A pH-Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
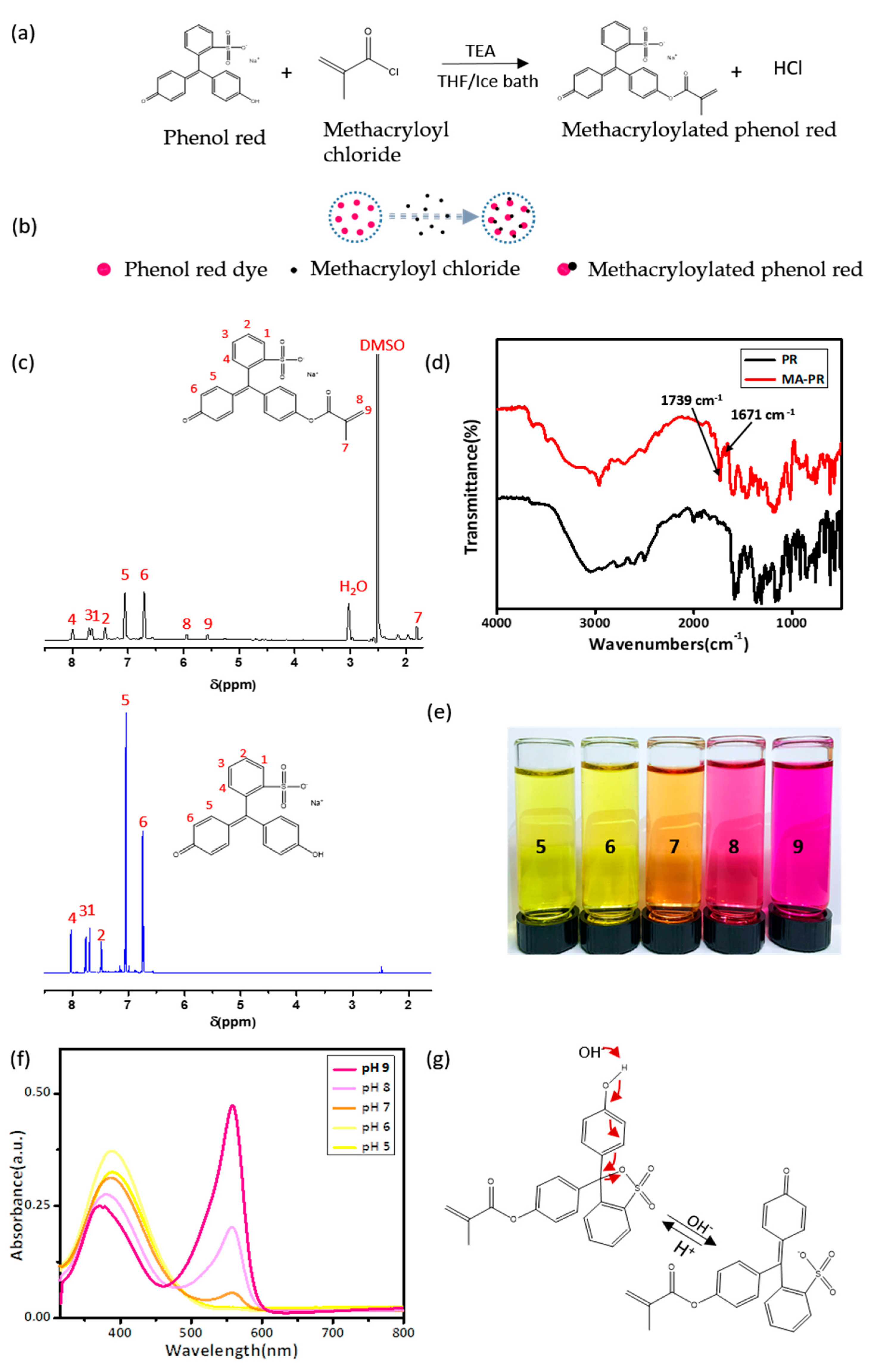
2.2. Synthesis and Characterization of Methacrylated Phenol Red (MA-PR)
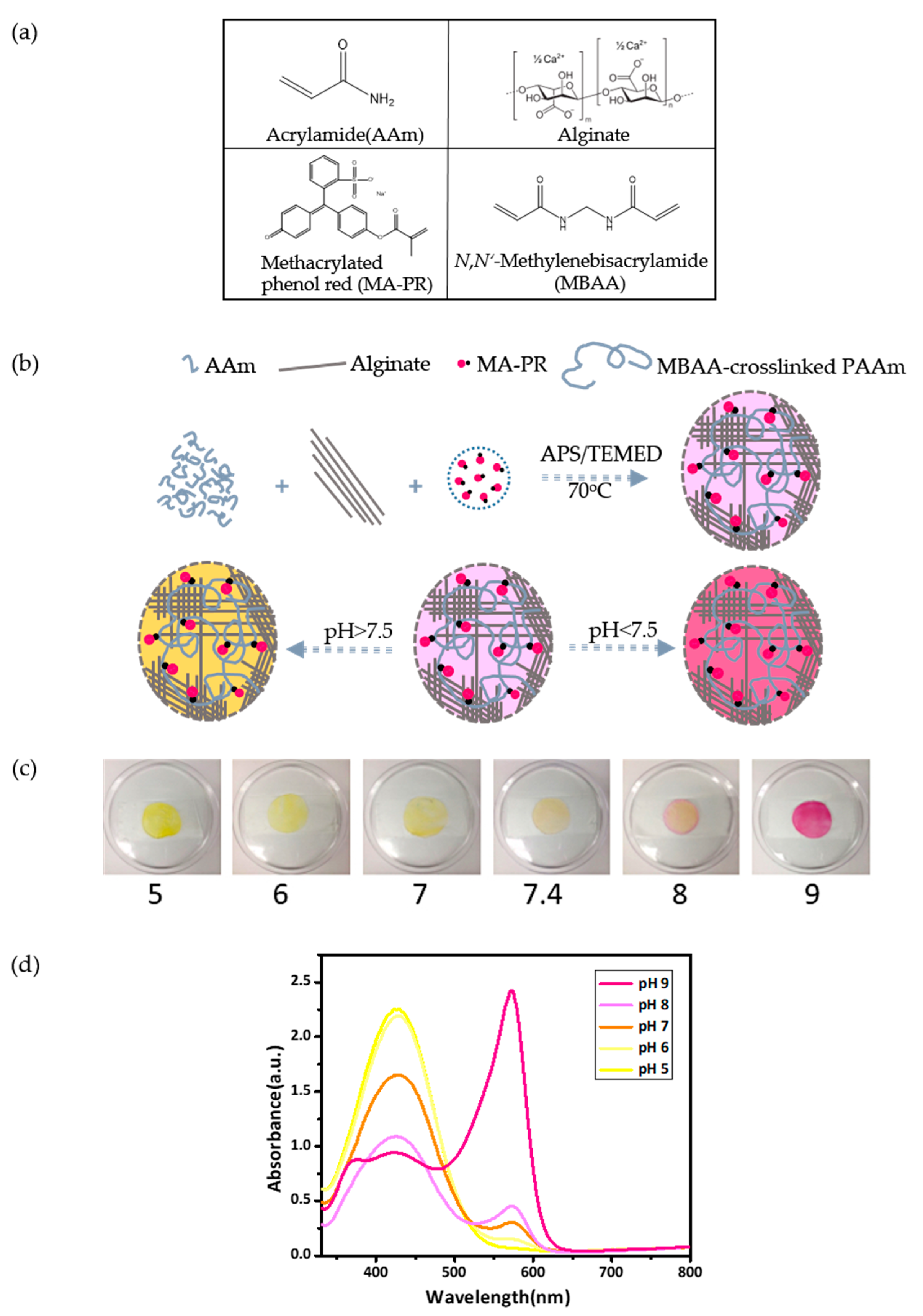
2.3. Preparation of P(AAm-MAPR)/Alginate Hydrogel Patches
2.4. Characterization
2.5. Mechanical Testing
2.6. Swelling Ratio Test
2.7. Water Vapour Transmission Test
3. Results and Discussion
3.1. Synthesis and Characterization of MA-PR
3.2. Preparation and Characterization of P(AAm-MAPR)/Alginate Hydrogel Patches
3.3. Physical Evaluation of Hydrogel Patches as a Function of P(AAm-MAPR) Crosslinking Density
3.4. Investigation of Mechanical Properties under Different Calcium and Water Contents
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carvalho, I.C.; Mansur, H.S. Engineered 3d-scaffolds of photocrosslinked chitosan-gelatin hydrogel hybrids for chronic wound dressings and regeneration. Mater. Sci. Eng. C 2017, 78, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing–Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of ph on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Heuss, E. Die Reaktion Des Schweisses Beim gesuNden Menschen; Voss: Hordaland, Norway, 1892; pp. 400–401. [Google Scholar]
- Schade, H.; Marchionini, A. Der säuremantel der haut (nach gaskettenmessungen). J. Mol. Med. 1928, 7, 12–14. [Google Scholar] [CrossRef]
- Dikstein, S.; Zlotogorski, A. Measurement of skin ph. Acta Dermato-Venereologica Supplementum 1993, 185, 18–20. [Google Scholar]
- Rippke, F.; Schreiner, V.; Schwanitz, H.J. The acidic milieu of the horny layer/new findings on physiology and pathophysiology of the skin ph-value. Dermatosen in Beruf und Umwelt 1999, 47, 230–245. [Google Scholar]
- Greener, B.; Hughes, A.; Bannister, N. The effect of ph on proteolytic activity in chronic wound fluids and methods for determination. In Proceedings of the Poster Z079 2nd WUWHS Congress, Paris, France, 1 May 2005. [Google Scholar]
- Greener, B.; Hughes, A.; Bannister, N.; Douglass, J. Proteases and ph in chronic wounds. J. Wound Care 2005, 14, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Noble, J.; Eagle, M. The use of proteases as prognostic markers for the healing of venous leg ulcers. J. Wound Care 1999, 8, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Hammad, L.; Creevy, J.; Shearman, C.; Mani, R. Physical changes in dermal tissues around chronic venous ulcers. In Proceedings of the 7th European Conference on Advances in Wound Management, Harrogate, UK, 18–20 November 1997. [Google Scholar]
- Gethin, G. The significance of surface ph in chronic wounds. Wounds 2007, 3, 52. [Google Scholar]
- Guinovart, T.; Valdés-Ramírez, G.; Windmiller, J.R.; Andrade, F.J.; Wang, J. Bandage-based wearable potentiometric sensor for monitoring wound ph. Electroanalysis 2014, 26, 1345–1353. [Google Scholar] [CrossRef]
- Schyrr, B.; Pasche, S.; Scolan, E.; Ischer, R.; Ferrario, D.; Porchet, J.-A.; Voirin, G. Development of a polymer optical fiber ph sensor for on-body monitoring application. Sens. Actuators B Chem. 2014, 194, 238–248. [Google Scholar] [CrossRef]
- Sridhar, V.; Takahata, K. A hydrogel-based passive wireless sensor using a flex-circuit inductive transducer. Sens. Actuators A Phys. 2009, 155, 58–65. [Google Scholar] [CrossRef]
- Bergveld, P.; Sibbald, A. Analytical and Biomedical Applications of Ion-Selective Field-Effect Transistors; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Van der Schueren, L.; De Clerck, K. Coloration and application of pH-sensitive dyes on textile materials. Color. Technol. 2012, 128, 82–90. [Google Scholar] [CrossRef]
- Cooney, C.G.; Towe, B.C. A miniaturized ph and pco 2 intravascular catheter using optical monitoring and a dual concentric-flow microdialysis approach. Sens. Actuators B Chem. 2000, 62, 177–181. [Google Scholar] [CrossRef]
- Ramos, M.L.P.; González, J.A.; Fabian, L.; Pérez, C.J.; Villanueva, M.E.; Copello, G.J. Sustainable and smart keratin hydrogel with pH-sensitive swelling and enhanced mechanical properties. Mater. Sci. Eng. C 2017, 78, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.; Bajpai, A.K.; Bajpai, J.; Singh, S.K. Evaluation of poly (vinyl alcohol) based cryogel–zinc oxide nanocomposites for possible applications as wound dressing materials. Mater. Sci. Eng. C 2016, 65, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.H.; Sun, J.Y.; Chen, Y.M.; Zhou, J.; Suo, Z. Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl. Mater. Interfaces 2013, 5, 10418–10422. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Agag, T.; Yagci, Y.; Ishida, H. Methacryloyl-functional benzoxazine: Photopolymerization and thermally activated polymerization. Macromolecules 2011, 44, 767–772. [Google Scholar] [CrossRef]
- Kandola, B.K.; Krishnan, L.; Deli, D.; Luangtriratana, P.; Ebdon, J.R. Fire and mechanical properties of a novel free-radically cured phenolic resin based on a methacrylate-functional novolac and of its blends with an unsaturated polyester resin. RSC Adv. 2015, 5, 33772–33785. [Google Scholar] [CrossRef]
- Kermis, H.R.; Kostov, Y.; Rao, G. Rapid method for the preparation of a robust optical ph sensor. Analyst 2003, 128, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.I.; Lim, J.S.; Kim, S.H.; Suh, D.H. Synthesis and luminescent properties of a novel eu-containing nanoparticle. Polymer 2006, 47, 5253–5258. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A novel wound dressing based on Ag/graphene polymer hydrogel: Effectively kill bacteria and accelerate wound healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Pathak, V.; Soni, B.; Mohan, Y.M. Cnws loaded poly(sa) hydrogels: Effect of high concentration of cnws on water uptake and mechanical properties. Carbohydr. Polym. 2014, 106, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Shezad, O.; Khan, S.; Khan, T.; Park, J.K. Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Carbohydr. Polym. 2010, 82, 173–180. [Google Scholar] [CrossRef]
- Zheng, A.; Xue, Y.; Wei, D.; Li, S.; Xiao, H.; Guan, Y. Synthesis and characterization of antimicrobial polyvinyl pyrrolidone hydrogel as wound dressing. Soft Mater. 2014, 12, 179–187. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly (vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Saha, N.; Humpolicek, P.; Saha, P. Permeability and biocompatibility of novel medicated hydrogel wound dressings. Soft Mater. 2010, 8, 338–357. [Google Scholar] [CrossRef]
- Lamke, L.-O.; Nilsson, G.; Reithner, H. The evaporative water loss from burns and the water-vapour permeability of grafts and artificial membranes used in the treatment of burns. Burns 1977, 3, 159–165. [Google Scholar] [CrossRef]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Wang, J.L.; Young, T.H.; Han, J.L.; Hsieh, K.H. Evaluation of chitosan/γ-poly (glutamic acid) polyelectrolyte complex for wound dressing materials. Carbohydr. Polym. 2011, 84, 812–819. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Calcium: A potential central regulator in wound healing in the skin. Wound Repair. Regen. 2002, 10, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Chen, X.; Eldin, M.S.M.; Kenawy, E.-R.S. Crosslinked poly (vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, X.; Nagao, M.; Elias, A.L.; Narain, R.; Chung, H.-J. A pH-Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings. Polymers 2017, 9, 558. https://doi.org/10.3390/polym9110558
Liu L, Li X, Nagao M, Elias AL, Narain R, Chung H-J. A pH-Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings. Polymers. 2017; 9(11):558. https://doi.org/10.3390/polym9110558
Chicago/Turabian StyleLiu, Li, Xinda Li, Masanori Nagao, Anastasia L. Elias, Ravin Narain, and Hyun-Joong Chung. 2017. "A pH-Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings" Polymers 9, no. 11: 558. https://doi.org/10.3390/polym9110558
APA StyleLiu, L., Li, X., Nagao, M., Elias, A. L., Narain, R., & Chung, H.-J. (2017). A pH-Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings. Polymers, 9(11), 558. https://doi.org/10.3390/polym9110558






