Poly(lactide-co-glycolide)/Hydroxyapatite Porous Scaffold with Microchannels for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PLGA/HA Homogeneous Hybrid Composite
2.2. PLGA/HA Scaffold Fabrication via PI Method
2.3. PLGA/HA Scaffold Fabrication via MM Method
2.4. Characterizations of Scaffolds
2.5. Cell Culture
2.5.1. Isolation of Rabbit Bone Marrow Mesenchymal Stem Cells (rBMSCs)
2.5.2. Cell Adhesion
2.5.3. Cell Proliferation
2.5.4. Cell Differentiation
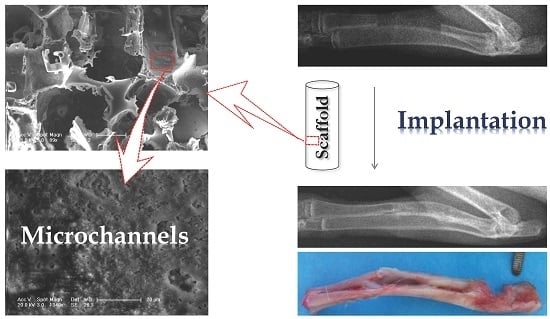
2.6. In Vivo Animal Study
2.6.1. Implantation for Radius Defect Repair
2.6.2. X-ray Examination
2.7. Statistical Analyses
3. Results and Discussion
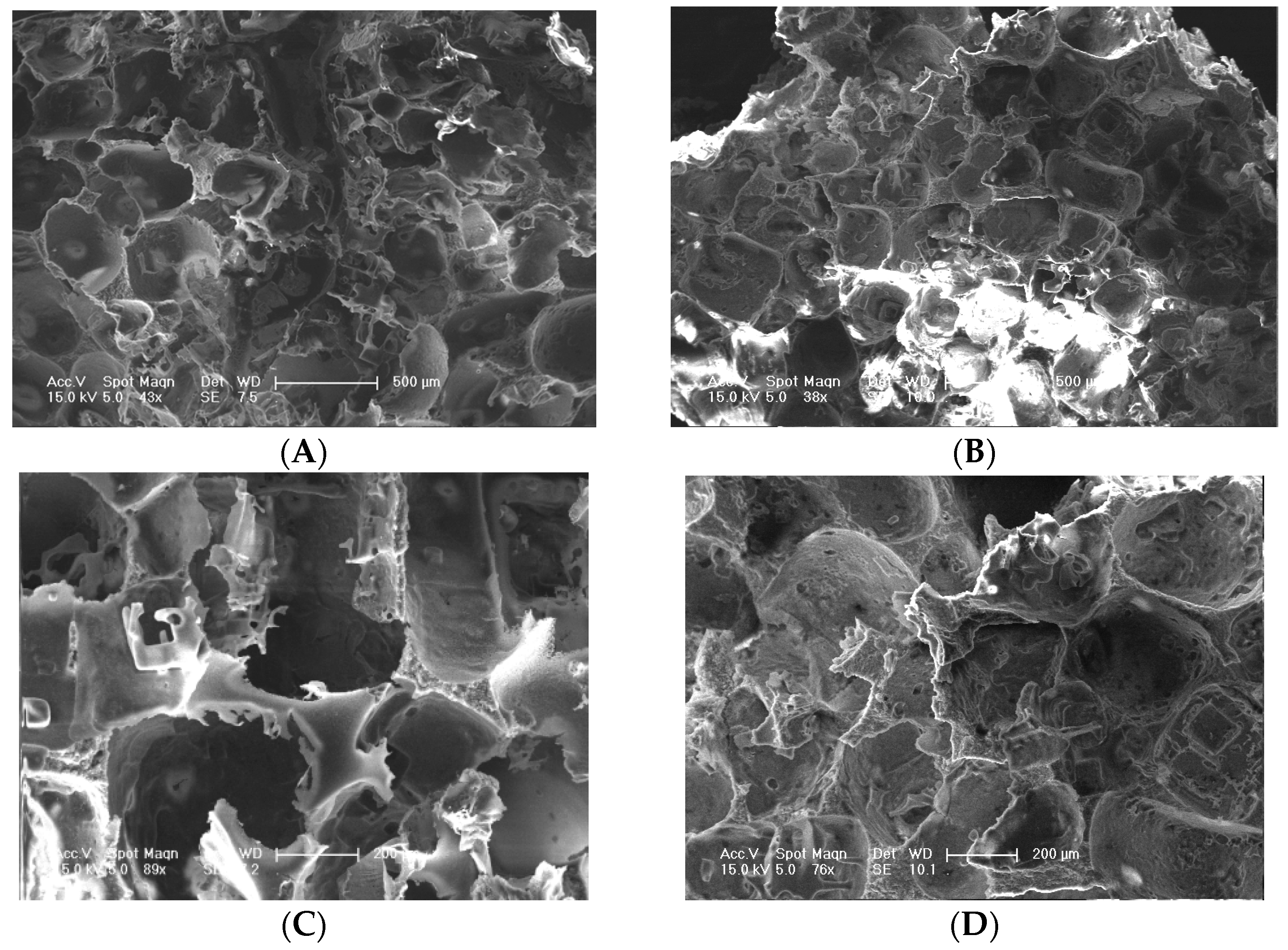
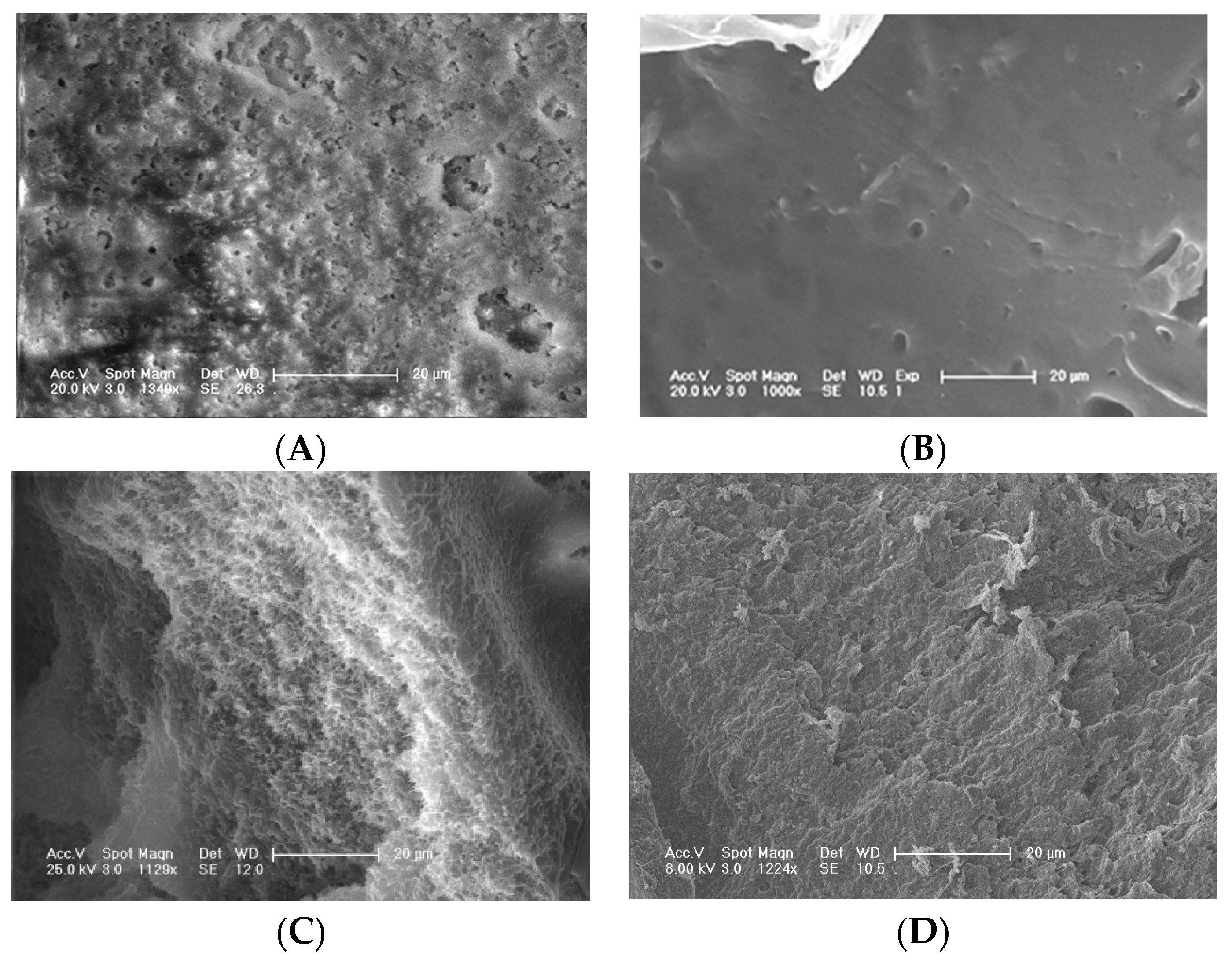
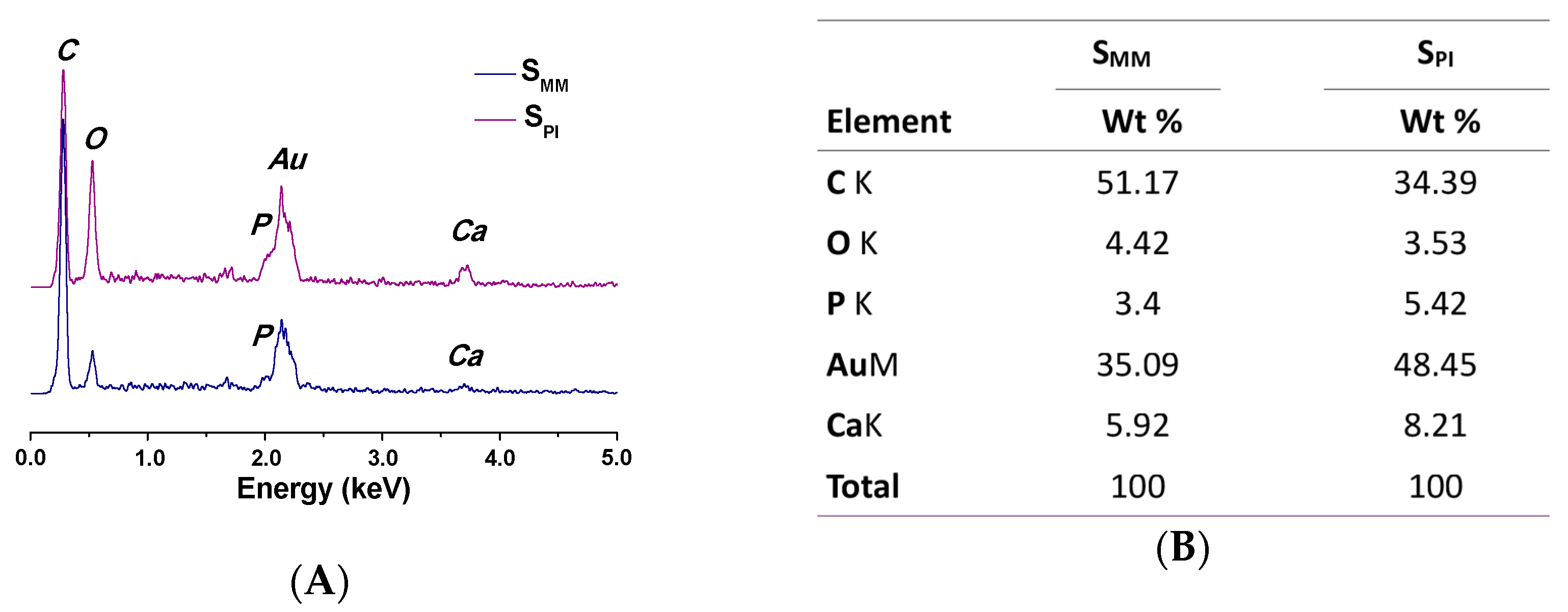
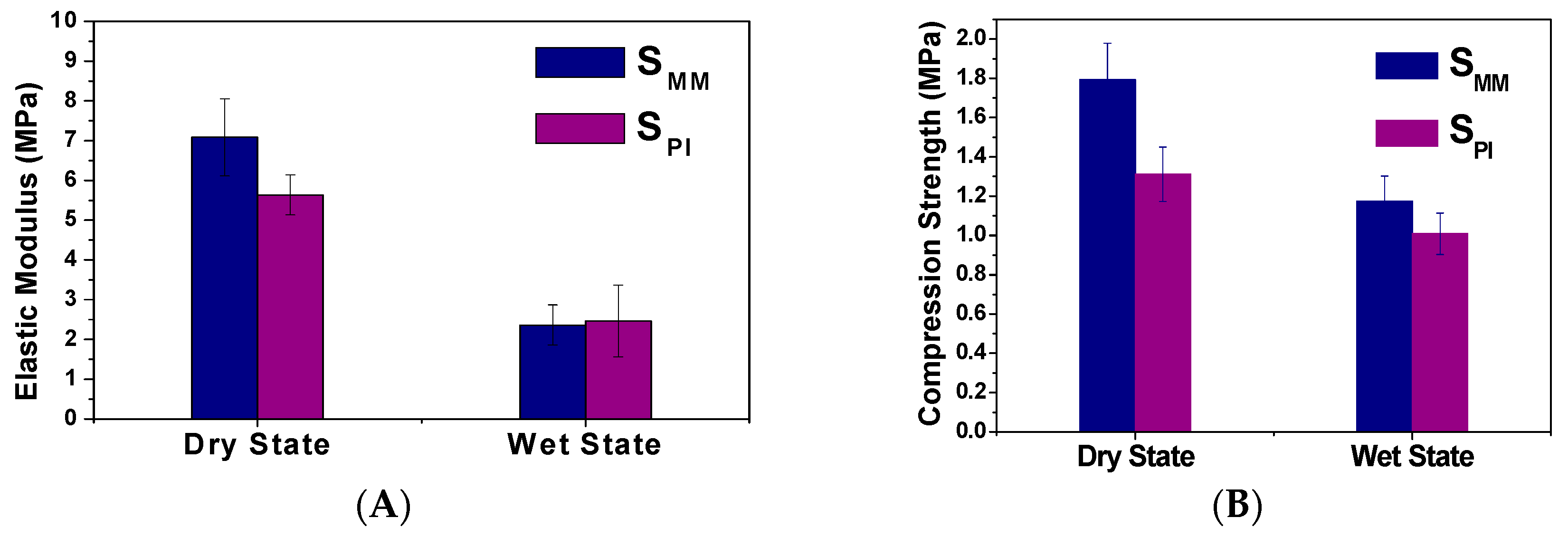
3.1. Scaffold Characterizations
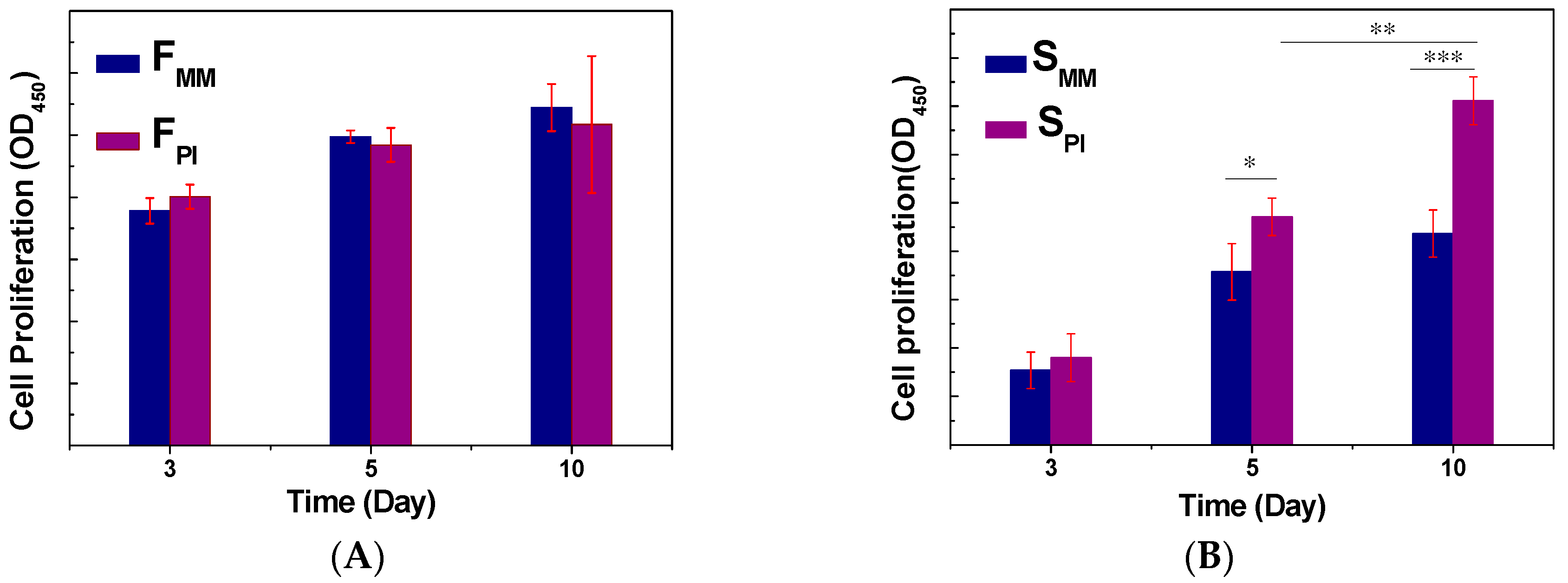
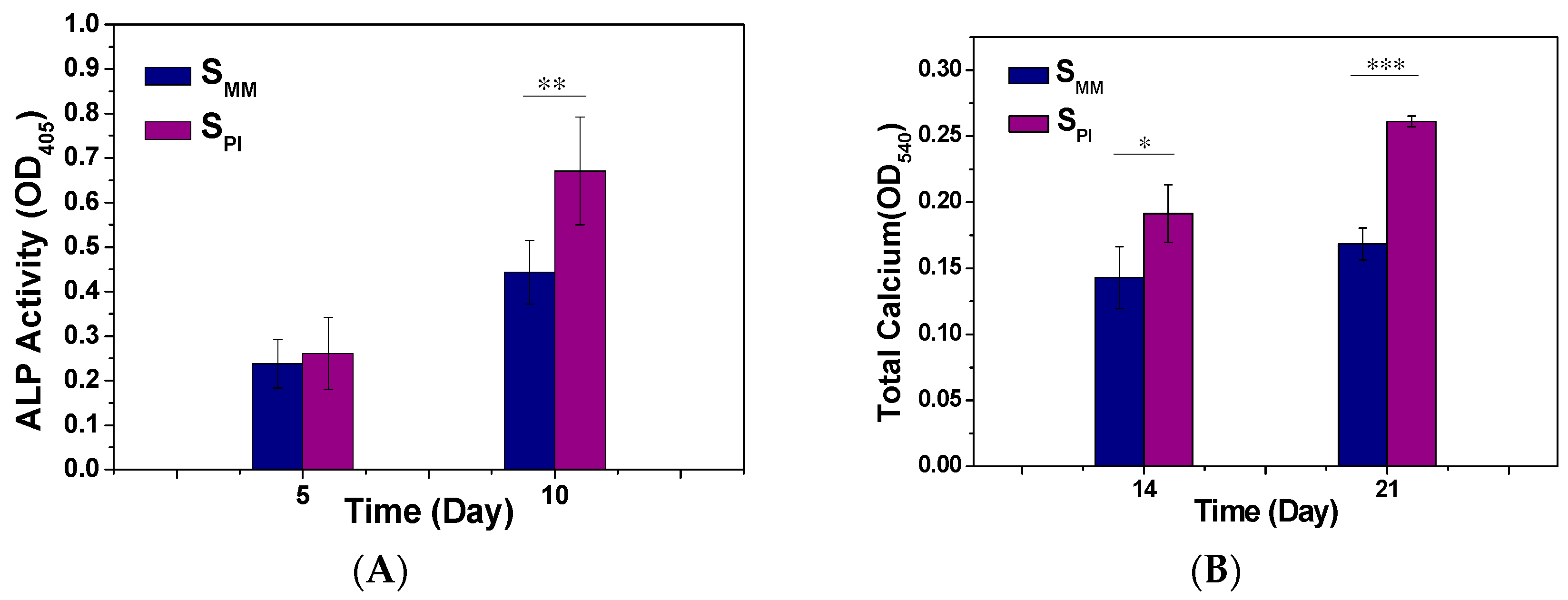
3.2. Cell Adhesion, Proliferation, and Differentiation
3.3. Radius Bone Defect Repair
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Seo, S.J.; Mahapatra, C.; Singh, R.K.; Knowles, J.C.; Kim, H.W. Strategies for osteochondral repair: Focus on scaffolds. J. Tissue Eng. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Zhang, R.; Xiao, G.; Franceschi, R. Engineering new bone tissue in vitro on highly porous poly(α-hydroxyl acids)/hydroxyapatite composite scaffolds. J. Biomed. Mater. Res. 2001, 54, 284–293. [Google Scholar] [CrossRef]
- Gloria, A.; Ronca, D.; Russo, T.; D’Amora, U.; Chierchia, M.; de Santis, R.; Nicolais, L.; Ambrosio, L. Technical features and criteria in designing fiber-reinforced composite materials: From the aerospace and aeronautical field to biomedical applications. J. Appl. Biomater. Biomech. 2011, 9, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Chaudhuri, J.B. Poly(lactic-co-glycolic acid) hollow fibre membranes for use as a tissue engineering scaffold. Biotechnol. Bioeng. 2007, 96, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.L.; English, J.P.; Cowsar, D.R.; Vanderbilt, D.P. Biodegradable in-situ forming implants and methods of producing the same. US Patent No. 4938763, 3 July 1990. [Google Scholar]
- Go, D.P.; Palmer, J.A.; Mitchell, G.M.; Gras, S.L.; O’Connor, A.J. Porous PLGA microspheres tailored for dual delivery of biomolecules via layer-by-layer assembly. J. Biomed. Mater. Res. A 2015, 103, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.C.; Mao, H.Q.; Wang, S.; Leong, K.W.; Ong, L.K.; Yu, H. Fabrication of poly(phosphoester) nerve guides by immersion precipitation and the control of porosity. Biomaterials 2001, 22, 1147–1156. [Google Scholar] [CrossRef]
- Shin, K.C.; Kim, B.S.; Kim, J.H.; Park, T.G.; Nam, J.D.; Lee, D.S. A facile preparation of highly interconnected macroporous PLGA scaffolds by liquid–liquid phase separation II. Polymers 2005, 46, 3801–3808. [Google Scholar] [CrossRef]
- Feng, J.H.; Park, T.G.; Lee, D.S. A facile preparation of highly interconnected macroporous poly(d,l-lactic acid-co-glycolic acid) (PLGA) scaffolds by liquid–liquid phase separation of a PLGA–dioxane–water ternary system. Polymers 2003, 44, 1911–1920. [Google Scholar]
- Oh, S.H.; Lee, J.H. Fabrication and characterization of hydrophilized porous PLGA nerve guide conduits by a modified immersion precipitation method. J. Biomed. Mater. Res. A 2007, 80, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.B.; Hong, Z.K.; Yu, T.; Chen, X.S.; Jing, X.B. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(l-lactide). Biomaterials 2009, 30, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.M.; Sandhu, H.S. Current approaches to experimental bone grafting. Orthop. Clin. North Am. 1987, 18, 213–225. [Google Scholar] [PubMed]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Jing, D.; Ding, J. “Wet-state” mechanical properties of three-dimensional polyester porous scaffolds. J. Biomed. Mater. Res. A 2006, 76, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Townsend-Nicholson, A.; Jayasinghe, S.N. Cell electrospinning: A unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules 2006, 7, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N. Cell electrospinning: A novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst 2013, 138, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, C.M.; Ray, R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 2001, 55, 141–150. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Cui, L.; Zhang, P.; Wang, X.; Wei, Y.; Chen, X. In vitro studies on regulation of osteogenic activities by electrical stimulus on biodegradable electroactive polyelectrolyte multilayers. Biomacromolecules 2014, 15, 3146–3157. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhang, N.; Wang, Z.; Wang, Y.; Liu, Y.; Ito, Y.; Zhang, P. Biodegradable microcarriers of poly(lactide-co-glycolide) and nano-hydroxyapatite decorated with IGF-1 via polydopamine coating for enhancing cell proliferation and osteogenic differentiation. Macromol. Biosci. 2015, 15, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.; D’Souza, R.N.; Hematti, P. Biomaterial-mesenchymal stem cell constructs for immunomodulation in composite tissue engineering. Tissue Eng. A 2014, 20, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.A.; Zouani, O.F.; Glinel, K.; Jonas, A.M.; Durrieu, M.C. Bioactive chemical nanopatterns impact human mesenchymal stem cell fate. Nano Lett. 2013, 13, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Gumusderelioglu, M.; Aday, S. Heparin-functionalized chitosan scaffolds for bone tissue engineering. Carbohyd. Res. 2011, 346, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Intranuovo, F.; Russo, T.; De Santis, R.; Gloria, A.; Ambrosio, L.; Ciurana, J.; Bartolo, P. The first systematic analysis of 3D rapid prototyped poly(ε-caprolactone) scaffolds manufactured through BioCell printing: the effect of pore size and geometry on compressive mechanical behaviour and in vitro hMSC viability. Biofabrication 2013, 5, 045004. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Wang, Y.; Xu, W.; Hu, Y.; Ding, J. Poly(lactide-co-glycolide)/Hydroxyapatite Porous Scaffold with Microchannels for Bone Regeneration. Polymers 2016, 8, 218. https://doi.org/10.3390/polym8060218
Zhang N, Wang Y, Xu W, Hu Y, Ding J. Poly(lactide-co-glycolide)/Hydroxyapatite Porous Scaffold with Microchannels for Bone Regeneration. Polymers. 2016; 8(6):218. https://doi.org/10.3390/polym8060218
Chicago/Turabian StyleZhang, Ning, Yang Wang, Wenpeng Xu, Yong Hu, and Jianxun Ding. 2016. "Poly(lactide-co-glycolide)/Hydroxyapatite Porous Scaffold with Microchannels for Bone Regeneration" Polymers 8, no. 6: 218. https://doi.org/10.3390/polym8060218