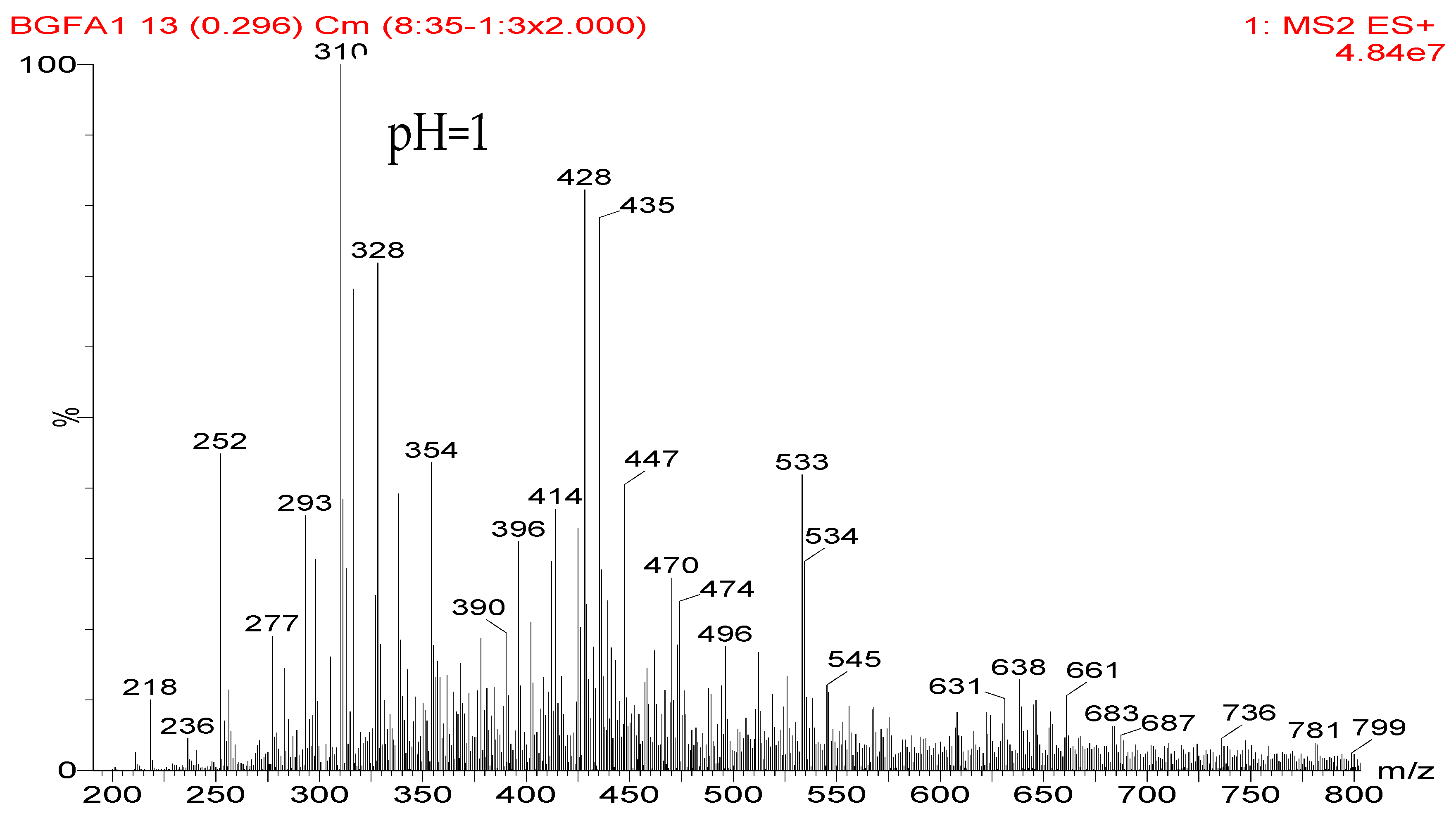
3.1. The ESI-MS Analysis
The ESI-MS spectra of FA/AG series samples prepared at different pHs, specifically pH = 1, 3, 5, 7, 9, 11, and 13, are given as
Figure 1. The assignments on the main ion peaks gotten under acid and alkaline conditions are given in
Table 1 and
Table 2, seperately. According to the mechanism of ESI-MS and the chemical structure of furfuryl alcohol and AG molecules, the ion peaks observed in the ESI-MS spectra mainly came from ions in three forms, namely [M + H]
+, [M + Na]
+, and [M + K]
+. The former came from the combination of amino groups in AG molecules and H
+ and the latter two came from the combination of oxygen atom from carbonyl groups in AG molecules and Na
+ or K
+. Since there were p–π conjugated structure in furfuryl alcolhol molecules, the furfural carbonium ions were possible to be detected.
In
Figure 1, more ion peaks could be detected for FA/AG series samples prepared under acid conditions than those prepared under alkaline conditions. In theory, hydroxymethyl furfural carbonium ions might be the active intermediates. Under acid conditions, with a higher H
+ concentration, the concentration of the carbonium ions would increase and then be helpful for the reaction between furfuryl alcohol and AG. While under alkaline conditions, the furfural carbenium ions become unstable [
23].
According to the calculation of the ion peaks, the peak of 218 Da came from the AG molecules, which meant the existence of the free AG. Although the intensity from this peak was not high, it could be detected in all of the samples. In this work, the acid conditions were adjusted by toluene-p-sulfonic acid with molecule weight 172 Da. The ions with 293 and 533 Da from the samples prepared under acid conditions were then be assigned as the combination of the toluene-p-sulfonic acid and FA or their self-condensates.
During the self-condensation of furfuryl alcohol molecules, formaldehyde might be given off for the relatively unstable methylene ether link, seen as below:
![Polymers 09 00711 i024]()
In
Table 1, the peak of 298 Da was assigned as the co-condensation product of furfuryl alcohol and AG and that of 617 Da came from the Na
+ complex with two molecules of the products. Seen from
Figure 1, the ions with 298 Da could be detected clearly for the samples prepared under pH 1 and 3. Then, the intensity of the ion peak decreased for the sample prepared under pH 5. Even no peak at 298 Da could be detected for the sample prepared under pH 7. Although the peak at around 298 Da reappeared for the samples prepared under pH 9, 11, and 13, judged by the intensity of this peak, the reaction between furfuryl alcohol and AG under alkaline conditions was not as easy as that under acid conditions. The peaks of 252 and 310 Da could be assigned as carbonium ions that were gotten from the co-condensation product of furfuryl alcohol and AG. The possible reaction for the formation of the carbonium ions of 310 Da was as below:
![Polymers 09 00711 i025]()
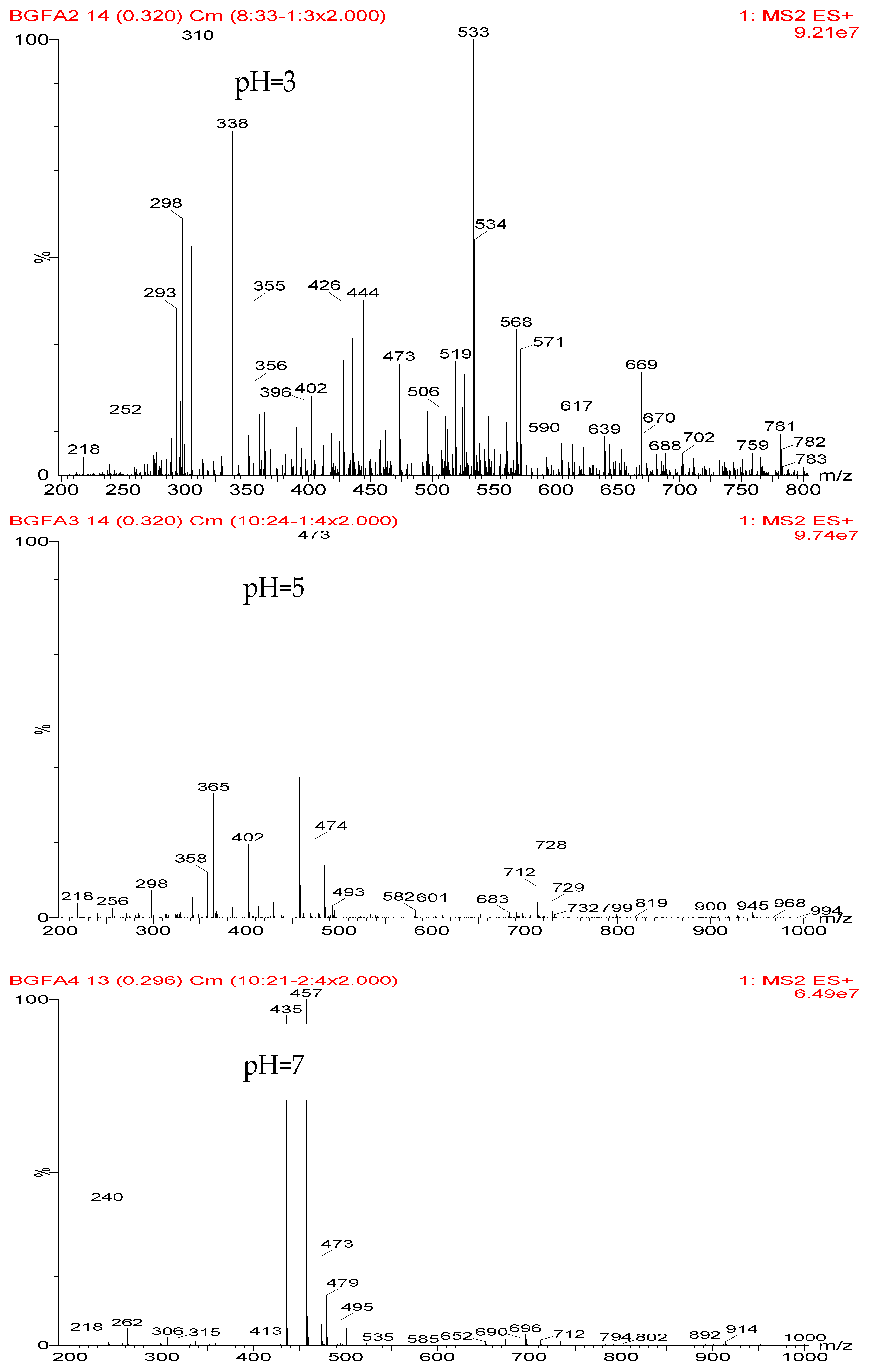
Table 2 was the assignments on the main ion peaks of the samples with furfuryl alcohol and AG prepared under alkaline conditions. It was regretful that there was no co-condensate with furfuryl alcohol and AG was detected. The peaks of 218, 240, and 256 Da came from one molecule of AG. The peaks of 435, 457, and 479 Da came from the complex of two molecules of AG with H
+. The peaks of 501, 718, and 740 Da were from the complex of two molecules of AG with Na
+. The peaks of 299, 315, 318, 397, and 413 Da were from furfuryl alcohol and its derivatives.
In all, the analysis on the ESI-MS spectra indicated that the co-condensation occured only under acidic conditions. However, since one AG molecule owns three different amino groups, specifically, primary amido, secondary amido, and aliphatic amino groups, and all of them have the possibility to react with furfuryl alcohol in theory, the analysis of the mechanism of the reaction between AG and furfuryl alcohol is still impossible with the limited information that is given by the ESI-MS results with so many isomers.
3.2. The 13C NMR Analysis
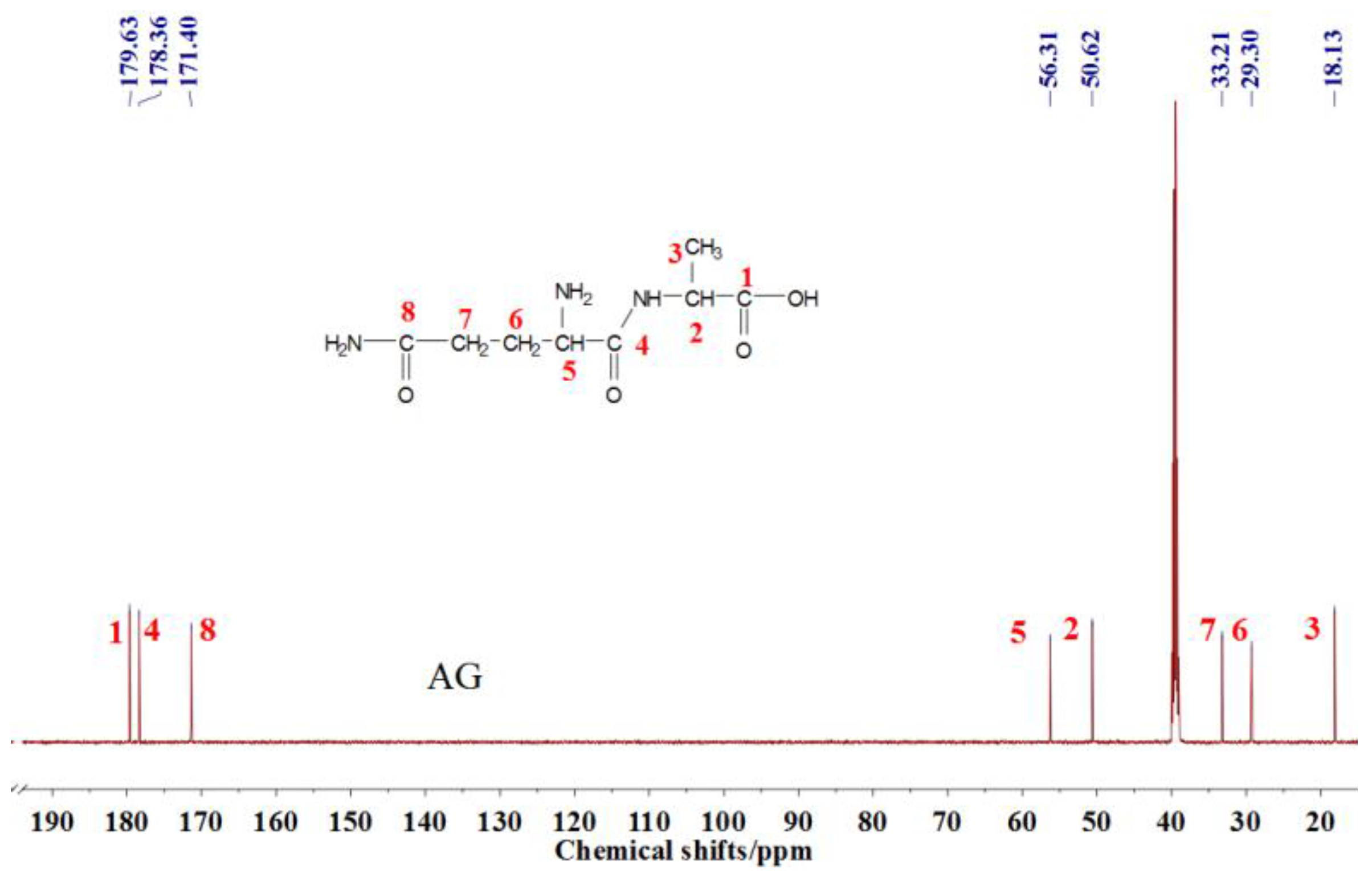
The
13C NMR result of AG is given in
Figure 2. The clarity of the
13C NMR results of the AG sample reflected the purity of the raw material used in this study. All the carbons in an AG molecule and their assignments were labeled in
Figure 2. Seen from the chemical structure of AG, both amino and carboxyl groups of AG molecules are possible to react with furfuryl alcohol molecules. When considering the unpaired electron of the amino group, the amino groups will show better nucleophilicity than carboxyl groups and be more liable to react with furfuryl alcohol molecules.
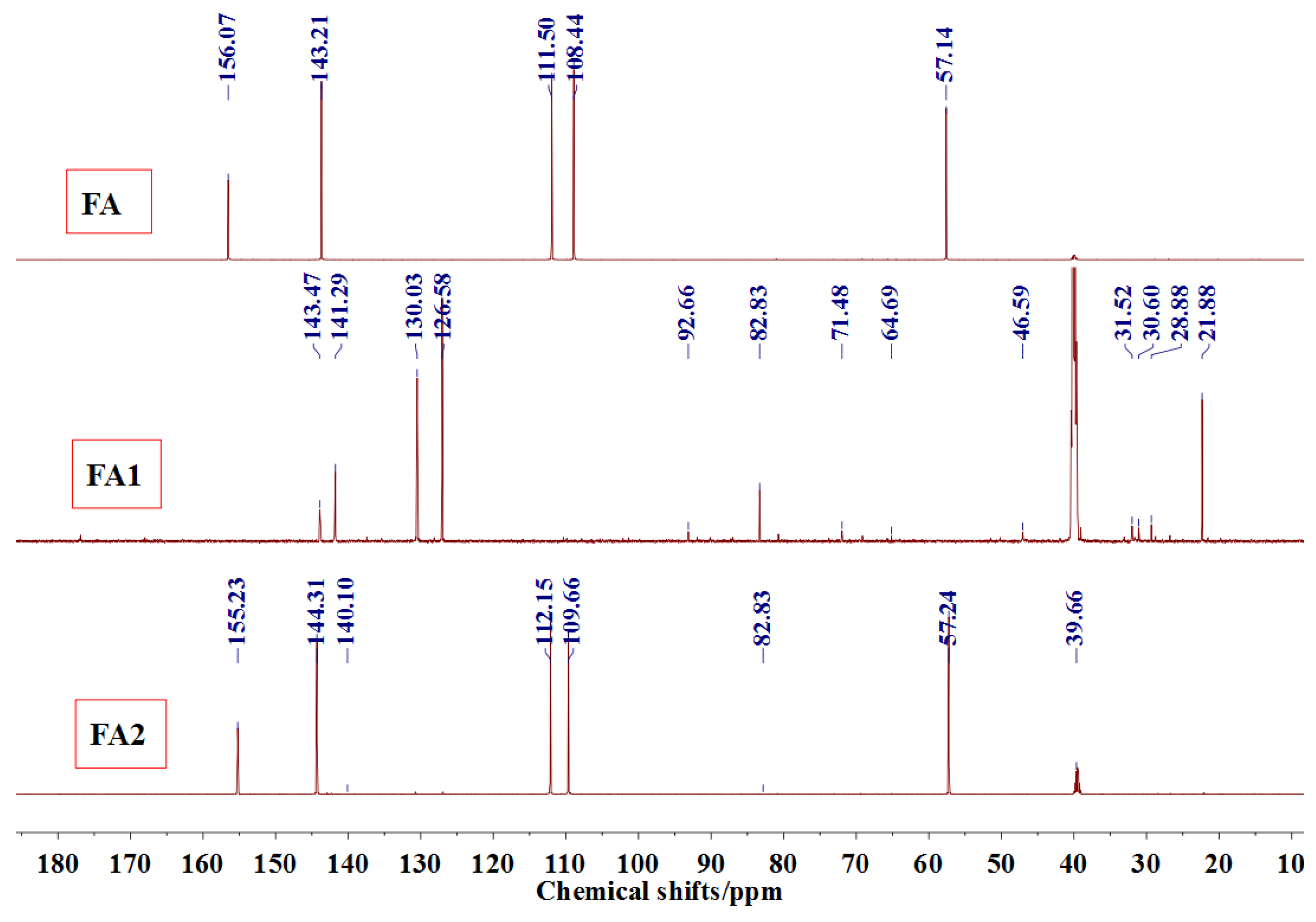
Figure 3 was the
13C NMR results of the material furfuryl alcohol (FA) and the samples FA1 and FA2. FA1, and FA2 were the products gotten with furfuryl alcohol under strong acid and alkaline conditions, respectively. The
13C NMR result of FA2 showed no obvious difference as that of FA, which indicated that there was no self-condensation between furfuryl alcohol molecules under strong alkaline conditions. On the contrary, the
13C NMR result of FA1 showed great difference as that of FA. In the
13C NMR of FA1, 28.88 and 31.52 ppm could be assigned as the methylene link from the self-condensation of furfuryl alcohol molecules. The shift at 64.69 ppm could be assigned as the methylene ether link, which came from the self-condensation of furfuryl alcohol, too. Since the peak area of methylene link was obviously bigger than that of ether link, methylene link might be the main form for the self-condensation of furfuryl alcohol under pH 1. In all, the
13C NMR proved that the self-condensation reaction of furfuryl alcohol under strong acid conditions. The shift at 82.83 ppm was assigned as the formaldehyde hydrate, namely methylene glycol, which indicated that the hydroxymethyl groups might be released from the furfuryl alcohol molecules or its self-condensation products under strong acid conditions. That also caused the appearance of the shift at 46.59, 71.48, and 92.66 ppm, which all came for formaldehyde and its derivatives.
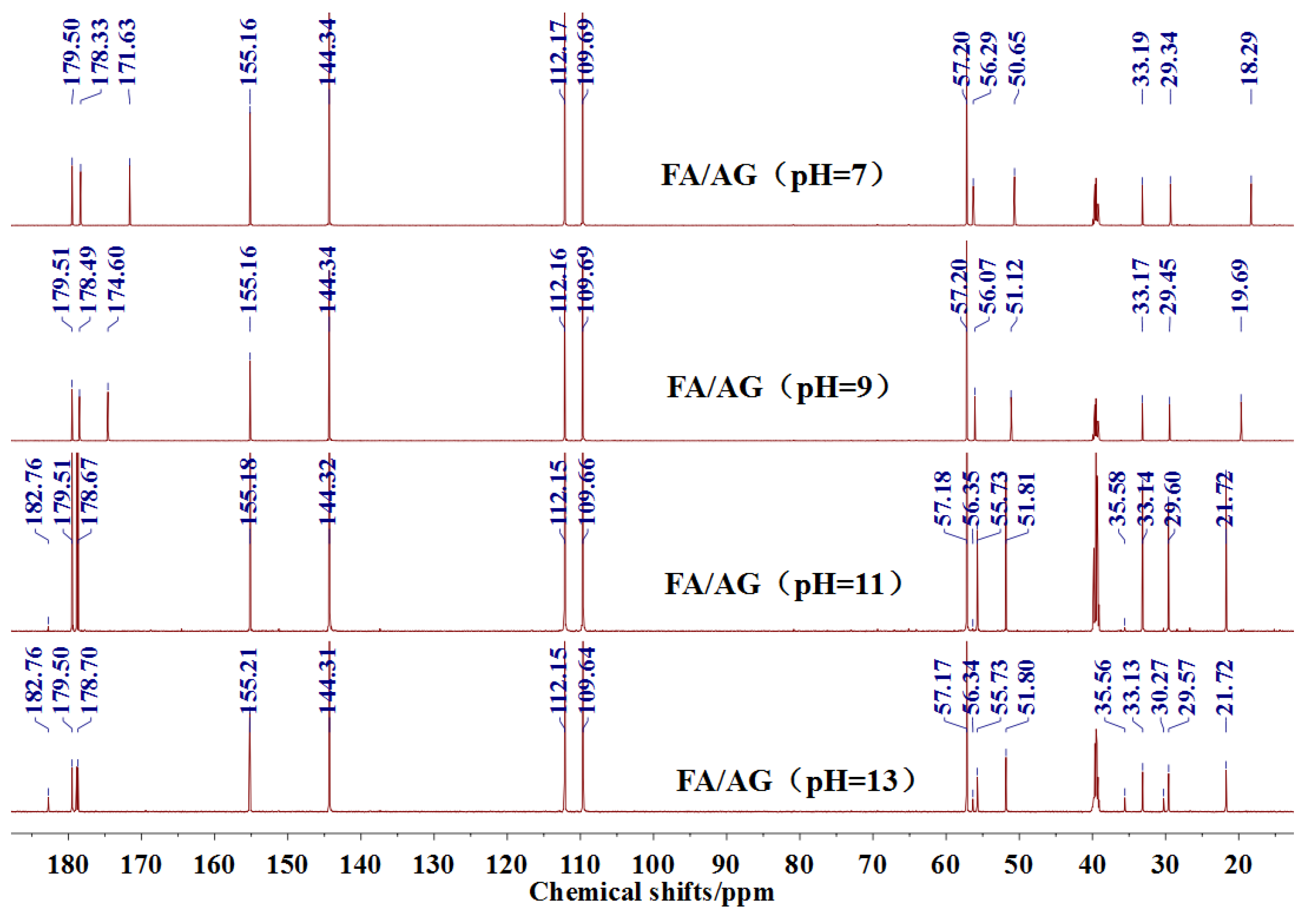
The
13C NMR results of the samples prepared with furfuryl alcohol and AG under alkaline conditions were given as
Figure 4.
Seen from
Figure 4, the
13C NMR results of the samples that were prepared with furfuryl alcohol and AG under pH 7 and 9 showed no much difference from that of furfuryl alcohol and AG themselves, meaning no reaction between furfuryl alcohol and AG under pH 7 and 9. For the FA/AG samples that were prepared under pH 11 and 13, new peaks around 35.6 ppm appeared. To determine the assignment of this new peak, the software of Mestrenove was used to predict the chemical shifts of all the possible products gotten with furfuryl alcohol and AG. The chemical shift was actually determined greatly by the inductivity of the aliphatic amino, primary and secondary amido groups from AG molecules. The calculation of the software Mestrenove on the
13C NMR chemical shifts of the possible reactions between AG and furfuryl alcohol was then the results of respecting this law. According to the calculation results, the possible chemical shift of Furan–
CH
2–NH(CO)–R from the reaction between the primary amido group of AG and furfuryl alcohol was 36–37 ppm. The possible chemical shift of R–NH–
CH
2–Furan from the reaction between the aliphatic amine group of AG and furfuryl alcohol was 43–44 ppm. The possible chemical shift of Furan–
CH
2–NR’(CO)–R from the reaction between secondary amido group of AG and furfuryl alcohol was 40–41 ppm. Therefore, the new peak at 35.58 ppm for the sample prepared at pH 11 and 35.56 ppm for the sample prepared at pH 13 could be assigned as the Furan–
CH
2–NH(CO)–R structure from the reaction between the primary amido group of AG and furfuryl alcohol. The peak at 57.20 ppm could be assigned as –CH
2 bond of furfuryl alcohol, which meant the existance of the free furfuryl alcohol in the system. It is normal and reasonable for the existence of the reaction monomer.
There was a peak at 182.76 ppm (pH = 11 and 13). Seen from the structure of AG, the self-condensation of intramolecular cyclisation of AG between carboxylic acid and primary amide could be occurred. However, the peak at 182.76 ppm should not be from carbonyl of the self-condensate of AG. When compared with the
Figure 2 of the
13C NMR spectra of AG, 182.76 ppm appeared at a relatively lower field. In theory, if the self-condensation of intramolecular cyclisation of AG occurred, the carbonyl of the self-condensate would appeared at a relatively higher field because of the p–π conjugated effect in the new formed –CO–NH– groups. Similary, the peak at 182.76 ppm should not come from the C1 reacted with methylol groups of FA for the p–π conjugated effect.
There was a great possibility for the peak at 182.76 ppm from the shift of C1 of AG that was caused by reaction between C8 of AG and FA. Their reaction mechanism was similar with that between urea and formaldehyde under alkaline conditions.
However, for the 13C NMR, the chemical shift of carbonyl groups would be much easier to be affected by its environments, such as the resolvent, pH, and others, than that of methylene groups. The judgements on the reaction based on the analysis of the peaks of methylene groups would be more reliable than that of carbonyls groups.
There was no peak from methylene and ether link caused by the self-condensation of furfuryl alcohol molecules in
Figure 4.
The analysis on
Figure 4 indicated that the reaction between furfuryl alcohol and AG was difficult to happen under alkaline conditions and only when the alkaline condition was as strong as pH = 11 or higher, were a few co-condensation products that were detected in a mixing system with furfuryl alcohol and AG. The stability of furfuryl alcohol under alkaline conditions might be responsible for that [
17,
24]. Under relatively weak alkaline conditions, it seemed to be difficult for AG molecule to form a relatively stable primary amide anions reaction intermediate or even there were some amide anions in the system, their concentration was not as high as to promote the condensation reaction with furfuryl alcohol. Under strong alkaline conditions, to form more amide anions would get easier, which would be helpful for their combination with furfuryl alcohol. For the aliphatic amino groups of AG, it was hard to form reaction intermediate. For the secondary amido groups, the steric hindrance might be the main reason for the stop of condensation between them and furfuryl alcohol molecules.
Based on the analysis above, the reaction mechanism between furfuryl alcohol and AG was presumed, as below.
In all, under alkaline conditions, the condensation reaction between FA and AG was slow.
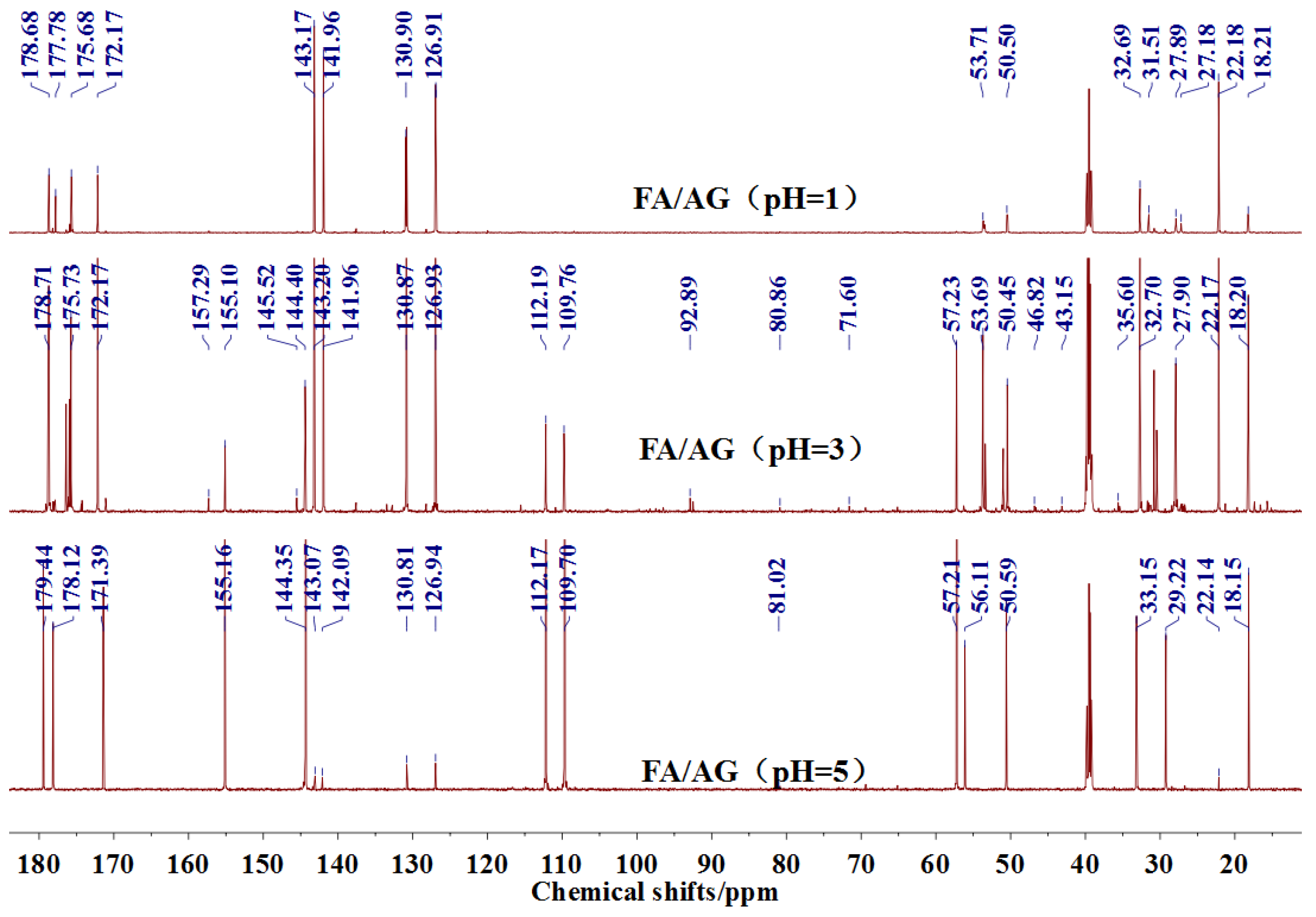
The
13C NMR results of the samples prepared with furfuryl alcohol and AG under pH 1, 3, and 5 were given as
Figure 5.
In the mixing system with furfuryl alcohol and AG, the reaction intermediate might come from the hydroxymethyl furfural carbonium ions. They could react with its carbon 5, its hydroxymethyl groups or the amino groups of AG molecules. As seen from
Figure 5, in the samples that were prepared with furfuryl alcohol and AG under pH 3, the peaks at 35.60 and 43.15 ppm were obviously observed. According to the analysis on the assignments of the chemical shift above, they could be assigned as Furan–
CH
2–NH(CO)–R and R–NH–
CH
2–Furan, respectively. Being similar with the results of the samples that were prepared under alkaline conditions, as seen from the bigger peak area of the former structure than that of the latter, the reaction between the primary amido groups of AG and furfuryl alcohol would be easier to proceed than that between the aliphatic amino groups of AG and furfuryl alcohol.
In theory, the amido groups in AG moleculs show weaker nucleophilicity than aliphatic amide groups because of the p–π conjugated effect of the amido groups. However, under acid conditions, aliphatic amide has the priority to attract hydrogen ions, and then be inactivated to other groups [
23]. Therefore, under acid conditions, when compared with the aliphatic amide groups, the non-protonized amido groups would show bigger possibility to react with the furfural carbonium ions to form the Furan–
CH
2–NH(CO)–R structure. Being as the same as the results of the samples tha were prepared under alkaline conditions, no peak at around 41 ppm from the structure of Furan–
CH
2–NR’(CO)–R was detected.
The possible reactions with furfuryl alcohol and AG under acid conditions were as below.
![Polymers 09 00711 i027]()
Seen from
Figure 5, there were both self- and co-condensation products in the mixing system with furfuryl alcohol and AG under acid conditions, and the sample prepared under pH 3 showed the biggest amount of the co-condensation reaction products. Obviously, the co-condensation reaction between furfuryl alcohol and AG had to compete with the self-condensation of furfuryl alcohol. It was reported that violent self-condensation reaction between furfuryl alcohol molecules would happen under strong acid conditions, especially when the value of pH was lower than 2, and large quantities of heat would be given off [
25,
26]. The strong acid conditions would catalyze the reaction between the hydroxymethyl furfural carbonium ions and the furfuryl alcohol’s carbon 5. The release of the large quantities of heat meant that the self-condensation products of furfuryl alcohol molecules owned relatively low energy in the opinion of thermodynamics.
Both the relative higher energy of the co-condensation products of furfuryl alcohol and AG, and the possible protonation would lead to the prevailing of the self-condensation reaction in the mixing system with furfuryl alcohol and AG under acid conditions. The more acid the system was, the more violent the self-condensation would be. However, at the same time, with more acid in the mixing system, there would be more hydroxymethyl furfural carbonium ions, which would be favorable to the co-condensation reaction between furfuryl alcohol and AG. Therefore, there was an equilibrium pH for the competition between the self-condensation and co-condensation in the mixing system. In this study, for more co-condensation products, it was better for furfuryl alcohol and AG to be mixed under pH 3.
3.3. FT-IR Analysis
Figure 6 gives the FT-IR results of AG. In
Figure 6, the absorption at 3334.3 and 3411.5 cm
−1 could be assigned as the symmetric and asymmetric vibration of N–H bond of –NH
2 groups. The absorption at 3227.6 cm
−1 came from the vibration of N–H bond from –NH– groups. The absorption at 1652.7 cm
−1 was from the C=O from amido groups. The absorption at 1606.4 cm
−1 was from the bending vibration of N–H bond from aliphatic amino groups. The absorption at 2939.0 and 1321 cm
−1 were, respectively, from O–H bond and C–O bond from carboxyl groups [
27,
28].
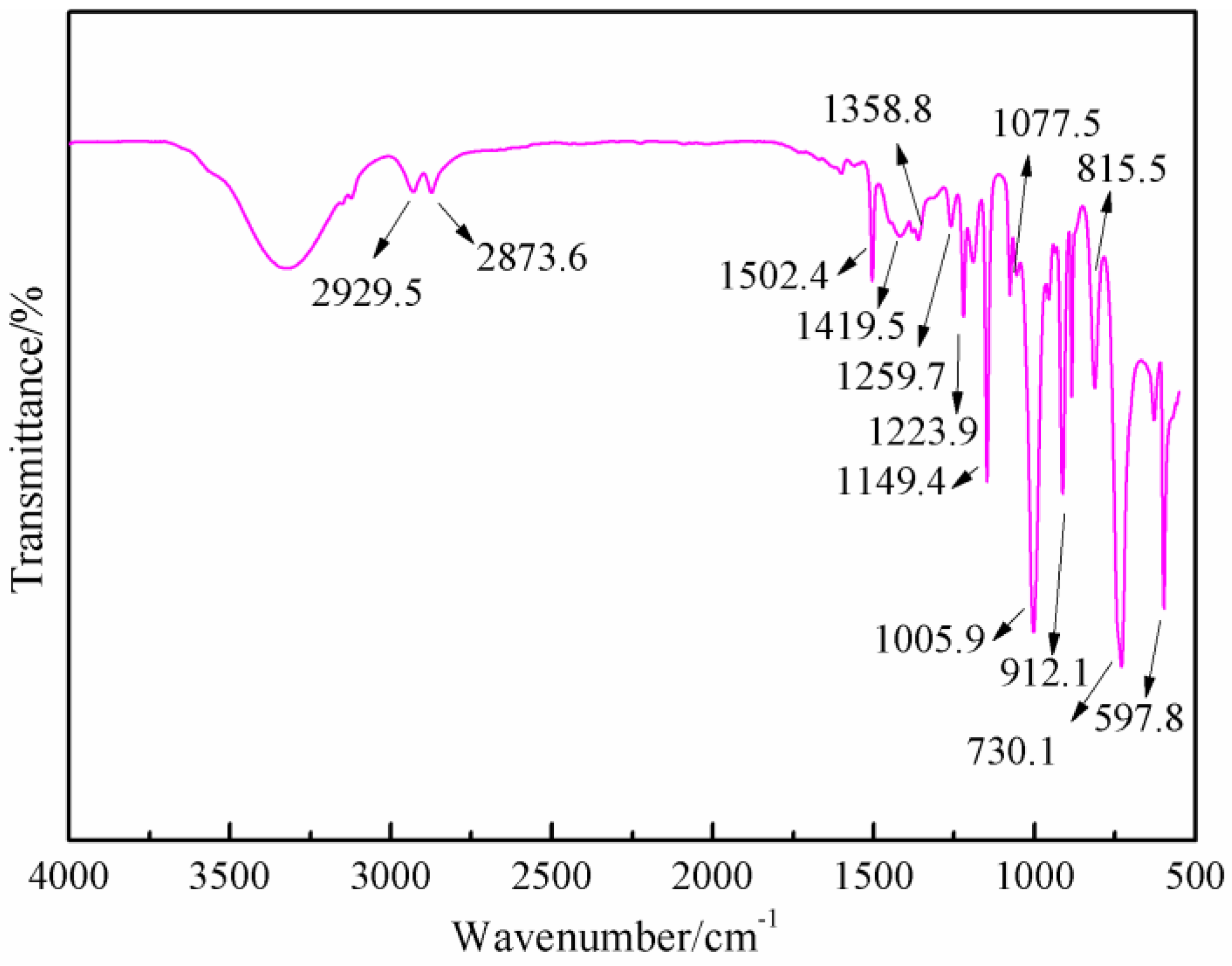
Figure 7 was the FT-IR results of furfuryl alcohol. The absorption at 3200 to 3300 cm
−1 was from the vibration of hydroxyl from –CH
2OH groups. The absorption at 2873.8 and 2929.5 cm
−1 could be assigned as the symmetric and asymmetric vibration of methylene bond from –CH
2OH groups. The absorption at 1005.9 cm
−1 was from C–O of methylol groups. The absorption at 815.5 cm
−1 was from the vibration of C-H of furan ring.
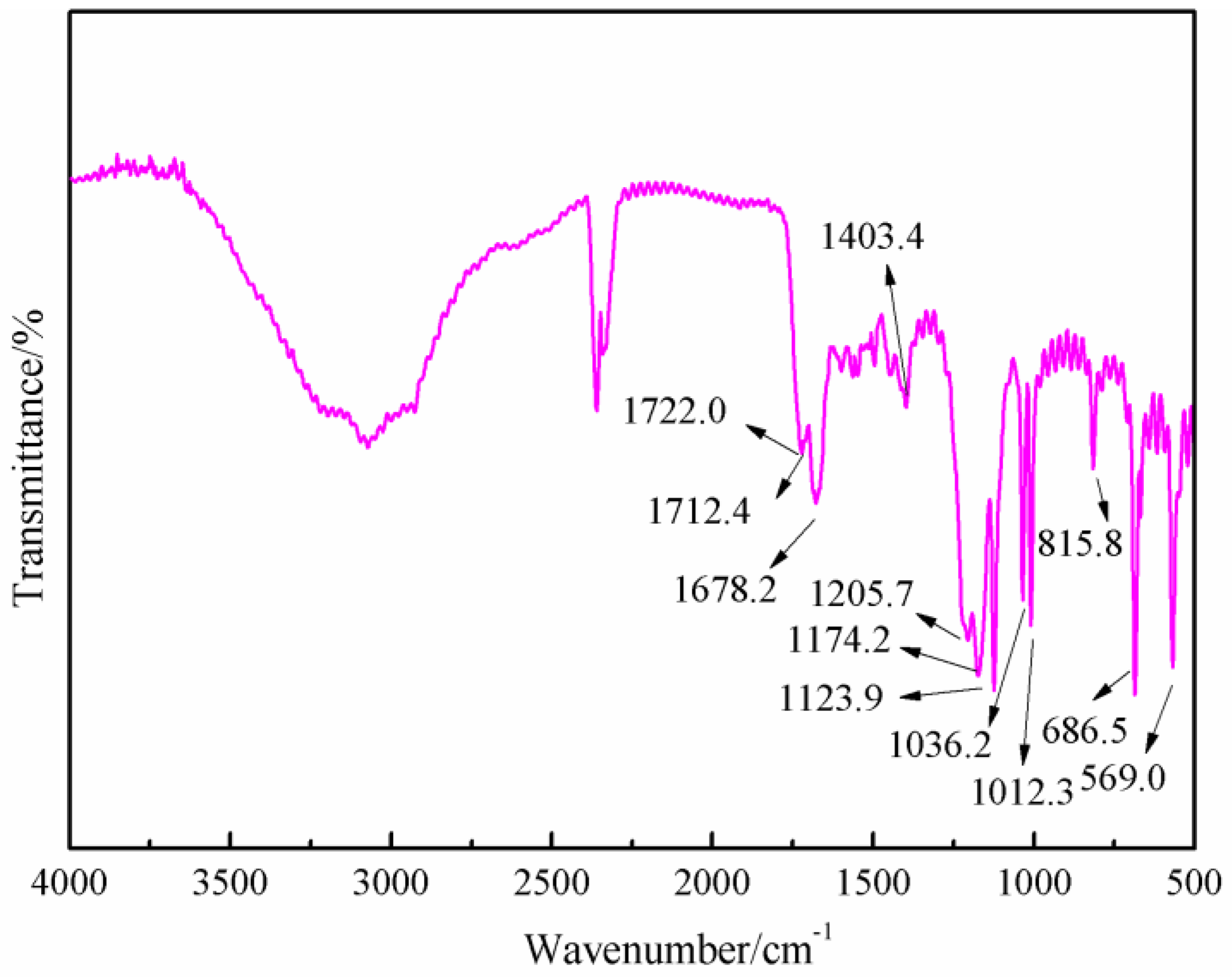
Figure 8 was the FT-IR results of the sample synthesized with furfuryl alcohol and AG under acid conditions, specifically at pH = 3. When compared with
Figure 6, the absorption at 1652.7 and 1606.4 cm
−1 from amido and aliphatic amino groups shifted, respectively, to 1722.0 and 1678.2 cm
−1, which indicated that the combination of the amido and aliphatic amino groups with some groups with stronger inductivity than that of hydrogen atom. The absorption of 1420.4 cm
−1 in
Figure 6 from the vibration of C–N groups shifted to 1404.3 cm
−1 in
Figure 8, which indicated that the possible new group combined with the amido groups would weaken the p–π conjugated effect between amino and carbonyl bond. New absorptions appeared at 1205 and 1174.2 cm
−1, which could be assigned as the symmetric and asymmetric vibration of methylene bond from –NH–CH
2–C=C– groups. In all, the FT-IR results were another proof of the reaction between furfuryl alcohol and AG.
















 (AG)
(AG) (AG-Na)
(AG-Na)
















