary isotactic and syndiotactic poly(methyl methacrylate)s as revealed by atomic force microscopy. Angew. Chem. Int. Ed. 2007, 46, 5348–5351. [Google Scholar] [CrossRef] [PubMed]
- Kumaki, J.; Kawauchi, T.; Ute, K.; Kitayama, T.; Yashima, E. Molecular weight recognition in the multiple-stranded helix of a synthetic polymer without specific monomer–monomer interaction. J. Am. Chem. Soc. 2008, 130, 6373–6380. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, T.; Kitaura, A.; Kumaki, J.; Kusanagi, H.; Yashima, E. Helix-sense-controlled synthesis of optically active poly(methyl methacrylate) stereocomplexes. J. Am. Chem. Soc. 2008, 130, 11889–11891. [Google Scholar] [CrossRef] [PubMed]
- Christofferson, A.J.; Yiapanis, G.; Ren, J.M.; Qiao, G.G.; Satoh, K.; Kamigaito, M.; Yarovsky, I. Molecular mapping of poly(methyl methacrylate)super-helix stereocomplexes. Chem. Sci. 2015, 6, 1370–1378. [Google Scholar] [CrossRef]
- Sugaya, H.; Sakai, Y. Polymethylmethacryalte: From polymer to dialyzer. Contrib. Nephrol. 1998, 125, 1–8. [Google Scholar]
- Brinkhuis, R.H.G.; Schouten, J.A. Thin-film behavior of poly(methy1 methacrylates). 6. Monolayer phase behavior in relation to stereocomplexation in mixed monolayers of i- and s-PMMA. Macromolecules 1993, 26, 2514–2519. [Google Scholar] [CrossRef]
- Serizawa, T.; Hamada, K.; Kitayama, T.; Fujimoto, N.; Hatada, K.; Akashi, M. Stepwise stereocomplex assembly of stereoregular poly(methyl methacrylate)s on a substrate. J. Am. Chem. Soc. 2000, 122, 1891–1899. [Google Scholar] [CrossRef]
- Hamada, K.; Serizawa, T.; Kitayama, T.; Fujimoto, N.; Hatada, K.; Akashi, M. Stepwise stereocomplex assembly of isotactic poly(methyl methacrylate) and syndiotactic poly(alkyl methacrylate)s on surfaces. Langmuir 2001, 17, 5513–5559. [Google Scholar] [CrossRef]
- Serizawa, T.; Hamada, K.; Akashi, M. Polymerization within a molecular-scale stereoregular template. Nature 2004, 429, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Kamei, D.; Ajiro, H.; Akashi, M. Specific recognition of syndiotactic poly(methacrylic acid) in porous isotactic poly(methyl methacrylate) thin films based on the effects of stereoregularity, temperature, and solvent. J. Polym. Sci. Part A 2010, 48, 3561–3567. [Google Scholar] [CrossRef]
- Kawauchi, T.; Kumaki, J.; Kitaura, A.; Okoshi, K.; Kusanagi, H.; Kobayashi, K.; Sugai, T.; Shinohara, H.; Yashima, E. Encapsulation of fullerenes in a helical PMMA cavity leading to a robust processable complex with a macromolecular helicity memory. Angew. Chem. Int. Ed. 2008, 47, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Iida, H.; Liu, L.; Irle, S.; Hu, W.; Yashima, E. Electrical switching behavior of a [60]fullerene-based molecular wire encapsulated in a syndiotactic poly(methyl methacrylate) helical cavity. Angew. Chem. Int. Ed. 2013, 52, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Ousaka, N.; Mamiya, F.; Iwata, Y.; Nishimura, K.; Yashima, E. “Helix-in-helix” superstructure formation through encapsulation of fullerene-bound helical peptides within a helical poly(methyl methacrylate) Cavity. Angew. Chem. Int. Ed. 2017, 56, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Hatada, K. Stereoregular uniform polymers. J. Polym. Sci. Part A 1999, 37, 245–260. [Google Scholar] [CrossRef]
- Hatada, K.; Kitayama, T. Structurally controlled polymerizations of methacrylates and acrylates. Polym. Int. 2000, 49, 11–47. [Google Scholar] [CrossRef]
- Doherty, M.A.; Hogen-Esch, T.E. Synthesis of a stereoblock poly(methyl methacrylate). Makromol. Chem. 1986, 187, 61–69. [Google Scholar] [CrossRef]
- Kitayama, T.; Fujimoto, N.; Yanagida, T.; Hadata, K. Synthesis of stereoblock poly(methyl methacrylate) via transformation of isotactic-specific living polymer anion to syndiotactic-specific anion. Polym. Int. 1994, 33, 165–170. [Google Scholar] [CrossRef]
- Hatada, K.; Nshiura, T.; Kitayama, T.; Ute, K.; Hirotani, S. Preparation of uniform stereoblock poly(methyl methacrylate). Polym. J. 1996, 28, 185–188. [Google Scholar] [CrossRef]
- Nshiura, T.; Kitayama, T.; Hatada, T. Intra- and Intermolecular Stereocomplex Formation of Uniform Stereoblock Poly(methyl methacrylate). Polym. J. 1996, 28, 1021–1023. [Google Scholar] [CrossRef]
- Hatada, K.; Kitayama, T.; Ute, K.; Nshiura, T. Preparation of uniform stereoregular polymer, stereoblock polymer and copolymer of methacrylate, and their stereocomplex formation. Macromol. Symp. 1998, 132, 221–230. [Google Scholar] [CrossRef]
- Nishiura, T.; Abe, Y.; Kitayama, T. Uniform poly(methyl methacrylate) stereostars: Synthesis, separation and stereocomplex formation. Polym. J. 2010, 42, 868–874. [Google Scholar] [CrossRef]
- Mariott, W.R.; Escudé, N.C.; Chen, E.Y.-X. Stereoregular P(MMA)-clay nanocomposites by metallocene catalysts: In situ synthesis and stereocomplex formation. J. Polym. Sci. Part A 2007, 45, 2581–2592. [Google Scholar] [CrossRef]
- Escudé, N.C.; Ning, Y.; Chen, E.Y.-X. In situ stereocomplexing polymerization of methyl methacrylate by diastereospecific metallocene catalyst pairs. Polym. Chem. 2012, 3, 3247–3255. [Google Scholar] [CrossRef]
- Vidal, F.; Falivene, L.; Caporaso, L.; Caballo, L.; Chen, E.Y.-X. Robust cross-linked stereocomplexes and C60 inclusion complexes of vinyl-functionalized stereoregular polymers derived from chemo/stereoselective coordination polymerization. J. Am. Chem. Soc. 2016, 138, 9533–9547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bailey, T.S.; Hong, M.; Chen, E.Y.-X. Stereoregular brush polymers and graft copolymers by chiral zirconocene-mediated coordination polymerization of P3HT macromers. Polymers 2017, 9, 139. [Google Scholar] [CrossRef]
- Goh, T.K.; Tan, J.F.; Guntari, S.N.; Satoh, K.; Blencowe, A.; Kamigaito, M.; Qiao, G.G. Nano-to-macroscale poly(methyl methacrylate) stereocomplex sssemblies. Angew. Chem. Int. Ed. 2009, 48, 8707–8711. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.M.; Satoh, K.; Goh, T.K.; Blencowe, A.; Nagai, K.; Ishitake, K.; Christofferson, A.J.; Yiapanis, G.; Yarovsky, I.; Kamigaito, M.; et al. Stereospecific cyclic poly(methyl methacrylate) and its topology-guided hierarchically controlled supramolecular sssemblies. Angew. Chem. Int. Ed. 2014, 53, 459–464. [Google Scholar] [CrossRef]
- Ren, J.M.; Subbiah, J.; Zhang, B.; Ishitake, K.; Satoh, K.; Kamigaito, M.; Qiao, G.G.; Wong, E.H.H.; Wong, W.W.H. Fullerene peapod nanoparticles as an organic semiconductor–electrode interface layer. Chem. Commun. 2016, 52, 3356–3359. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.M.; Ishitake, K.; Satoh, K.; Blencowe, A.; Fu, Q.; Wong, E.H.H.; Kamigaito, M.; Qiao, G.G. Stereoregular high-density bottlebrush polymer and its organic nanocrystal stereocomplex through triple-helix formation. Macromolecules 2016, 49, 788–795. [Google Scholar] [CrossRef]
- Usuki, N.; Satoh, K.; Kamigaito, M. Synthesis of syndiotactic macrocyclic poly(methyl methacrylate) via transformation of the growing terminal in stereospecific anionic polymerization. Macromol. Chem. Phys. 2017, 218, 1700041. [Google Scholar] [CrossRef]
- Kohsaka, Y.; Kurata, T.; Kitayama, T. End-functional stereoregular poly(methyl methacrylate) with clickable C=C bonds: Facile synthesis and thiol–ene reaction. Polym. Chem. 2013, 4, 5043–5047. [Google Scholar] [CrossRef]
- Kohsaka, Y.; Kurata, T.; Yamamoto, K.; Ishihara, S.; Kitayama, T. Synthesis and post-polymerization reaction of end-clickable stereoregular polymethacrylates via termination of stereospecific living anionic polymerization. Polym. Chem. 2015, 6, 1078–1087. [Google Scholar] [CrossRef]
- Kohsaka, Y.; Yamamoto, K.; Ishihara, S.; Kitayama, T. Stereoregular poly(methyl methacrylate) with double-clickable-end: Synthesis and click reaction. Polym. Chem. 2015, 6, 3601–3607. [Google Scholar] [CrossRef]
- Nishioka, A.; Watanabe, H.; Abe, K.; Sono, Y. Grignard reagent-catalyzed polymerization of methyl methacrylate. J. Polym. Sci. 1960, 48, 241–272. [Google Scholar] [CrossRef]
- Liquiori, A.M.; Anzuino, G.; D’Alagni, M.; Vitagliano, V.; Costantino, L. Identification of a stereoblock poly(methyl methacrylate) sample with a stereocomplex. J. Polym. Sci. Part A-2 1968, 6, 509–516. [Google Scholar] [CrossRef]
- Dixit, S.S.; Deshpande, A.B.; Anand, L.C.; Kapur, S.L. Stereoblock polymerization of methyl methacrylate with VOCl3–Al(C2H5)3 catalyst system. J. Polym. Sci. Part A-1 1969, 7, 1973–1982. [Google Scholar] [CrossRef]
- Dixit, S.S.; Deshpande, A.B.; Kapur, S.L. Kinetics and mechanism of polymerization yielding stereoblock poly(methyl methacrylate) with VCl4-Al(C2H5)3 catalyst system. J. Polym. Sci. Part A-1 1970, 8, 1289–1297. [Google Scholar] [CrossRef]
- Miyamoto, T.; Inagaki, H. The stereocomplex formation in poly(methyl methacrylate) and the stereospecific polymerization of its monomer. Polym. J. 1970, 1, 46–54. [Google Scholar] [CrossRef]
- Buter, R.; Tan, Y.T.; Challa, G. Characterization of stereoblock polymers of poly(methyl methacrylate) by thin-layer chromatography. Polymer 1973, 14, 171–172. [Google Scholar] [CrossRef]
- Miyamoto, T.; Tomoshige, S.; Inagaki, H. Separation of isotactic and syndiotactic poly(methyl methacrylate) by a competitive adsorption method. Polym. J. 1974, 6, 564–570. [Google Scholar] [CrossRef]
- Allen, P.E.M.; Bateup, B.O. Polymerization of methylmethacrylate by organometallic compounds—VIII. Initiation by n-, i-, s- or t-butylmagnesium compounds—Evidence for an eneidic pseudoanionic mechanism. Eur. Polym. J. 1978, 14, 1001–1010. [Google Scholar] [CrossRef]
- Allen, P.E.M.; Mair, C. Isotactic and stereoblock poly(methyl methacrylates) produced by butylmagnesium initiators. Eur. Polym. J. 1984, 20, 697–705. [Google Scholar] [CrossRef]
- Allen, P.E.M.; Host, D.M.; Truong, V.T.; Williams, D.R.G. Some physical and mechanical properties of isotactic-atactic poly(methyl methacrylate) blends and stereoblocks. Eur. Polym. J. 1985, 21, 603–610. [Google Scholar] [CrossRef]
- Aoshima, H.; Satoh, K.; Kamigaito, M. Direct mechanistic transformations from isotactic or syndiotactic living anionic polymerizations of methyl methacrylate into metal-catalyzed living radical polymerizations. ACS Macro Lett. 2013, 2, 72–76. [Google Scholar] [CrossRef]
- Hatada, K.; Ute, K.; Tanaka, K.; Kitayama, T.; Okamoto, Y. Preparation of highly isotactic poly(methyl methacrylate) of low polydispersity. Polym. J. 1985, 17, 977–980. [Google Scholar] [CrossRef]
- Hatada, K.; Ute, K.; Tanaka, K.; Okamoto, Y.; Kitayama, T. Living and highly isotactic polymerization of methyl methacrylate by t-C4H9MgBr in toluene. Polym. J. 1986, 18, 1037–1047. [Google Scholar] [CrossRef]
- Cao, Z.-K.; Okamoto, Y.; Hatada, K. Synthesis of syndiotactic poly(methyl methacrylate)s with Bulky Alkyllithiums. Koubunshi Ronbunshu 1986, 12, 857–861. [Google Scholar] [CrossRef]
| Code | Mn (it) | mm/mr/rr (it) | Mn (st) | mm/mr/rr (st) | Mn (it-b-st) | Mw/Mn (it-b-st) | mm/mr/rr (it-b-st) | it-/st-Calcd | it-/st-NMR |
|---|---|---|---|---|---|---|---|---|---|
| it-25-b-st-50 | 2700 | 91/7/2 | 6100 | 1/22/77 | 8700 | 1.12 | 27/16/57 | 31/69 | 28/72 |
| it-25-b-st-100 | 2700 | 91/7/2 | 12000 | 1/22/77 | 14700 | 1.09 | 18/20/62 | 18/82 | 19/81 |
| it-50-b-st-50 | 4800 | 92/7/1 | 6100 | 1/22/77 | 9600 | 1.26 | 43/13/44 | 44/56 | 45/55 |
| it-50-b-st-100 | 4800 | 92/7/1 | 12000 | 1/22/77 | 14000 | 1.20 | 27/16/57 | 29/71 | 32/68 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usuki, N.; Satoh, K.; Kamigaito, M. Synthesis of Isotactic-block-Syndiotactic Poly(methyl Methacrylate) via Stereospecific Living Anionic Polymerizations in Combination with Metal-Halogen Exchange, Halogenation, and Click Reactions. Polymers 2017, 9, 723. https://doi.org/10.3390/polym9120723
Usuki N, Satoh K, Kamigaito M. Synthesis of Isotactic-block-Syndiotactic Poly(methyl Methacrylate) via Stereospecific Living Anionic Polymerizations in Combination with Metal-Halogen Exchange, Halogenation, and Click Reactions. Polymers. 2017; 9(12):723. https://doi.org/10.3390/polym9120723
Chicago/Turabian StyleUsuki, Naoya, Kotaro Satoh, and Masami Kamigaito. 2017. "Synthesis of Isotactic-block-Syndiotactic Poly(methyl Methacrylate) via Stereospecific Living Anionic Polymerizations in Combination with Metal-Halogen Exchange, Halogenation, and Click Reactions" Polymers 9, no. 12: 723. https://doi.org/10.3390/polym9120723