Polymers in Cartilage Defect Repair of the Knee: Current Status and Future Prospects
Abstract
:1. Introduction
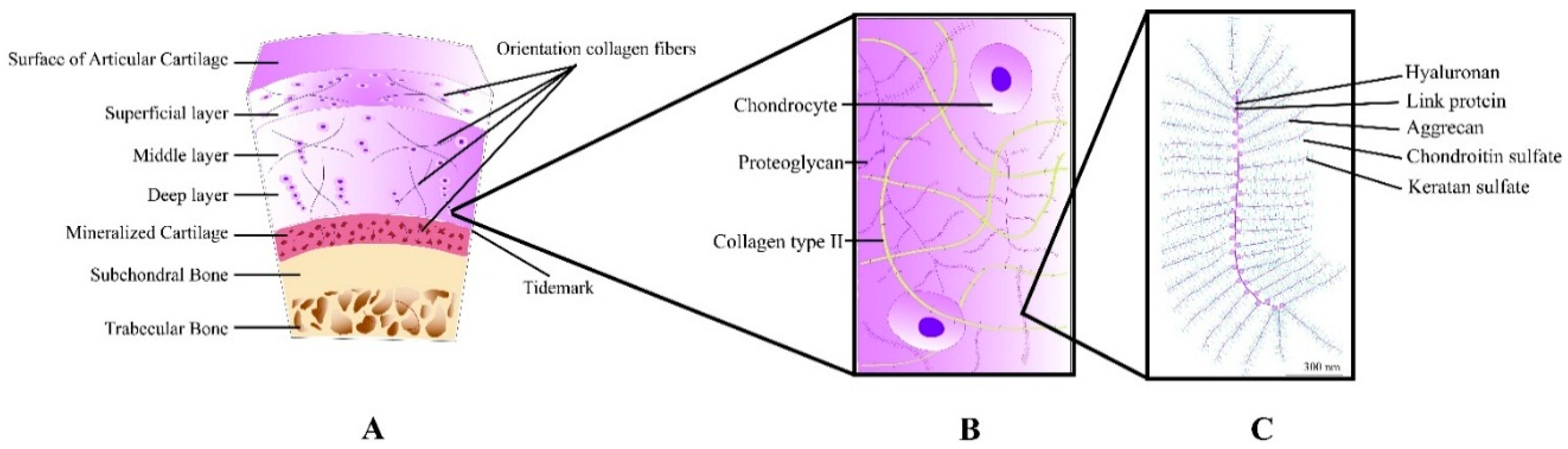
2. Cartilage: Structure and Repair
3. Construct Components
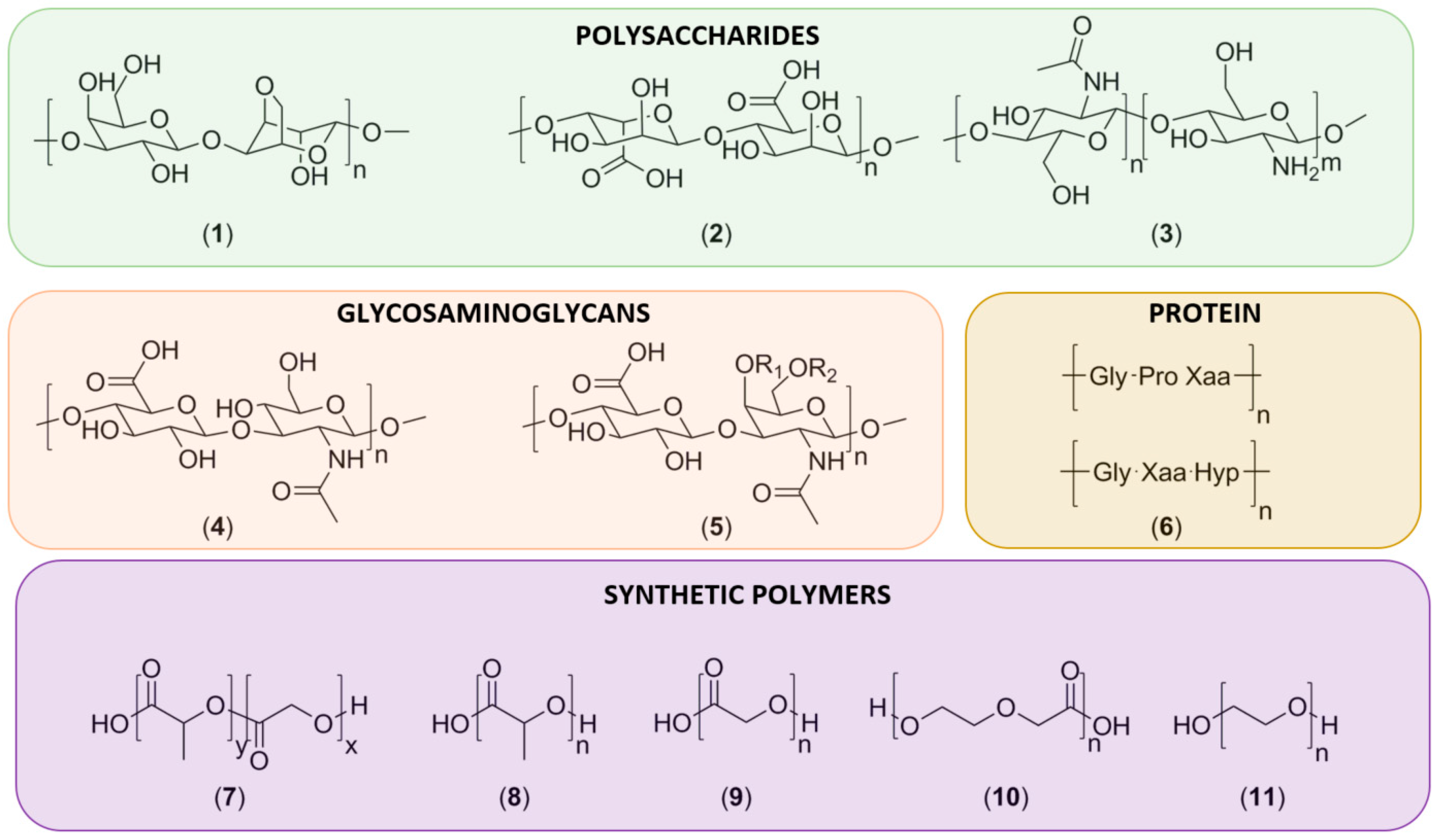
3.1. Natural Polymers
3.1.1. Polysaccharides
3.1.2. Glycosaminoglycans
3.1.3. Proteins
3.2. Synthetic Polymers
3.2.1. Poly(lactic-co-glycolic) Acid, Polylactic Acid and Polyglycolic Acid
3.2.2. Polydioxanone
3.2.3. Poly(ethylene glycol)
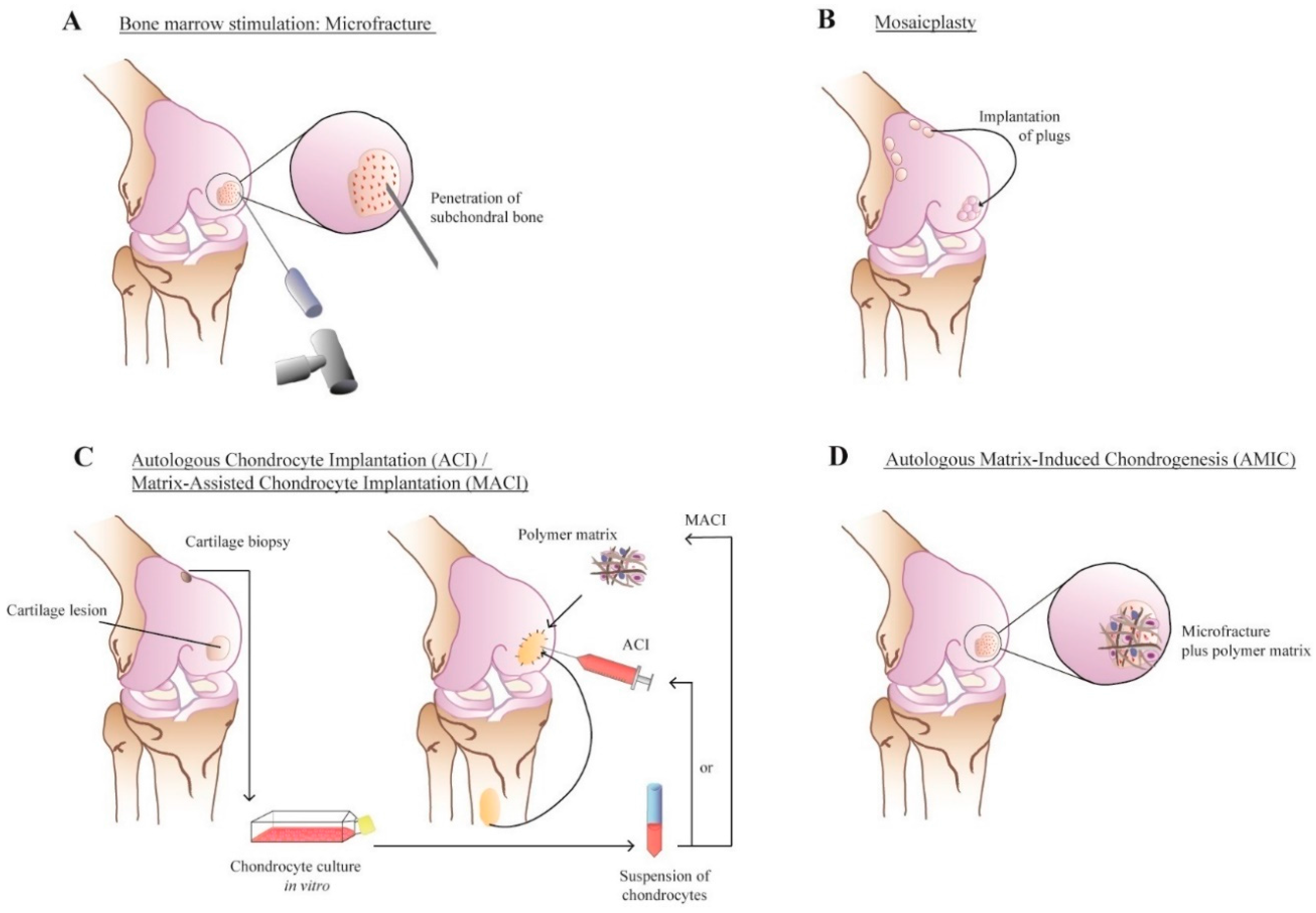
3.3. Polymers Used in Preclinical Settings
3.4. Advances in Construct Fabrication Techniques
4. Commercially Available Products
4.1. PLA/PLGA-Based Constructs
4.2. Collagen-Based Constructs
4.3. Other Natural Polymer-Based Constructs
4.4. Clinical Evidence in the Pipeline
4.5. Resurfacing Treatment Options: Closing the Bridge between Regenerative Treatments and Arthroplasties?
5. Discussion and Future Prospects
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACI | Autologous Chondrocyte Implantation |
| MACI | Matrix-Assisted Chondrocyte Implantation |
| ECM | Extracellular Matrix |
| AMIC | Autologous Matrix-Induced Chondrogenesis |
| MSC | Mesenchymal Stem Cells |
| OATS | Osteochondral Autograft Transfer System |
| PRP | Platelet-Rich Plasma |
| BMC | Bone Marrow Concentrate |
| PDGF | Platelet-Derived Growth Factor |
| TGF-β | Transforming Growth Factor beta |
| FGF | Fibroblast Growth Factor |
| EGF | Epidermal Growth Factor |
| GAG | Glycosaminoglycan |
| PLGA | Poly(Lactic-co-Glycolic Acid) |
| PEG | Polyethylene Glycol |
| RGD | tripeptide (arg-gly-asp) |
| PCL | Polycaprolactone |
| PGA | Polyglycolic Acid |
| PLA | Polylactic Acid |
| PDS | Polydioxanone |
| FDA | Food and Drug Administration |
| NP | Nanoparticle |
| PVA | Polyvinyl Acid |
| PROM | Patient Reported Outcome Measure |
References
- Hjelle, K.; Solheim, E.; Strand, T.; Muri, R.; Brittberg, M. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy 2002, 18, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Kim, T.K.; Taboas, A.; Malik, A.; Manson, P.; Elisseeff, J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003, 9, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Widuchowski, W.; Widuchowski, J.; Trzaska, T. Articular cartilage defects: Study of 25,124 knee arthroscopies. Knee 2007, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Julin, J.; Jamsen, E.; Puolakka, T.; Konttinen, Y.T.; Moilanen, T. Younger age increases the risk of early prosthesis failure following primary total knee replacement for osteoarthritis. A follow-up study of 32,019 total knee replacements in the finnish arthroplasty register. Acta Orthop. 2010, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Vogel, L.A.; Carotenuto, G.; Basti, J.J.; Levine, W.N. Physical activity after total joint arthroplasty. Sports Health 2011, 3, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the united states from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Crema, M.D.; Nevitt, M.C.; Guermazi, A.; Felson, D.T.; Wang, K.; Lynch, J.A.; Marra, M.D.; Torner, J.; Lewis, C.E.; Roemer, F.W. Progression of cartilage damage and meniscal pathology over 30 months is associated with an increase in radiographic tibiofemoral joint space narrowing in persons with knee OA—The most study. Osteoarthr. Cartil. 2014, 22, 1743–1747. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takeda, H.; Nakagawa, T.; Nakamura, K.; Engebretsen, L. Prevention and management of knee osteoarthritis and knee cartilage injury in sports. Br. J. Sports Med. 2011, 45, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.W.; Lalor, P.A.; Aberman, H.M.; Simon, T.M. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J. Bone Jt. Surg. Am. 2001, 83, 53–64. [Google Scholar]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Steinwachs, M.; Kreuz, P.C. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: A prospective study with a 3-year follow-up. Arthroscopy 2007, 23, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Orth, P.; Cucchiarini, M.; Kohn, D.; Madry, H. Alterations of the subchondral bone in osteochondral repair—Translational data and clinical evidence. Eur. Cell Mater. 2013, 25, 299–316. [Google Scholar] [PubMed]
- Gudas, R.; Stankevicius, E.; Monastyreckiene, E.; Pranys, D.; Kalesinskas, R.J. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Krych, A.J.; Harnly, H.W.; Rodeo, S.A.; Williams, R.J. Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: A retrospective comparative study. J. Bone Jt. Surg. Am. 2012, 94, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Steinwachs, M.R.; Waibl, B.; Mumme, M. Arthroscopic treatment of cartilage lesions with microfracture and bst-cargel. Arthrosc. Tech. 2014, 3, e399–e402. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Faxen, E.; Peterson, L. Carbon fiber scaffolds in the treatment of early knee osteoarthritis. A prospective 4-year followup of 37 patients. Clin. Orthop. Relat. Res. 1994, 307, 155–164. [Google Scholar] [PubMed]
- Zeifang, F.; Oberle, D.; Nierhoff, C.; Richter, W.; Moradi, B.; Schmitt, H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: A randomized clinical trial. Am. J. Sports Med. 2010, 38, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.M.; Emans, P.J.; Coolsen, M.M.; Voss, L.; Surtel, D.A.; Cremers, A.; van Rhijn, L.W.; Welting, T.J. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Matsumoto, T.; Mifune, Y.; Fukui, T.; Kubo, S.; Matsushita, T.; Asahara, T.; Kurosaka, M.; Kuroda, R. Therapeutic strategy of third-generation autologous chondrocyte implantation for osteoarthritis. Ups. J. Med. Sci. 2011, 116, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Behrens, P.; Bitter, T.; Kurz, B.; Russlies, M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee 2006, 13, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Suzer, F.; Thermann, H. Autologous matrix-induced chondrogenesis in the knee: A review. Cartilage 2014, 5, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Solheim, E.; Hegna, J.; Oyen, J.; Harlem, T.; Strand, T. Results at 10 to 14 years after osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee. Knee 2013, 20, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Ozdemir, M.R.; Ozkan, Y. Osteochondral autografting (mosaicplasty) in grade iv cartilage defects in the knee joint: 2- to 7-year results. Int. Orthop. 2006, 30, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Enea, D.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Manzotti, S.; Gigante, A. One-step cartilage repair in the knee: Collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee 2015, 22, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.J.; Farr, J.; Winalski, C.S.; Hosea, T.; Richmond, J.; Mandelbaum, B.; de Deyne, P.G. Outcomes after a single-stage procedure for cell-based cartilage repair: A prospective clinical safety trial with 2-year follow-up. Am. J. Sports Med. 2011, 39, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Almqvist, K.F.; Dhollander, A.A.; Verdonk, P.C.; Forsyth, R.; Verdonk, R.; Verbruggen, G. Treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am. J. Sports Med. 2009, 37, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Buda, R.; Vannini, F.; Cavallo, M.; Grigolo, B.; Cenacchi, A.; Giannini, S. Osteochondral lesions of the knee: A new one-step repair technique with bone-marrow-derived cells. J. Bone Jt. Surg. Am. 2010, 92, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy 2015, 31, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Smyth, N.A.; Murawski, C.D.; Haleem, A.M.; Hannon, C.P.; Savage-Elliott, I.; Kennedy, J.G. Establishing proof of concept: Platelet-rich plasma and bone marrow aspirate concentrate may improve cartilage repair following surgical treatment for osteochondral lesions of the talus. World J. Orthop. 2012, 3, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. 2008, 13, 3532–3548. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.H.; Chaoji, V.; Maiguel, D.; Faridi, M.H.; Barth, C.J.; Salem, S.M.; Singhal, M.; Stoub, D.; Krastins, B.; Ogihara, M.; et al. Proteomic and phospho-proteomic profile of human platelets in basal, resting state: Insights into integrin signaling. PLoS ONE 2009, 4, e7627. [Google Scholar] [CrossRef] [PubMed]
- Senzel, L.; Gnatenko, D.V.; Bahou, W.F. The platelet proteome. Curr. Opin. Hematol. 2009, 16, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Smyth, S.S.; McEver, R.P.; Weyrich, A.S.; Morrell, C.N.; Hoffman, M.R.; Arepally, G.M.; French, P.A.; Dauerman, H.L.; Becker, R.C. Platelet functions beyond hemostasis. J. Thromb. Haemost. 2009, 7, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Akeda, K.; An, H.S.; Pichika, R.; Attawia, M.; Thonar, E.J.; Lenz, M.E.; Uchida, A.; Masuda, K. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine 2006, 31, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tummala, P.; King, A.; Lee, B.; Kraus, M.; Tse, V.; Jacobs, C.R. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng. C Methods 2009, 15, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Min, H.J.; Park, H.J.; Lee, S.; Seong, S.C.; Lee, M.C. Synovial membrane-derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy 2013, 29, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Feng, Y.; Zhang, C.Q.; Chen, S.B.; Cheng, X.G. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int. Orthop. 2010, 34, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Trattnig, S.; Lintner, F. Anatomy, biochemistry, and physiology of articular cartilage. Investig. Radiol. 2000, 35, 573–580. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Quinn, T.M.; Hauselmann, H.J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr. Cartil. 2002, 10, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Venkatesan, J.K.; Ekici, M.; Schmitt, G.; Madry, H. Human mesenchymal stem cells overexpressing therapeutic genes: From basic science to clinical applications for articular cartilage repair. Biomed. Mater. Eng. 2012, 22, 197–208. [Google Scholar] [PubMed]
- Siliski, J.M. Traumatic Disorders of the Knee; Springer-Verlag: New York, NY, USA, 1994. [Google Scholar]
- Wilson, W.; Huyghe, J.M.; van Donkelaar, C.C. Depth-dependent compressive equilibrium properties of articular cartilage explained by its composition. Biomech. Model. Mechanobiol. 2007, 6, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Guo, X.E. Mechano-electrochemical properties of articular cartilage: Their inhomogeneities and anisotropies. Annu. Rev. Biomed. Eng. 2002, 4, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, R.A. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J. Anat. 1971, 109, 411–421. [Google Scholar] [PubMed]
- Nordin, M.; Frankel, V.H.; Frankel, V.H. Basic Biomechanics of the Musculoskeletal System, 2nd ed.; Lea & Febiger: Philadelphia, PA, USA, 1989. [Google Scholar]
- Bevill, S.L.; Thambyah, A.; Broom, N.D. New insights into the role of the superficial tangential zone in influencing the microstructural response of articular cartilage to compression. Osteoarthr. Cartil. 2010, 18, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Wu, Y.; Ito, K.; van Donkelaar, C.C. The importance of superficial collagen fibrils for the function of articular cartilage. Biomech. Model. Mechanobiol. 2014, 13, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bartell, L.R.; Fortier, L.A.; Bonassar, L.J.; Cohen, I. Measuring microscale strain fields in articular cartilage during rapid impact reveals thresholds for chondrocyte death and a protective role for the superficial layer. J. Biomech. 2015, 48, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.L.; Barrett, A.R.; Das, M.; Petersen, P.B.; Bonassar, L.J.; Cohen, I. Structure-function relations and rigidity percolation in the shear properties of articular cartilage. Biophys. J. 2014, 107, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M. Variation of collagen fiber alignment in a joint surface: A scanning electron microscope study of the tibial plateau in dog, rabbit, and man. J. Orthop. Res. 1991, 9, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Responte, D.J.; Natoli, R.M.; Athanasiou, K.A. Collagens of articular cartilage: Structure, function, and importance in tissue engineering. Crit. Rev. Biomed. Eng. 2007, 35, 363–411. [Google Scholar] [CrossRef] [PubMed]
- Havelka, S.; Horn, V.; Spohrova, D.; Valouch, P. The calcified-noncalcified cartilage interface: The tidemark. Acta Biol. Hung. 1984, 35, 271–279. [Google Scholar] [PubMed]
- Hunziker, E.B.; Rosenberg, L.C. Repair of partial-thickness defects in articular cartilage: Cell recruitment from the synovial membrane. J. Bone Jt. Surg. Am. 1996, 78, 721–733. [Google Scholar]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Jt. Surg. Am. 1993, 75, 532–553. [Google Scholar]
- Henderson, I.; Lavigne, P.; Valenzuela, H.; Oakes, B. Autologous chondrocyte implantation: Superior biologic properties of hyaline cartilage repairs. Clin. Orthop. Relat. Res. 2007, 455, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.J.; Goodrich, L.R.; Chen, C.T.; Hidaka, C.; Nixon, A.J. Biochemical and biomechanical properties of lesion and adjacent articular cartilage after chondral defect repair in an equine model. Am. J. Sports Med. 2005, 33, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Gaissmaier, C.; Koh, J.L.; Weise, K. Growth and differentiation factors for cartilage healing and repair. Injury 2008, 39, S88–S96. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Pulsatelli, L.; Facchini, A. Signaling pathways in cartilage repair. Int. J. Mol. Sci. 2014, 15, 8667–8698. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.; Tschon, M.; Palazzo, B.; Nitti, P.; Martini, L.; Rimondini, L. Agarose gel as biomaterial or scaffold for implantation surgery: Characterization, histological and histomorphometric study on soft tissue response. Connect. Tissue Res. 2012, 53, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yang, W.; Mao, X. Agarose/collagen composite scaffold as an anti-adhesive sheet. Biomed. Mater. 2007, 2, S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Karoubi, G.; Ormiston, M.L.; Stewart, D.J.; Courtman, D.W. Single-cell hydrogel encapsulation for enhanced survival of human marrow stromal cells. Biomaterials 2009, 30, 5445–5455. [Google Scholar] [CrossRef] [PubMed]
- Rahfoth, B.; Weisser, J.; Sternkopf, F.; Aigner, T.; von der Mark, K.; Brauer, R. Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthr. Cartil. 1998, 6, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Wan, L.Q.; Jiang, J.; Arnold, D.E.; Guo, X.E.; Lu, H.H.; Mow, V.C. Calcium concentration effects on the mechanical and biochemical properties of chondrocyte-alginate constructs. Cell. Mol. Bioeng. 2008, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Diduch, D.R.; Jordan, L.C.; Mierisch, C.M.; Balian, G. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy 2000, 16, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2012, 49, 780–792. [Google Scholar] [CrossRef]
- Dumitriu, S. Polymeric Biomaterials, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; Volume p xiv, p. 1168. [Google Scholar]
- Kubota, N.; Tatsumoto, N.; Sano, T.; Toya, K. A simple preparation of half N-acetylated chitosan highly soluble in water and aqueous organic solvents. Carbohydr. Res. 2000, 324, 268–274. [Google Scholar] [CrossRef]
- Sashiwa, H.; Yajima, H.; Aiba, S. Synthesis of a chitosan-dendrimer hybrid and its biodegradation. Biomacromolecules 2003, 4, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Venkatrajah, B.; Malathy, V.V.; Elayarajah, B.; Rajendran, R.; Rammohan, R. Synthesis of carboxymethyl chitosan and coating on wound dressing gauze for wound healing. Pak. J. Biol. Sci. 2013, 16, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Domard, A.; Rinaudo, M.; Terrassin, C. New method for the quaternization of chitosan. Int. J. Biol. Macromol. 1986, 8, 105–107. [Google Scholar] [CrossRef]
- De Vasconcelos, C.L.; Bezerril, P.M.; dos Santos, D.E.; Dantas, T.N.; Pereira, M.R.; Fonseca, J.L. Effect of molecular weight and ionic strength on the formation of polyelectrolyte complexes based on poly(methacrylic acid) and chitosan. Biomacromolecules 2006, 7, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Wen, N.; Cao, J.K.; Wang, H.B.; Lu, S.H.; Liu, T.; Lin, Q.X.; Duan, C.M.; Wang, C.Y. The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthr. Cartil. 2010, 18, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan-pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Filova, E.; Jakubcova, B.; Danilova, I.; KuZelova Kostakova, E.; Jarosikova, T.; Chernyavskiy, O.; Hejda, J.; Handl, M.; Beznoska, J.; Necas, A.; et al. Polycaprolactone foam functionalized with chitosan microparticles—A suitable scaffold for cartilage regeneration. Physiol. Res. 2015, 1, 121–131. [Google Scholar]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Dumitriu, S.; Popa, V.I. Polymeric Biomaterials; CRC Press: Boca Raton, FL, USA, 2013; pp. 318–324. [Google Scholar]
- Toh, W.S.; Lee, E.H.; Guo, X.M.; Chan, J.K.; Yeow, C.H.; Choo, A.B.; Cao, T. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials 2010, 31, 6968–6980. [Google Scholar] [CrossRef] [PubMed]
- Shiedlin, A.; Bigelow, R.; Christopher, W.; Arbabi, S.; Yang, L.; Maier, R.V.; Wainwright, N.; Childs, A.; Miller, R.J. Evaluation of hyaluronan from different sources: Streptococcus zooepidemicus, rooster comb, bovine vitreous, and human umbilical cord. Biomacromolecules 2004, 5, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Soltes, L.; Mendichi, R.; Lath, D.; Mach, M.; Bakos, D. Molecular characteristics of some commercial high-molecular-weight hyaluronans. Biomed. Chromatogr. 2002, 16, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Nettles, D.L.; Vail, T.P.; Morgan, M.T.; Grinstaff, M.W.; Setton, L.A. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann. Biomed. Eng. 2004, 32, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Doherty, P.; Radice, M.; Brun, P.; Abatangelo, G.; Williams, D.F. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials 1998, 19, 2101–2127. [Google Scholar] [CrossRef]
- Barbucci, R.; Magnani, A.; Rappuoli, R.; Lamponi, S.; Consumi, M. Immobilisation of sulphated hyaluronan for improved biocompatibility. J. Inorg. Biochem. 2000, 79, 119–125. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Han, Y. Chondroitin sulfate-based biomaterials for tissue engineering. Turk. J. Biol. 2016, 40, 290–299. [Google Scholar] [CrossRef]
- Bryant, S.J.; Arthur, J.A.; Anseth, K.S. Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomater. 2005, 1, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Sechriest, V.F.; Miao, Y.J.; Niyibizi, C.; Westerhausen-Larson, A.; Matthew, H.W.; Evans, C.H.; Fu, F.H.; Suh, J.K. Gag-augmented polysaccharide hydrogel: A novel biocompatible and biodegradable material to support chondrogenesis. J. Biomed. Mater. Res. 2000, 49, 534–541. [Google Scholar] [CrossRef]
- Van Susante, J.L.C.; Pieper, J.; Buma, P.; van Kuppevelt, T.H.; van Beuningen, H.; van der Kraan, P.M.; Veerkamp, J.H.; van den Berg, W.B.; Veth, R.P.H. Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials 2001, 22, 2359–2369. [Google Scholar] [CrossRef]
- Lodish, H.F. Molecular Cell Biology, 7th ed.; W.H. Freeman and Co.: New York, NY, USA, 2013; p. 1158. [Google Scholar]
- Nehrer, S.; Breinan, H.A.; Ramappa, A.; Shortkroff, S.; Young, G.; Minas, T.; Sledge, C.B.; Yannas, I.V.; Spector, M. Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J. Biomed. Mater. Res. 1997, 38, 95–104. [Google Scholar] [CrossRef]
- Freyria, A.M.; Ronziere, M.C.; Cortial, D.; Galois, L.; Hartmann, D.; Herbage, D.; Mallein-Gerin, F. Comparative phenotypic analysis of articular chondrocytes cultured within type I or type II collagen scaffolds. Tissue Eng. A 2009, 15, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Menage, J.; Sandell, L.J.; Evans, E.H.; Richardson, J.B. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee 2009, 16, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Rajan, N.; Habermehl, J.; Cote, M.F.; Doillon, C.J.; Mantovani, D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat. Protoc. 2006, 1, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, S.; Ohyabu, Y.; Hatayama, H. Temperature-responsive gelation of type I collagen solutions involving fibril formation and genipin crosslinking as a potential injectable hydrogel. Int. J. Biomater. 2013, 2013, 620765. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Griffith, M.; Hincke, M. Characterization and inhibition of fibrin hydrogel-degrading enzymes during development of tissue engineering scaffolds. Tissue Eng. 2007, 13, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef] [PubMed]
- Brandstedt, S.; Rank, F.; Olson, P.S. Wound healing and formation of granulation tissue in normal and defibrinogenated rabbits. An experimental model and histological study. Eur. Surg. Res. 1980, 12, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Marx, G. Evolution of fibrin glue applicators. Transfus. Med. Rev. 2003, 17, 287–298. [Google Scholar] [CrossRef]
- Brittberg, M.; Sjogren-Jansson, E.; Lindahl, A.; Peterson, L. Influence of fibrin sealant (tisseel) on osteochondral defect repair in the rabbit knee. Biomaterials 1997, 18, 235–242. [Google Scholar] [CrossRef]
- Frisman, I.; Orbach, R.; Seliktar, D.; Bianco-Peled, H. Structural investigation of PEG-fibrinogen conjugates. J. Mater. Sci. Mater. Med. 2010, 21, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Trattnig, S.; Ohel, K.; Mlynarik, V.; Juras, V.; Zbyn, S.; Korner, A. Morphological and compositional monitoring of a new cell-free cartilage repair hydrogel technology—Gelrinc by MR using semi-quantitative mocart scoring and quantitative T2 index and new zonal T2 index calculation. Osteoarthr. Cartil. 2015, 23, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Fussenegger, M.; Meinhart, J.; Hobling, W.; Kullich, W.; Funk, S.; Bernatzky, G. Stabilized autologous fibrin-chondrocyte constructs for cartilage repair in vivo. Ann. Plast. Surg. 2003, 51, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Scaffolds for tissue fabrication. Materialstoday 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Jeetah, R.; Bhaw-Luximon, A.; Jhurry, D. Polydioxanone-based bio-materials for tissue engineering and drug/gene delivery applications. Eur. J. Pharm. Biopharm. 2015, 97, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.K.; Oh, S.H.; Lee, J.H.; Im, G.I. Repair of osteochondral defects with a construct of mesenchymal stem cells and a polydioxanone/poly(vinyl alcohol) scaffold. Biotechnol. Appl. Biochem. 2008, 49, 155–164. [Google Scholar] [CrossRef] [PubMed]
- DeLee, J.; Drez, D.; Miller, M.D. Delee & Drez’s Orthopaedic Sports Medicine Principles and Practice, 3rd ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- BioTissue. Bioseed®-c, the Chondrocytes Graft for Joint Cartilage Repair. Available online: http://www.biotissue.de/bioseed/health-professionals/bioseed-c/ (accessed on 11 February 2016).
- Ossendorf, C.; Kaps, C.; Kreuz, P.C.; Burmester, G.R.; Sittinger, M.; Erggelet, C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res. Ther. 2007, 9, R41. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, P.C.; Muller, S.; Ossendorf, C.; Kaps, C.; Erggelet, C. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: Four-year clinical results. Arthritis Res. Ther. 2009, 11, R33. [Google Scholar] [CrossRef] [PubMed]
- Erggelet, C.; Kreuz, P.C.; Mrosek, E.H.; Schagemann, J.C.; Lahm, A.; Ducommun, P.P.; Ossendorf, C. Autologous chondrocyte implantation versus aci using 3D-bioresorbable graft for the treatment of large full-thickness cartilage lesions of the knee. Arch. Orthop. Trauma Surg. 2010, 130, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Marlovits, S.; Singer, P.; Zeller, P.; Mandl, I.; Haller, J.; Trattnig, S. Magnetic resonance observation of cartilage repair tissue (mocart) for the evaluation of autologous chondrocyte transplantation: Determination of interobserver variability and correlation to clinical outcome after 2 years. Eur. J. Radiol. 2006, 57, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Varghese, S.; Li, H.; Elisseeff, J. Regulation of osteogenic and chondrogenic differentiation of mesenchymal stem cells in PEG-ECM hydrogels. Cell Tissue Res. 2011, 344, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Elisseeff, J.; Anseth, K.; Sims, D.; McIntosh, W.; Randolph, M.; Langer, R. Transdermal photopolymerization for minimally invasive implantation. Proc. Natl. Acad. Sci. USA 1999, 96, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Kudva, A.K.; Saxena, N.S.; Roy, K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 2011, 32, 6946–6952. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, M.; Liu, K.; Wan, Y.; Li, X.; Feng, G. Novel chitosan hydrogel formed by ethylene glycol chitosan, 1,6-diisocyanatohexan and polyethylene glycol-400 for tissue engineering scaffold: In vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2014, 25, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Kirilak, Y.; Pavlos, N.J.; Willers, C.R.; Han, R.; Feng, H.; Xu, J.; Asokananthan, N.; Stewart, G.A.; Henry, P.; Wood, D.; et al. Fibrin sealant promotes migration and proliferation of human articular chondrocytes: Possible involvement of thrombin and protease-activated receptors. Int. J. Mol. Med. 2006, 17, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.M.; Fisher, J.P. Chondrocyte signaling and artificial matrices for articular cartilage engineering. Adv. Exp. Med. Biol. 2006, 585, 67–86. [Google Scholar] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Yan, L.P.; Silva-Correia, J.; Oliveira, M.B.; Vilela, C.; Pereira, H.; Sousa, R.A.; Mano, J.F.; Oliveira, A.L.; Oliveira, J.M.; Reis, R.L. Bilayered silk/silk-nanocap scaffolds for osteochondral tissue engineering: In vitro and in vivo assessment of biological performance. Acta Biomater. 2015, 12, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Yodmuang, S.; McNamara, S.L.; Nover, A.B.; Mandal, B.B.; Agarwal, M.; Kelly, T.A.N.; Chao, P.H.G.; Hung, C.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015, 11, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Ramakrishna, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Liu, X.; Smith, L.A.; Hu, J.; Ma, P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009, 30, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Grad, S.; Gorna, K.; Gogolewski, S.; Goessl, A.; Alini, M. Fibrin-polyurethane composites for articular cartilage tissue engineering: A preliminary analysis. Tissue Eng. 2005, 11, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Eyrich, D.; Wiese, H.; Maier, G.; Skodacek, D.; Appel, B.; Sarhan, H.; Tessmar, J.; Staudenmaier, R.; Wenzel, M.M.; Goepferich, A.; et al. In vitro and in vivo cartilage engineering using a combination of chondrocyte-seeded long-term stable fibrin gels and polycaprolactone-based polyurethane scaffolds. Tissue Eng. 2007, 13, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.W.; Scheer, M.J.; Simon, T.M. Cartilage substitutes: Overview of basic science and treatment options. J. Am. Acad. Orthop. Surg. 2001, 9, 37–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackson, D.W.; Aberman, H.M.; Kunishima, D.H.; Simon, T.M. Surface restoration of large medial femoral condyle articular cartilage lesions using a laminated polymer plug—An experimental study in goats. Orthop. Res. Inst. Lab. 2001, 1, 53–54. [Google Scholar]
- Hannink, G.; de Mulder, E.L.; van Tienen, T.G.; Buma, P. Effect of load on the repair of osteochondral defects using a porous polymer scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2082–2089. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.; Hutmacher, D.W.; Schantz, J.T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. A 2009, 90, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.H.; Jensen, B.E.; Smith, A.A.; Zelikin, A.N. Poly(vinyl alcohol) physical hydrogels: New vista on a long serving biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Stammen, J.A.; Williams, S.; Ku, D.N.; Guldberg, R.E. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001, 22, 799–806. [Google Scholar] [CrossRef]
- Nakashima, S.; Sawae, Y.; Murakami, T. Study on mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Mech. Prop. Novel PVA Hydrog. Shear Unconfin. Compress. 2005, 48, 555–561. [Google Scholar]
- Stile, R.A.; Burghardt, W.R.; Healy, K.E. Synthesis and characterization of injectable poly(N-isopropylacrylamide)-based hydrogels that support tissue formation in vitro. Macromolecules 1999, 32, 7370–7379. [Google Scholar] [CrossRef]
- Chen, J.P.; Cheng, T.H. Thermo-responsive chitosan-graft-poly(N-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromol. Biosci. 2006, 6, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. From nano- to macro-scale: Nanotechnology approaches for spatially controlled delivery of bioactive factors for bone and cartilage engineering. Nanomedicine 2012, 7, 1045–1066. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. B Rev. 2011, 17, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Darling, E.M.; Athanasiou, K.A. Biomechanical strategies for articular cartilage regeneration. Ann. Biomed. Eng. 2003, 31, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Haaparanta, A.M.; Jarvinen, E.; Cengiz, I.F.; Ella, V.; Kokkonen, H.T.; Kiviranta, I.; Kellomaki, M. Preparation and characterization of collagen/PLA, chitosan/PLA, and collagen/chitosan/PLA hybrid scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, D.; de Vos, P.; Suter, N.; Jayasinghe, S.N. Bio-electrospraying and cell electrospinning: Progress and opportunities for basic biology and clinical sciences. Adv. Healthc. Mater. 2012, 1, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N. Cell electrospinning: A novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst 2013, 138, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, G.; Steffens, D.; Braghirolli, D.I.; Fernandes, R.A.; Netto, C.A.; Pranke, P. Viability of mesenchymal stem cells during electrospinning. Braz. J. Med. Biol. Res. 2012, 45, 125–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Townsend-Nicholson, A.; Jayasinghe, S.N. Cell electrospinning: A unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules 2006, 7, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Lee, W.C.; Goh, J.C.; Toh, S.L. Bio-electrospraying: A potentially safe technique for delivering progenitor cells. Biotechnol. Bioeng. 2010, 106, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, R.; Ramakrishna, S. Electrosprayed nanoparticles for drug delivery and pharmaceutical applications. Biomatter 2013, 3, e24281. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Nanoparticle-based bioactive agent release systems for bone and cartilage tissue engineering. Regener. Ther. 2015, 1, 109–118. [Google Scholar] [CrossRef]
- Daher, R.J.; Chahine, N.O.; Greenberg, A.S.; Sgaglione, N.A.; Grande, D.A. New methods to diagnose and treat cartilage degeneration. Nat. Rev. Rheumatol. 2009, 5, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.M.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Encapsulation of adipose-derived stem cells and transforming growth factor-β1 in carrageenan-based hydrogels for cartilage tissue engineering. J. Bioact. Compat. Polym. 2011, 26, 493–507. [Google Scholar] [CrossRef]
- Park, J.S.; Shim, M.S.; Shim, S.H.; Yang, H.N.; Jeon, S.Y.; Woo, D.G.; Lee, D.R.; Yoon, T.K.; Park, K.H. Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-beta3. Biomaterials 2011, 32, 8139–8149. [Google Scholar] [CrossRef] [PubMed]
- Mullen, L.M.; Best, S.M.; Brooks, R.A.; Ghose, S.; Gwynne, J.H.; Wardale, J.; Rushton, N.; Cameron, R.E. Binding and release characteristics of insulin-like growth factor-1 from a collagen-glycosaminoglycan scaffold. Tissue Eng. C Methods 2010, 16, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; La, W.G.; Bhang, S.H.; Kim, H.J.; Im, G.I.; Lee, H.; Park, J.H.; Kim, B.S. Hyaline cartilage regeneration by combined therapy of microfracture and long-term bone morphogenetic protein-2 delivery. Tissue Eng. A 2011, 17, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Luvizuto, E.R.; Tangl, S.; Zanoni, G.; Okamoto, T.; Sonoda, C.K.; Gruber, R.; Okamoto, R. The effect of BMP-2 on the osteoconductive properties of beta-tricalcium phosphate in rat calvaria defects. Biomaterials 2011, 32, 3855–3861. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomedicine 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Zhai, D.Y.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. Enhanced msc chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32, 6425–6434. [Google Scholar] [CrossRef] [PubMed]
- Sukarto, A.; Amsden, B.G. Low melting point amphiphilic microspheres for delivery of bone morphogenetic protein-6 and transforming growth factor-β3 in a hydrogel matrix. J. Control. Release 2012, 158, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Liu, Y.; Holloway, J.L.; Maher, S.A.; Cao, Y.; Liu, W.; Zhou, G.; Lowman, A.M. A novel method for the direct fabrication of growth factor-loaded microspheres within porous nondegradable hydrogels: Controlled release for cartilage tissue engineering. J. Control. Release 2012, 157, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xu, X.; Ye, Y.; Song, K.; Cheng, Y.; Di, J.; Hu, Q.; Li, J.; Ju, H.; Jiang, Q.; et al. Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano 2016, 10, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martinez Avila, H.; Hagg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Tseng, C.S.; Dai, L.G.; Hsu, S.H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 2016, 83, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.; Fosang, A.; Donati, D.M.; Wallace, G.G.; Choong, P.F. 3D bioprinting of cartilage for orthopedic surgeons: Reading between the lines. Front. Surg. 2015, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Gulino, A.; Agnello, S.; Giammona, G. Injectable in situ forming hydrogels based on natural and synthetic polymers for potential application in cartilage repair. R. Soc. Chem. 2015, 5, 19715–19723. [Google Scholar] [CrossRef]
- Dubruel, P.; Vlierberghe, S.V. (Eds.) Biomaterials for bone regeneration novel techniques and applications. In Woodhead Publishing Series in Biomaterials Number 75; Woodhead Publ.: Cambridge, UK; Waltham, MA, USA, 2014.
- Ligon, S.C.; Husar, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2014, 114, 557–589. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Yang, D.H.; Lee, J.B.; Heo, D.N.; Kwon, Y.D.; Youn, I.C.; Choi, K.; Hong, J.H.; Kim, G.T.; Choi, Y.S.; et al. Photo-cured hyaluronic acid-based hydrogels containing simvastatin as a bone tissue regeneration scaffold. Biomaterials 2011, 32, 8161–8171. [Google Scholar] [CrossRef] [PubMed]
- Elisseeff, J.; Anseth, K.; Sims, D.; McIntosh, W.; Randolph, M.; Yaremchuk, M.; Langer, R. Transdermal photopolymerization of poly(ethylene oxide)-based injectable hydrogels for tissue-engineered cartilage. Plast. Reconstr. Surg. 1999, 104, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S. Injectable Hydrogels for Regenerative Engineering; Imperial College Press: London, UK, 2015. [Google Scholar]
- Johnson, K.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A stem cell-based approach to cartilage repair. Science 2012, 336, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Siclari, A.; Mascaro, G.; Gentili, C.; Cancedda, R.; Boux, E. A cell-free scaffold-based cartilage repair provides improved function hyaline-like repair at one year. Clin. Orthop. Relat. Res. 2012, 470, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Siclari, A.; Mascaro, G.; Kaps, C.; Boux, E. A 5-year follow-up after cartilage repair in the knee using a platelet-rich plasma-immersed polymer-based implant. Open Orthop. J. 2014, 8, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Siclari, A.; Mascaro, G.; Gentili, C.; Kaps, C.; Cancedda, R.; Boux, E. Cartilage repair in the knee with subchondral drilling augmented with a platelet-rich plasma-immersed polymer-based implant. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Enea, D.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Manzotti, S.; Kaps, C.; Gigante, A. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee 2013, 20, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Becher, C.; Ettinger, M.; Ezechieli, M.; Kaps, C.; Ewig, M.; Smith, T. Repair of retropatellar cartilage defects in the knee with microfracture and a cell-free polymer-based implant. Arch. Orthop. Trauma Surg. 2015, 135, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.C.; Heveran, C.M.; Cannon, W.D., Jr.; Foo, L.F.; Potter, H.G. An autologous cartilage tissue implant neocart for treatment of grade iii chondral injury to the distal femur: Prospective clinical safety trial at 2 years. Am. J. Sports Med. 2009, 37, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.C.; DeBerardino, T.M.; Williams, R.J., 3rd. Neocart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: An FDA phase-II prospective, randomized clinical trial after two years. J. Bone Jt. Surg. Am. 2012, 94, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, A.; van Niekerk, L.; Triantafillopoulos, I. Autologous chondrocyte implantation for knee cartilage injuries: Moderate functional outcome and performance in patients with high-impact activities. Orthopedics 2012, 35, e6–e14. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, T.R.; Pietschmann, M.F.; Horng, A.; Rossbach, B.P.; Ficklscherer, A.; Jansson, V.; Muller, P.E. Graft hypertrophy of matrix-based autologous chondrocyte implantation: A two-year follow-up study of novocart 3d implantation in the knee. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Zak, L.; Albrecht, C.; Wondrasch, B.; Widhalm, H.; Vekszler, G.; Trattnig, S.; Marlovits, S.; Aldrian, S. Results 2 years after matrix-associated autologous chondrocyte transplantation using the novocart 3D scaffold: An analysis of clinical and radiological data. Am. J. Sports Med. 2014, 42, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Broese, M.; Simon, A.; Liodakis, E.; Ettinger, M.; Guenther, D.; Zeichen, J.; Krettek, C.; Jagodzinski, M.; Haasper, C. Cares (MACT) versus microfracture in treating symptomatic patellofemoral cartilage defects: A retrospective matched-pair analysis. J. Orthop. Sci. 2013, 18, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Rackwitz, L.; Andereya, S.; Siebenlist, S.; Fensky, F.; Reichert, J.; Loer, I.; Barthel, T.; Rudert, M.; Noth, U. A prospective multicenter study on the outcome of type i collagen hydrogel-based autologous chondrocyte implantation (cares) for the repair of articular cartilage defects in the knee. Am. J. Sports Med. 2011, 39, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Schuttler, K.F.; Schenker, H.; Theisen, C.; Schofer, M.D.; Getgood, A.; Roessler, P.P.; Struewer, J.; Rominger, M.B.; Efe, T. Use of cell-free collagen type i matrix implants for the treatment of small cartilage defects in the knee: Clinical and magnetic resonance imaging evaluation. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Behrens, P. Matrixgekoppelte mikrofrakturierung. Ein neues konzept zur knorpeldefektbehandlung. Arthroskopie 2005, 18, 193–197. [Google Scholar] [CrossRef]
- Gille, J.; Behrens, P.; Volpi, P.; de Girolamo, L.; Reiss, E.; Zoch, W.; Anders, S. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: Data of the amic registry. Arch. Orthop. Trauma Surg. 2013, 133, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Jakob, R.P.; Gautier, E.; Magnussen, R.A.; Hoogewoud, H.; Jacobi, M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Delcogliano, M.; de Caro, F.; Scaravella, E.; Ziveri, G.; De Biase, C.F.; Marotta, D.; Marenghi, P.; Delcogliano, A. Use of innovative biomimetic scaffold in the treatment for large osteochondral lesions of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Venieri, G.; Perdisa, F.; Marcacci, M. Tibial plateau lesions. Surface reconstruction with a biomimetic osteochondral scaffold: Results at 2 years of follow-up. Injury 2014, 45, S121–S125. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Di Martino, A.; Iacono, F.; Marcacci, M. Arthroscopic second-generation autologous chondrocyte implantation: A prospective 7-year follow-up study. Am. J. Sports Med. 2011, 39, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Gobbi, A.; Filardo, G.; Delcogliano, M.; Zaffagnini, S.; Marcacci, M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: Prospective nonrandomized study at 5 years. Am. J. Sports Med. 2009, 37, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Berruto, M.; Benazzo, F.; Zanon, G.; Della Villa, S.; Marcacci, M. Articular cartilage treatment in high-level male soccer players: A prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am. J. Sports Med. 2011, 39, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Condello, V.; Collarile, M.; Di Martino, A.; Zorzi, C.; Marcacci, M. Second-generation autologous chondrocyte implantation: Results in patients older than 40 years. Am. J. Sports Med. 2011, 39, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Selmi, T.A.; Verdonk, P.; Chambat, P.; Dubrana, F.; Potel, J.F.; Barnouin, L.; Neyret, P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: Outcome at two years. J. Bone Jt. Surg. Br. 2008, 90, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Clave, A.; Potel, J.F.; Servien, E.; Neyret, P.; Dubrana, F.; Stindel, E. Third-generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2-year randomized trial. J. Orthop. Res. 2016, 34, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.Y.; Kim, B.W.; Yeo, W.J.; Kim, H.B.; Suh, D.S.; Kim, J.S.; Kim, Y.S.; Seo, Y.H.; Cho, J.Y.; Chun, C.W.; et al. Gel-type autologous chondrocyte (chondron) implantation for treatment of articular cartilage defects of the knee. BMC Musculoskelet. Disord. 2010, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.V.V.; Worland, R.L.; Keenan, J.; Norambuena, N. Patient demographics as a predictor of the ten-year survival rate in primary total knee replacement. J. Bone Jt. Surg. Br. 2003, 85, 52–56. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, S.W.; Kim, S.R.; Oh, I.S.; Won, M.H. Autologous chondrocyte implantation in the knee using fibrin. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Stanish, W.D.; McCormack, R.; Forriol, F.; Mohtadi, N.; Pelet, S.; Desnoyers, J.; Restrepo, A.; Shive, M.S. Novel scaffold-based bst-cargel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J. Bone Jt. Surg. Am. 2013, 95, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Goldshmid, R.; Cohen, S.; Shachaf, Y.; Kupershmit, I.; Sarig-Nadir, O.; Seliktar, D.; Wechsler, R. Steric interference of adhesion supports in vitro chondrogenesis of mesenchymal stem cells on hydrogels for cartilage repair. Sci. Rep. 2015, 5, 12607. [Google Scholar] [CrossRef] [PubMed]
- Dhollander, A.A.; Almqvist, K.F.; Moens, K.; Vandekerckhove, P.J.; Verdonk, R.; Verdonk, P.; Victor, J. The use of a prosthetic inlay resurfacing as a salvage procedure for a failed cartilage repair. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2208–2212. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, A.B.; Feucht, M.J.; Meidinger, G.; Schottle, P.B.; Cotic, M. Prospective evaluation of anatomic patellofemoral inlay resurfacing: Clinical, radiographic, and sports-related results after 24 months. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Bollars, P.; Bosquet, M.; Vandekerckhove, B.; Hardeman, F.; Bellemans, J. Prosthetic inlay resurfacing for the treatment of focal, full thickness cartilage defects of the femoral condyle: A bridge between biologics and conventional arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Carranza, N.; Berg, H.E.; Lagerstedt, A.S.; Nurmi-Sandh, H.; Schupbach, P.; Ryd, L. Fixation of a double-coated titanium-hydroxyapatite focal knee resurfacing implant: A 12-month study in sheep. Osteoarthr. Cartil. 2014, 22, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Carranza, N.; Ryd, L.; Hultenby, K.; Hedlund, H.; Nurmi-Sandh, H.; Lagerstedt, A.S.; Schupbach, P.; Berg, H.E. Treatment of full thickness focal cartilage lesions with a metallic resurfacing implant in a sheep animal model, 1 year evaluation. Osteoarthr. Cartil. 2016, 24, 484–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Investigation of a Customized Femoral Resurfacing Implant (Episealer® Knee Condyle Device) to Assess the Safety Profile and Performance for 2 Years Post-Operatively. Available online: https://clinicaltrials.gov/ct2/show/NCT01690689 (accessed on 21 March 2016).
- Cartilage autograft Implantation System (Cais) for the Repair of Knee Cartilage through cartilage regeneration (cais). Available online: https://clinicaltrials.gov/ct2/show/NCT00881023 (accessed on 21 March 2016).
- Knee Articular Cartilage Repair: Cartilage Autograft Implantation System versus Conventional Microfracture (Cais). Available online: https://clinicaltrials.gov/ct2/show/NCT01498029 (accessed on 21 March 2016).
- Prospective Feasibility, Non-Randomized, Single Arm Multicentre, Multinational Interventional Clinical Investigation Using Instruct Therapy for the Repair of Knee Cartilage Defects. Available online: http://ichgcp.net/clinical-trials-registry/NCT01041885 (accessed on 21 March 2016).
- Gille, J. Evaluation of an acellular osteochondral graft for cartilage lesions (“eagle”) european post market study. Available online: ClinicalTrials.gov (accessed on 21 March 2016).
- Custers, R.J.; Dhert, W.J.; Saris, D.B.; Verbout, A.J.; van Rijen, M.H.; Mastbergen, S.C.; Lafeber, F.P.; Creemers, L.B. Cartilage degeneration in the goat knee caused by treating localized cartilage defects with metal implants. Osteoarthr. Cartil. 2010, 18, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Bennell, K.; Cicuttini, F.M. Effect of physical activity on cartilage development in healthy kids. Br. J. Sports Med. 2003, 37, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M.; Koyama, E.; Iwamoto, M. Mechanisms of synovial joint and articular cartilage formation: Recent advances, but many lingering mysteries. Birth Defects Res. C Embryo Today 2005, 75, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Steinmeyer, J.; Ackermann, B.; Raiss, R.X. Intermittent cyclic loading of cartilage explants modulates fibronectin metabolism. Osteoarthr. Cartil. 1997, 5, 331–341. [Google Scholar] [CrossRef]
- Fehrenbacher, A.; Steck, E.; Rickert, M.; Roth, W.; Richter, W. Rapid regulation of collagen but not metalloproteinase 1, 3, 13, 14 and tissue inhibitor of metalloproteinase 1, 2, 3 expression in response to mechanical loading of cartilage explants in vitro. Arch. Biochem. Biophys. 2003, 410, 39–47. [Google Scholar] [CrossRef]
- Fitzgerald, J.B.; Jin, M.; Dean, D.; Wood, D.J.; Zheng, M.H.; Grodzinsky, A.J. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic amp. J. Biol. Chem. 2004, 279, 19502–19511. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, P.; Siegrist, M.; Hunziker, E.B.; Wong, M. The mechanosensitivity of cartilage oligomeric matrix protein (comp). Biorheology 2003, 40, 101–109. [Google Scholar] [PubMed]
- Wong, M.; Siegrist, M.; Cao, X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999, 18, 391–399. [Google Scholar] [CrossRef]
- Parkkinen, J.J.; Ikonen, J.; Lammi, M.J.; Laakkonen, J.; Tammi, M.; Helminen, H.J. Effects of cyclic hydrostatic pressure on proteoglycan synthesis in cultured chondrocytes and articular cartilage explants. Arch. Biochem. Biophys. 1993, 300, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.L.; Kim, Y.J.; Doong, J.Y.; Grodzinsky, A.J.; Plaas, A.H.; Sandy, J.D. Biosynthetic response of cartilage explants to dynamic compression. J. Orthop. Res. 1989, 7, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E.; Wang, N.; Stamenovic, D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep. Prog. Phys. 2014, 77, 046603. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Tensegrity I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 116, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003, 116, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Kock, L.; van Donkelaar, C.C.; Ito, K. Tissue engineering of functional articular cartilage: The current status. Cell Tissue Res. 2012, 347, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Timur, U.T.; Edip, S.; Haak, E.; Wruck, C.; Weinans, H.; Jahr, H. TGF-β2 is involved in the preservation of the chondrocyte phenotype under hypoxic conditions. Ann. Anat. 2015, 198, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Timur, U.T.; Caron, M.; Welting, T.J.; Emans, P.J.; Jahr, H. TGF-β2 knockdown under osmolarity improves collagen expression in chondrocytes. Poster presentation EORS: Bristol, UK, 2015. [Google Scholar]
- Singh, S. Effects of Different pH and Oxygen Levels on Proliferation and Chondrogenic Differentiation of Human Mesenchymal Stem Cells Cultured in Hydrogels. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2014. [Google Scholar]
- Ghosh, K.; Ingber, D.E. Micromechanical control of cell and tissue development: Implications for tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Solorio, L.D.; Vieregge, E.L.; Dhami, C.D.; Dang, P.N.; Alsberg, E. Engineered cartilage via self-assembled hmsc sheets with incorporated biodegradable gelatin microspheres releasing transforming growth factor-beta1. J. Control. Release 2012, 158, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Bouffi, C.; Thomas, O.; Bony, C.; Giteau, A.; Venier-Julienne, M.C.; Jorgensen, C.; Montero-Menei, C.; Noel, D. The role of pharmacologically active microcarriers releasing TGF-β3 in cartilage formation in vivo by mesenchymal stem cells. Biomaterials 2010, 31, 6485–6493. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jha, A.K.; Duncan, R.L.; Jia, X. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011, 7, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Ertan, A.B.; Yilgor, P.; Bayyurt, B.; Calikoglu, A.C.; Kaspar, C.; Kok, F.N.; Kose, G.T.; Hasirci, V. Effect of double growth factor release on cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2013, 7, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.L.; Lipton, J.I.; Bonassar, L.J.; Lipson, H. Additive manufacturing for in situ repair of osteochondral defects. Biofabrication 2010, 2, 035004. [Google Scholar] [CrossRef] [PubMed]
- Plecko, M.; Sievert, C.; Andermatt, D.; Frigg, R.; Kronen, P.; Klein, K.; Stubinger, S.; Nuss, K.; Burki, A.; Ferguson, S.; et al. Osseointegration and biocompatibility of different metal implants-a comparative experimental investigation in sheep. BMC Musculoskelet. Disord. 2012, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Fahlgren, A.; Bostrom, M.P.; Yang, X.; Johansson, L.; Edlund, U.; Agholme, F.; Aspenberg, P. Fluid pressure and flow as a cause of bone resorption. Acta Orthop. 2010, 81, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Khaled, E.G.; Saleh, M.; Hindocha, S.; Griffin, M.; Khan, W.S. Tissue engineering for bone production-stem cells, gene therapy and scaffolds. Open Orthop. J. 2011, 5, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, S.; Nair, L.S.; El-Amin, S.; Nguyen, M.T.; Singh, A.; Greish, Y.E.; Allcock, H.R.; Brown, P.W.; Laurencin, C.T. Development and characterization of biodegradable nanocomposite injectables for orthopaedic applications based on polyphosphazenes. J. Biomater. Sci. Polym. Ed. 2011, 22, 733–752. [Google Scholar] [CrossRef] [PubMed]
- Biopolytm. Available online: http://www.biopolyortho.com/Biopoly.aspx (accessed on 28 March 2016).
- Dye, S.F.; Wojtys, E.M.; Fu, F.H.; Fithian, D.C.; Gillquist, I. Factors contributing to function of the knee joint after injury or reconstruction of the anterior cruciate ligament. Instr. Course Lect. 1999, 48, 185–198. [Google Scholar] [PubMed]
- Saris, D.B.; Dhert, W.J.; Verbout, A.J. Joint homeostasis. The discrepancy between old and fresh defects in cartilage repair. J. Bone Jt. Surg. Br. 2003, 85, 1067–1076. [Google Scholar] [CrossRef]
- Mithoefer, K.; Williams, R.J., 3rd; Warren, R.F.; Potter, H.G.; Spock, C.R.; Jones, E.C.; Wickiewicz, T.L.; Marx, R.G. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J. Bone Jt. Surg. Am. 2005, 87, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.M.; Emans, P.J.; Cremers, A.; Surtel, D.A.; Coolsen, M.M.; van Rhijn, L.W.; Welting, T.J. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by bMP-7. Osteoarthr. Cartil. 2013, 21, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Zweers, M.C.; de Boer, T.N.; van Roon, J.; Bijlsma, J.W.; Lafeber, F.P.; Mastbergen, S.C. Celecoxib: Considerations regarding its potential disease-modifying properties in osteoarthritis. Arthritis Res. Ther. 2011, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Mihov, G.; Welting, T.; Thies, J.; Emans, P. Drugs and polymers for delivery systems in OA joints: Clinical needs and opportunities. Polymers 2014, 6, 799–819. [Google Scholar] [CrossRef]
- Wylie, J.D.; Hartley, M.K.; Kapron, A.L.; Aoki, S.K.; Maak, T.G. What is the effect of matrices on cartilage repair? A systematic review. Clin. Orthop. Relat. Res. 2015, 473, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Falah, M.; Nierenberg, G.; Soudry, M.; Hayden, M.; Volpin, G. Treatment of articular cartilage lesions of the knee. Int. Orthop. 2010, 34, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Ossendorf, C.; Steinwachs, M.R.; Kreuz, P.C.; Osterhoff, G.; Lahm, A.; Ducommun, P.P.; Erggelet, C. Autologous chondrocyte implantation (ACI) for the treatment of large and complex cartilage lesions of the knee. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, K.; Peterson, L.; Zenobi-Wong, M.; Mandelbaum, B.R. Cartilage issues in football-today’s problems and tomorrow’s solutions. Br. J. Sports Med. 2015, 49, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.A.; Kim, S.-J.; Nakamura, N.; Brittberg, M. Techniques in Cartilage Repair Surgery; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Minas, T.; Gomoll, A.H.; Rosenberger, R.; Royce, R.O.; Bryant, T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am. J. Sports Med. 2009, 37, 902–908. [Google Scholar] [CrossRef] [PubMed]
| Polymer Type | Scaffold Type | Degradability | Degradation Time | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Natural | ||||||
| Agarose | Hydrogel (thermal) | Hydrolysis | Slow | Injectable Favorable solution-gel transition temperature | No direct cell adhesion Non-load-bearing | [63,64,65,66] |
| Alginate | Hydrogel (non-covalent cross-links) | Hydrolysis | Slow | Injectable | No direct cell adhesion Non-load-bearing Source dependent variation Difficulty controlling structural uniformity | [63,67,68,69] |
| Chitosan | Hydrogel (non-covalent cross-links) or solid scaffold | Enzymatic, hydrolysis | Slow, dependent on deacetylation degree | Chemically modifiable structure Allows cell interaction | Source dependent variation | [70,71,72,73,74,75,76,77,78,79,80,81] |
| Hyaluronic acid | Hydrogel | Enzymatic, hydrolysis | Fast | Natural component in synovial fluid/cartilage, High low friction | Source dependent variation Non-load-bearing | [82,83,84,85,86,87] |
| Chondroitin sulfate | Hydrogel | Enzymatic, hydrolysis | Fast | Natural component in synovial fluid/cartilage, low friction | Source dependent variation Non-load-bearing | [88,89,90,91,92] |
| Collagen | Hydrogel or solid scaffold | Enzymatic | Fast (weeks) | Natural cartilage component, Fully degradable Injectable (in situ gel formation) | Fast degradation, unstable mechanical properties due to degradation | [93,94,95,96,97,98] |
| Fibrin | Hydrogel (enzymatically cross-linked) | Enzymatic | Fast (weeks) | Injectable (in situ gel formation) | Sensitive to gel shrinkage Non-load-bearing Fast degradation | [99,100,101,102,103,104,105,106] |
| Synthetic | ||||||
| PLGA, PLA, PGA | Solid scaffold | Enzymatic, hydrolysis (bulk degradation) | Tunable (weeks to months) | Monomer ratio determines degradation rate Fully degradable Load-bearing | Inert, acidic degradation products | [107,108] |
| PDS | Solid scaffold | Enzymatic, hydrolysis | Months | Fully degradable Load-bearing | Inert, acidic degradation products | [109,110,111,112,113,114,115,116,117] |
| PEG | Cross-linked hydrogel | Non-degradable polymer; degradable cross-links possible | Non-degradable | Injectable (in situ gel formation) | Inert Non-load-bearing | [118,119,120,121] |
| Construct Type | Group | Product | Company | Composition | Procedure | Typical Clinical Findings | References |
|---|---|---|---|---|---|---|---|
| Degradables | PLGA-based | BioSeed®-C | BioTissue, AG | PGA-PLA scaffold reinforced with PDS and seeded with autologous chondrocytes and suspended in fibrin | Two-step procedure; MACI | No clinical superiority compared to ACI-p; radiologically better than ACI-p. | [115,116] |
| Chondrotissue® | BioTissue AG | Non-woven PGA textile treated with hyaluronic acid combined with either PRP or BMC. | One-step procedure; AMIC | Promising outcomes from case series with evidence of hyaline cartilaginous tissue; no comparative studies available. | [26,177,178,181] | ||
| Collagen-based | NeoCart® | Histogenics Corporation | Scaffold using bovine type I collagen seeded with autologous chondrocytes cultured in a bioreactor | Two-step procedure; MACI | Good clinical outcomes and superior to microfracture in comparative study. | [182,183] | |
| NovoCART® 3D | TETEC® Tissue Engineering Technologies AG | 3D collagen-chondroitin sulfate scaffold seeded with autologous chondrocytes | Two-step procedure; MACI | Performed better than ACI-p in high demanding patients, effect was not significant; high rate of graft hypertrophy in case series studies. | [184,185,186] | ||
| CaReS® | Arthro Kinetics | Hydrogel using type I collagen from rat tails seeded with autologous chondrocytes cultured in autologous blood | Two-step procedure; MACI | Superior results when compared to microfracture in matched-pair analysis after 3 years | [188,189] | ||
| Chondro-Gide® | Geistlich Pharma AG, Wolhusen, Switzerland | Collagen type I/III matrix sutured to debrided microfractured defect and supported by fibrin glue | One-step procedure; AMIC | No comparative studies available. | [190,191,192] | ||
| Maioregen® | Fin-Ceramica Faenza S.p.A., Italy | Threelayered nanostructured scaffold with a top layer consisting of type I collagen, a middle layer of 60% type I collagen and 40% hydroxyapatite and a bottom layer with 60% hydroxyapatie and 40% type I collagen. | One-step procedure; AMIC | No comparative studies available. | [193,194] | ||
| Other natural polymer-based constructs | Hyalograft® C | Anika Therapeutics, Inc. | Hyaluronan (HYAFF-11S), a benzylic ester of hyaluronic acid, scaffold seeded with autologous chondrocytes and fixated using fibrin glue | Two-step procedure; MACI | Performed better than microfracture after 2 years up to 7 years; faster improvements compared to Chondro-Gide® | [195,196,197,198] | |
| Cartipatch® | Tissue Bank of France | Hydrogel using an ultrapurified agarose-alginate suspension (GelForGel) seeded with autologous chondrocytes cultured in monolayer conditiones in autologous serum | Two-step procedure; MACI | Inferior results compared to mosaicplasty after 2 years in comparative study. | [199,200] | ||
| Chondron™ | Sewon Cellontech Co. Ltd | Hydrogel using autologous chondrocytes mixed with fibrin glue (ratio 1:1). | Two-step procedure; MACI | No comparative studies available. | [201,202,203] | ||
| BST-CarGel® | Piramal Healthcare Ltd | Chitosan mixed with autologous blood | One-step procedure; AMIC | Little evidence; clinically equal to microfracture but radiologically superior in comparative study | [204] | ||
| GelrinC™ | Regentis Biomaterials | PEG-fibrinogen hydrogel applied as liquid formulation and cured in-situ using long wave UV light | One-step procedure; AMIC | No comparative studies available. | [105,205] | ||
| Non-degrad-ables | Metals | HemiCAP® | Arthosurface INC. | Titanium cancellous screw with cobalt-chrome articular surface | One-step procedure; FKR | No comparative studies available; possible feasible treatment option for failed regenerative treatments. | [206,207,208] |
| Episealer® Condyle Solo | Episurf medical AB | Cobalt-chrome monobloc with titanium-hydroxyapatie coating | One-step procedure; FKR | No clinical evidence yet. | [209,210,211] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeuken, R.M.; Roth, A.K.; Peters, R.J.R.W.; Van Donkelaar, C.C.; Thies, J.C.; Van Rhijn, L.W.; Emans, P.J. Polymers in Cartilage Defect Repair of the Knee: Current Status and Future Prospects. Polymers 2016, 8, 219. https://doi.org/10.3390/polym8060219
Jeuken RM, Roth AK, Peters RJRW, Van Donkelaar CC, Thies JC, Van Rhijn LW, Emans PJ. Polymers in Cartilage Defect Repair of the Knee: Current Status and Future Prospects. Polymers. 2016; 8(6):219. https://doi.org/10.3390/polym8060219
Chicago/Turabian StyleJeuken, Ralph M., Alex K. Roth, Ruud J. R. W. Peters, Corrinus C. Van Donkelaar, Jens C. Thies, Lodewijk W. Van Rhijn, and Pieter J. Emans. 2016. "Polymers in Cartilage Defect Repair of the Knee: Current Status and Future Prospects" Polymers 8, no. 6: 219. https://doi.org/10.3390/polym8060219
APA StyleJeuken, R. M., Roth, A. K., Peters, R. J. R. W., Van Donkelaar, C. C., Thies, J. C., Van Rhijn, L. W., & Emans, P. J. (2016). Polymers in Cartilage Defect Repair of the Knee: Current Status and Future Prospects. Polymers, 8(6), 219. https://doi.org/10.3390/polym8060219