Developing (Quantitative Structure Property Relationships) QSPR Techniques to Predict the Char Formation of Polybenzoxazines
Abstract
:1. Introduction
2. Methodology
- The training data set was chosen from the Handbook of Benzoxazine Resins. This training set is a secondary data set and consists of thirty-two benzoxazines with corresponding actual char yield measured by different research groups.
- All monomers were built using the builder menu in MOE and a conformational search using Low Mode Molecular Dynamics [16] was carried out on each monomer before energy minimising the lowest energy conformer of each model to convergence.
- A series of descriptors [17] were calculated for each monomer, which cover molecular volume, shape, charge, etc.
- A QSPR equation as developed to relate the descriptors to the experimentally determined char yield using partial least squares (PLS) [18].
- Descriptors which play a major role in influencing the model were chosen. The linear model equation with the highest coefficient of determination (r2) was selected and further analysis was done on this model.
- The descriptors were then used to calculate the prediction values and the average percentage error of the data produced was calculated in-silico.
- The Leave-One-Out-Cross-Validation test [19] was carried out by the model to evaluate whether it could be taken further and capable to produce accurate prediction values. This test was done by taking out one of the materials in the training set and applying the model to that chosen material.
- The experimental data of the material used in the validation test was compared against the predicted/calculated data. The percentage error and difference error between the two values was calculated and a conclusion was made based on the comparison values.
3. Results and Discussion
4. Van Krevelen Calculations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BAF-a | bisphenolAF/aniline benzoxazine benzoxazine |
| PH-apa | N-aminophenyl-acetylene phenol benzoxazine |
| BF-apa | N-aminophenyl-acetylene bisphenol F benzoxazine |
| HQ-apa | N-aminophenyl-acetylene hydroquinone benzoxazine |
| BA-apa | N-aminophenyl-acetylene bisphenol A benzoxazine |
| BP-apa | N-aminophenyl-acetylene 4,4′-dihydroxy biphenyl benzoxazine |
| TP-apa | N-aminophenyl-acetylene 4,4′-thiodiphenol benzoxazine |
| BAF-apa | N-aminophenyl-acetylene bisphenol AF benzoxazine |
| BS-apa | N-aminophenyl-acetylene bisphenol S benzoxazine |
| BO-apa | N-aminophenyl-acetylene bisphenol O benzoxazine |
| BZ-apa | N-aminophenyl-acetylene 4,4′-dihydroxybenzophenone benzoxazine |
| NP-apa | N-aminophenyl-acetylene 2,7-dihydroxynaphtalene benzoxazine |
| PC-a | p-cresol/aniline benzoxazine |
| BA-a | bisphenol A/aniline benzoxazine |
| HQ-a | hydroquinone/aniline benzoxazine |
| 15N-a | 1,5-dihydronaphtalene/aniline benzoxazine |
| TP-a | 4,4′-thiodiphenol/aniline benzoxazine |
| TrisP-a | 1,1,1-tris(p-hydroxyphenyl)-ethane/aniline benzoxazine |
| 22P-a | 2,2′-dihydroxybiphenyl/aniline benzoxazine |
| 4,4′O-a | 4,4′-dihydroxybenzophenone/aniline benzoxazine |
| P-ad2 | ethlenediamine bisphenol benzoxazine |
| P-ad4 | N-1,4-diaminobutane bisphenol benzoxazine |
| P-ad6 | N-1,6-diaminohexane bisphenol benzoxazine |
| P-ad8 | N-1,8-diaminooctane bisphenol benzoxazine |
| P-ad12 | N-1,12-diaminododecane bisphenol benzoxazine |
| MIB-a | 1-(4-hydro-phenyl)-pyrrole-2,5-dione/aniline benzoxazine |
| NOB-a | p-hydroxyphenylnadimide/aniline benzoxazine |
| BHPPO-a | bis-(4-hydroxyphenyl)phenylphospine oxide benzoxazine |
| BHPPO-m | methylamine bis-(4-hydroxyphenyl)phenylphospine oxide benzoxazine |
| BHPPO-ea | 3-ethylaniline bis-(4-hydroxyphenyl)phenylphospine oxide benzoxazine |
| BPPPO-a | bis-(4-benzyloxyphenoxy-4′-phenyl)phenyl phosphine/aniline benzoxazine |
| BPPPO-m | methylamine bis-(4-benzyloxyphenoxy-4′-phenyl)phenyl phosphine benzoxazine |
| BPPPO-ea | 3-ethylaniline bis-(4-benzyloxyphenoxy-4′-phenyl)phenyl phosphine benzoxazine |
References
- Hamerton, I.; Mooring, L. The use of thermosets in aerospace applications. In Thermosets: Structure, Properties and Applications; Guo, Q., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 189–227. [Google Scholar]
- Lu, S.-Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Ishida, H. Overview and historical background of polybenzoxazine research. In Handbook of Benzoxazine Resins; Ishida, H., Agag, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 8–66. [Google Scholar]
- Alger, M.S. High-temperature and fire resistant polymers. In Specialty Polymers; Dyson, R.W., Ed.; Blackie Academic and Professional: Glasgow, UK, 1998; pp. 189–227. [Google Scholar]
- Gardziella, A.; Pilato, L.A.; Knop, A. Phenolic Resins: Chemistry, Applications, Standardisation, Safety and Ecology; Springer: Berlin, Germany, 2000; p. 560. [Google Scholar]
- Hamerton, I. Recent Developments in Epoxy Resins; iSmithers Rapra Publishing: Shawbury, UK, 1996. [Google Scholar]
- Aerospace Product Selector Guide. Available online: http://www.henkel-adhesives.com/com/content_data/372069_ASA15010_Structural_Adhesive_A4_02.pdf (accessed on 20 April 2016).
- Hamerton, I.; Thompson, S.; Howlin, B.J.; Stone, C.A. New method to predict the thermal degradation behavior of polybenzoxazines from empirical data using structure property relationships. Macromolecules 2013, 46, 7605–7615. [Google Scholar] [CrossRef]
- Ginzburg, V.V.; Weinhold, J.D.; Trefonas, P. Computational modeling of block-copolymer directed self-assembly. J. Polym. Sci. Part B 2015, 53, 90–95. [Google Scholar] [CrossRef]
- Kandare, E.; Griffin, G.; Feih, S.; Gibson, P.; Lattimer, B.; Mouritz, A. Fire structural modelling of fibre–polymer laminates protected with an intumescent coating. Compos. Part A 2012, 43, 793–802. [Google Scholar] [CrossRef]
- Li, C.; Strachan, A. Molecular scale simulations on thermoset polymers: A review. J. Polym. Sci. Part B 2015, 53, 103–122. [Google Scholar] [CrossRef]
- Patel, H.C.; Tokarski, J.S.; Hopfinger, A.J. Molecular modeling of polymers 16. gaseous diffusion in polymers: A quantitative structure-property relationship (QSPR) analysis. Pharm. Res. 1997, 14, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Sild, S.; Karelson, M. Correlation and prediction of the refractive indices of polymers by QSPR. J. Chem. Inf. Model. 1998, 38, 1171–1176. [Google Scholar] [CrossRef]
- Bicerano, J. Prediction of Polymer Properties, 3rd ed.; Marcel Dekker: New York, NY, USA, 2002; p. 784. [Google Scholar]
- Mhlanga, P.; Wan Hassan, W.A.; Hamerton, I.; Howlin, B.J. Using combined computational techniques to predict the glass transition temperatures of aromatic polybenzoxazines. PLoS ONE 2013, 8, e53367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labute, P. LowModeMD—Implicit low-mode velocity filtering applied to conformational search of macrocycles and protein loops. J. Chem. Inf. Model. 2010, 50, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Labute, P. A widely applicable set of descriptors. J. Mol. Graph. Model. 2000, 18, 464–477. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Rafaeilzadeh, P.; Tang, L.; Liu, H. Cross-validation. In Encyclopedia of Database Systems; Liu, L., Ozsu, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 532–538. [Google Scholar]
- Kim, H.J.; Brunovska, Z.; Ishida, H. Synthesis and thermal characterization of polybenzoxazines based on acetylene-functional monomers. Polym. J. 1999, 40, 6565–6573. [Google Scholar] [CrossRef]
- Low, H.Y.; Ishida, H. Structural effects of phenols on the thermal and thermo-oxidative degradation of polybenzoxazines. Polym. J. 1999, 40, 4365–4376. [Google Scholar] [CrossRef]
- Ishida, H.; Ohba, S. Synthesis and characterization of maleimide and norbornene functionalized benzoxazines. Polym. J. 2005, 46, 5588–5595. [Google Scholar] [CrossRef]
- Choi, S.W.; Ohba, S.; Brunovska, Z.; Hemvichian, K.; Ishida, H. Synthesis, characterization and thermal degradation of functional benzoxazine monomers and polymers containing phenylphosphine oxide. Polym. Degrad. Stab. 2006, 91, 1166–1178. [Google Scholar] [CrossRef]
- Allen, D.J.; Ishida, H. Physical and mechanical properties of flexible polybenzoxazine resins: Effect of aliphatic diamine chain length. J. Appl. Polym. Sci. 2006, 101, 2798–2809. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Synthesis and characterization of novel benzoxazine monomers containing allyl groups and their high performance thermosets. Macromolecules 2003, 36, 6010–6017. [Google Scholar] [CrossRef]
- Shen, S.B.; Ishida, H. Dynamic mechanical and thermal characterization of high-performance polybenzoxazines. J. Polym. Sci. Part B 1999, 37, 3257–3268. [Google Scholar] [CrossRef]
- Principles of Thermal Analysis and Calorimetry; Haines, P.J. (Ed.) Royal Society of Chemistry (RSC): Cambridge, UK, 2002; pp. 69–72.
- Jain, R.; Narula, A.K.; Choudhary, V. Studies on curing and thermal behavior of diglycidyl ether of bisphenol-A and benzoxazine mixtures. J. Appl. Polym. Sci. 2007, 106, 3327–3334. [Google Scholar] [CrossRef]
- Santhosh Kumar, K.S.; Reghunadhan Nair, C.P.; Sadhana, R.; Ninan, K.N. Benzoxazine–bismaleimide blends: Curing and thermal properties. Eur. Polym. J. 2007, 43, 5084–5096. [Google Scholar] [CrossRef]
- Nour-Eddine, E.M.; Yuan, Q.; Huang, F. Investigation of curing and thermal behavior of benzoxazine and lignin mixtures. J. Appl. Polym. Sci. 2012, 125, 1773–1781. [Google Scholar] [CrossRef]
- Lorjai, P.; Wongkasemjit, S.; Chaisuwan, T.; Jamieson, A.M. Significant enhancement of thermal stability in the non-oxidative thermal degradation of bisphenol-A/aniline based polybenzoxazine aerogel. Polym. Degrad. Stab. 2011, 96, 708–718. [Google Scholar] [CrossRef]
- Thompson, S. Examining the Thermal Degradation of Polybenzoxazines and Their Resultant Chars. Ph.D. Thesis, University of Surrey, Guildford, UK, 2013; pp. 105–112. [Google Scholar]
- McNamara, L.T. Characterisation of A Selection of Commercial Benzoxazines and Novel Toughtening Strategies. Ph.D. Thesis, University of Surrey, Guildford, UK, 2013; pp. 102–105. [Google Scholar]
- Wan Hassan Wan, H. Characterisation and Molecular Modelling of Selected Benzoxazines and Their Polymers. Ph.D. Thesis, University of Surrey, Guildford, UK, 2014; p. 206. [Google Scholar]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers, Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
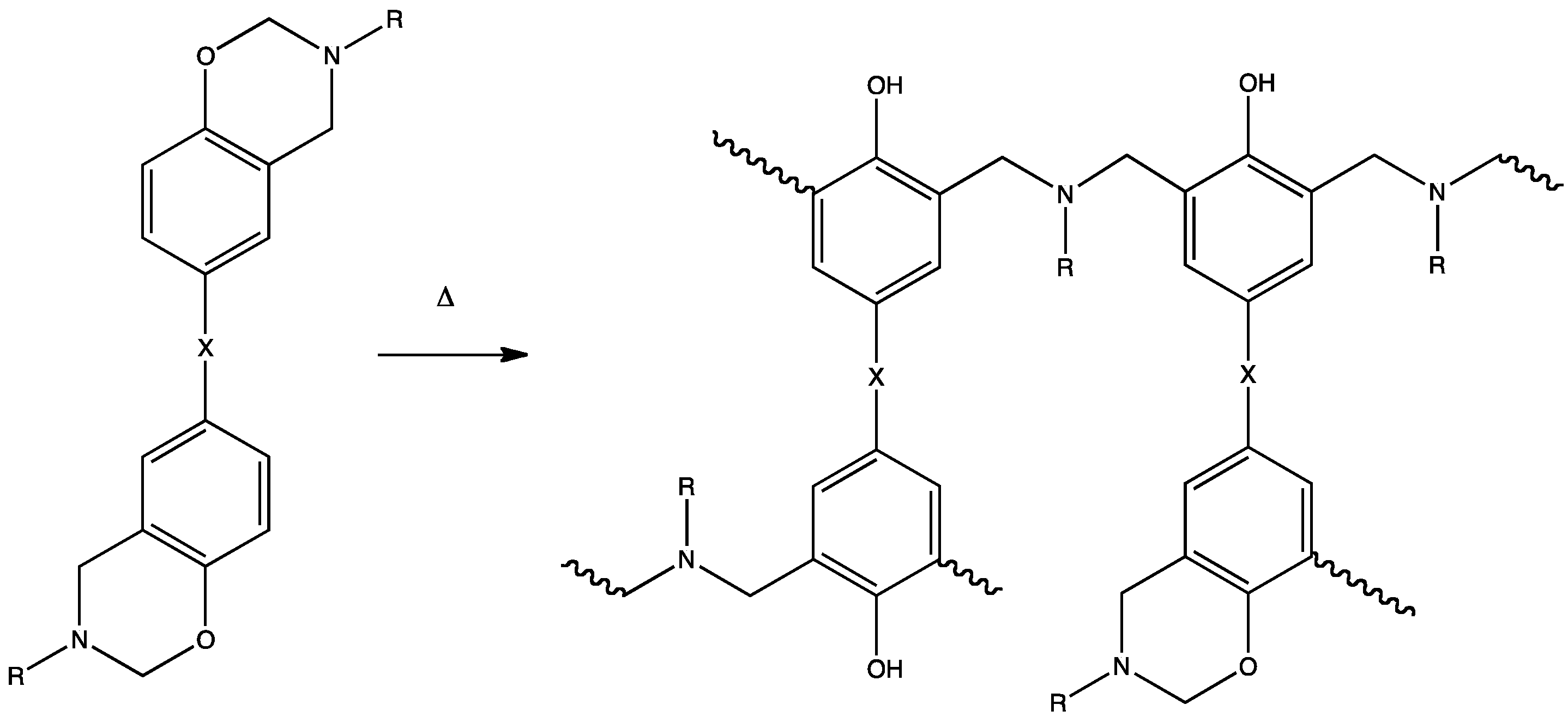
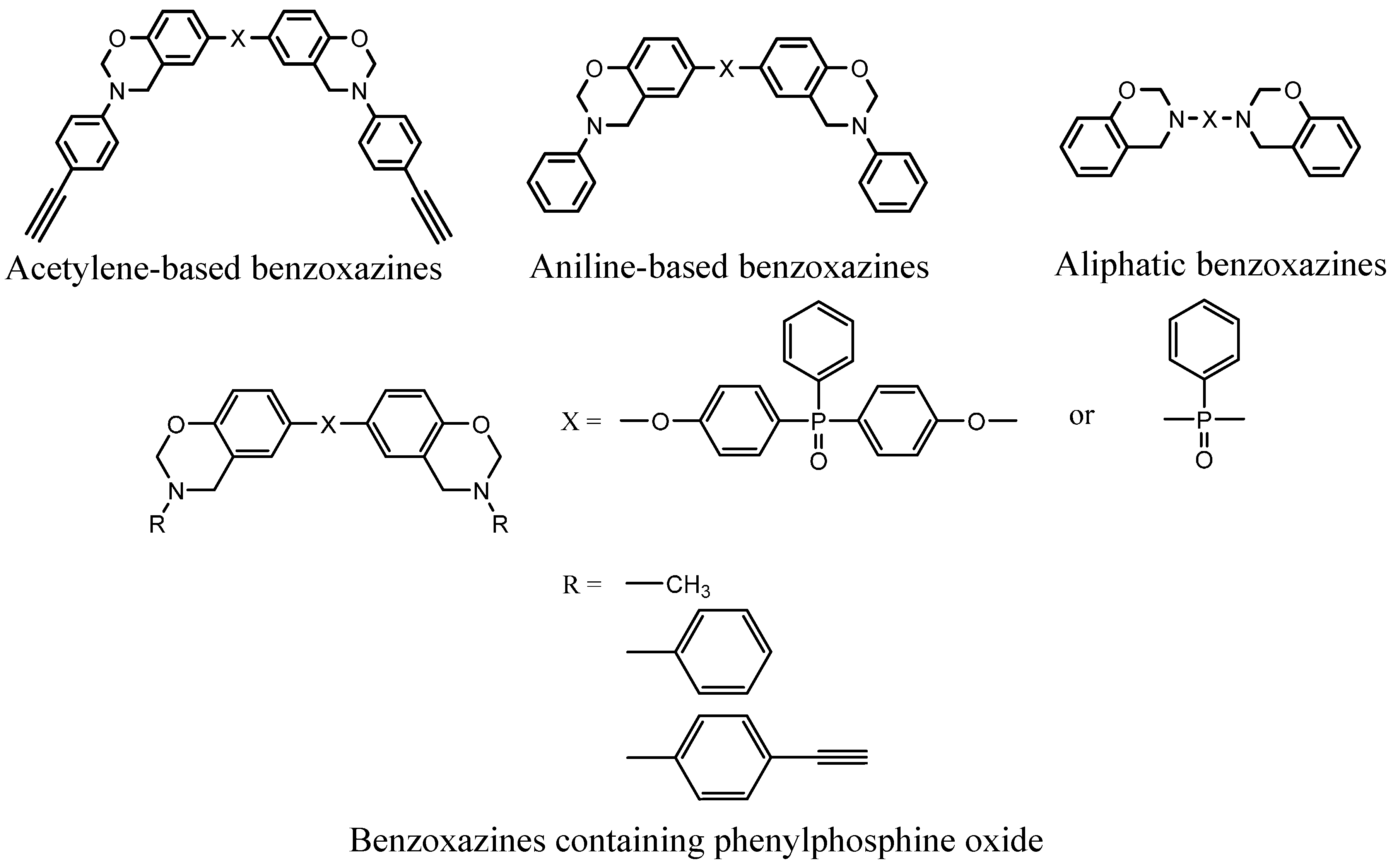
| No. | Materials | Char yield (%) | No. | Materials | Char yield (%) |
|---|---|---|---|---|---|
| 1 | HQ-apa | 81 [20] | 17 | TP-a | 57 [21] |
| 2 | BZ-apa | 80 [20] | 18 | MIB-a | 56 [22] |
| 3 | PH-apa | 79 [3], (81) [20] | 19 | BPPPO-a | 51 [23] |
| 4 | TP-apa | 79 [20] | 20 | BHPPO-m | 48 [23] |
| 5 | BF-apa | 78 [20] | 21 | TrisP-a | 47 [21] |
| 6 | BS-apa | 78 [20] | 22 | BHPPO-a | 46 [3], (41) [23] |
| 7 | NP-apa | 76 [20] | 23 | HQ-a | 44 [21] |
| 8 | BPPPO-ea | 76 [23] | 24 | P-ad2 | 41 [24] |
| 9 | BO-apa | 75 [20] | 25 | P-ad4 | 32 [24] |
| 10 | BA-apa | 74 [20] | 26 | BA-a | 32 [20,25] |
| 11 | BP-apa | 73 [20] | 27 | BPPPO-m | 30 [23] |
| 12 | BAF-apa | 71 [20] | 28 | PC-a | 20 [21] |
| 13 | 15N-a | 71 [21] | 29 | P-ad6 | 19 [24] |
| 14 | 4,4'O-a | 65 [26] | 30 | P-ad8 | 1 [24] |
| 15 | BHPPO-ea | 64 [23] | 31 | P-ad12 | 6 [24] |
| 16 | BAF-a | 57 [3] | 32 | NOB-a | 58 [22] |
| Temperature (°C) | Heating rate (K/min) | Measured char yield (%), Yc | Mean, Ȳc | Difference error, Yc − Ȳc | Average difference error | Percentage error (%) | Average percentage error (%) |
|---|---|---|---|---|---|---|---|
| 800 | 20 | 24.30 [28] | 28.72 | 4.42 | 4.06 | 18.19 | 14.00 |
| 20 | 32.00 [20] | 3.28 | 10.25 | ||||
| 10 | 35.60 [29] | 6.88 | 19.33 | ||||
| 10 | 25.70 [30] | 3.02 | 11.75 | ||||
| 900 | 20 | 26.00 [31] | 2.72 | 10.46 |
| Temperature (°C) | Measured char yield (%), Yc | Mean, Ȳc | Difference error, Yc − Ȳc | Average difference error | Percentage error (%) | Average percentage error (%) |
|---|---|---|---|---|---|---|
| 800 | 26.62 [32] | 27.78 | 1.16 | 3.12 | 4.36 | 10.48 |
| 25.00 [33] | 2.78 | 11.12 | ||||
| 26.00 [34] | 1.78 | 6.85 | ||||
| 35.60 [29] | 7.82 | 21.97 | ||||
| 25.70 [30] | 2.08 | 8.09 |
| Temperature (°C) | Measured char yield (%), Yc | Mean, Ȳc | Difference error, Yc − Ȳc | Average difference error | Percentage error (%) | Average percentage error (%) |
|---|---|---|---|---|---|---|
| 800 | 26.62 [32] | 25.87 | 0.75 | 0.58 | 2.80 | 2.26 |
| 25.00 [33] | 0.87 | 3.49 | ||||
| 26.00 [34] | 0.13 | 0.49 |
| Percentage char yield = −139.65 (+8.92 × b_rotN) (−7.00× lip_violation) (+1.15 × logP(o/w)) (−10.78 × opr_nrot) (−0.44 × PEOE_VSA-2) (−0.35 × PEOE_VSA-3) (+118.61 × petitjeanSC) (+174.65 × Q_VSA_FNEG) (+0.65 × SMR_VSA6) (−4.82 × std_dim2) | ||
| Relative importance | Descriptors’ abbreviations | Description |
| 1.00 | opr_nrot | Oprea Rotatable Bond Count |
| 0.75 | b_rotN | Number of rotatable bonds |
| 0.61 | SMR_VSA6 | Bin 6 SMR (0.485, 0.560) |
| 0.56 | Q_VSA_FNEG | Fractional negative vdw surface area |
| 0.34 | PEOE_VSA-2 | Total negative 2 Å2 vdw surface area |
| 0.24 | petitjeanSC | (diameter − radius)/radius |
| 0.19 | PEOE_VSA-3 | Total negative 3 Å2 vdw surface area |
| 0.18 | lip_violation | Lipinski Violation Count |
| 0.09 | std_dim2 | Standard dimension 2 Å |
| 0.08 | logP(o/w) | Log octanol/water partition coefficient |
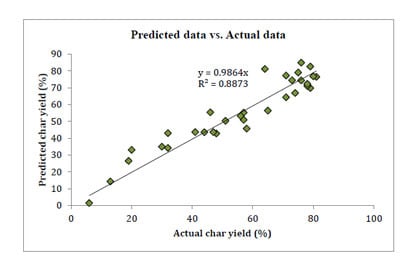
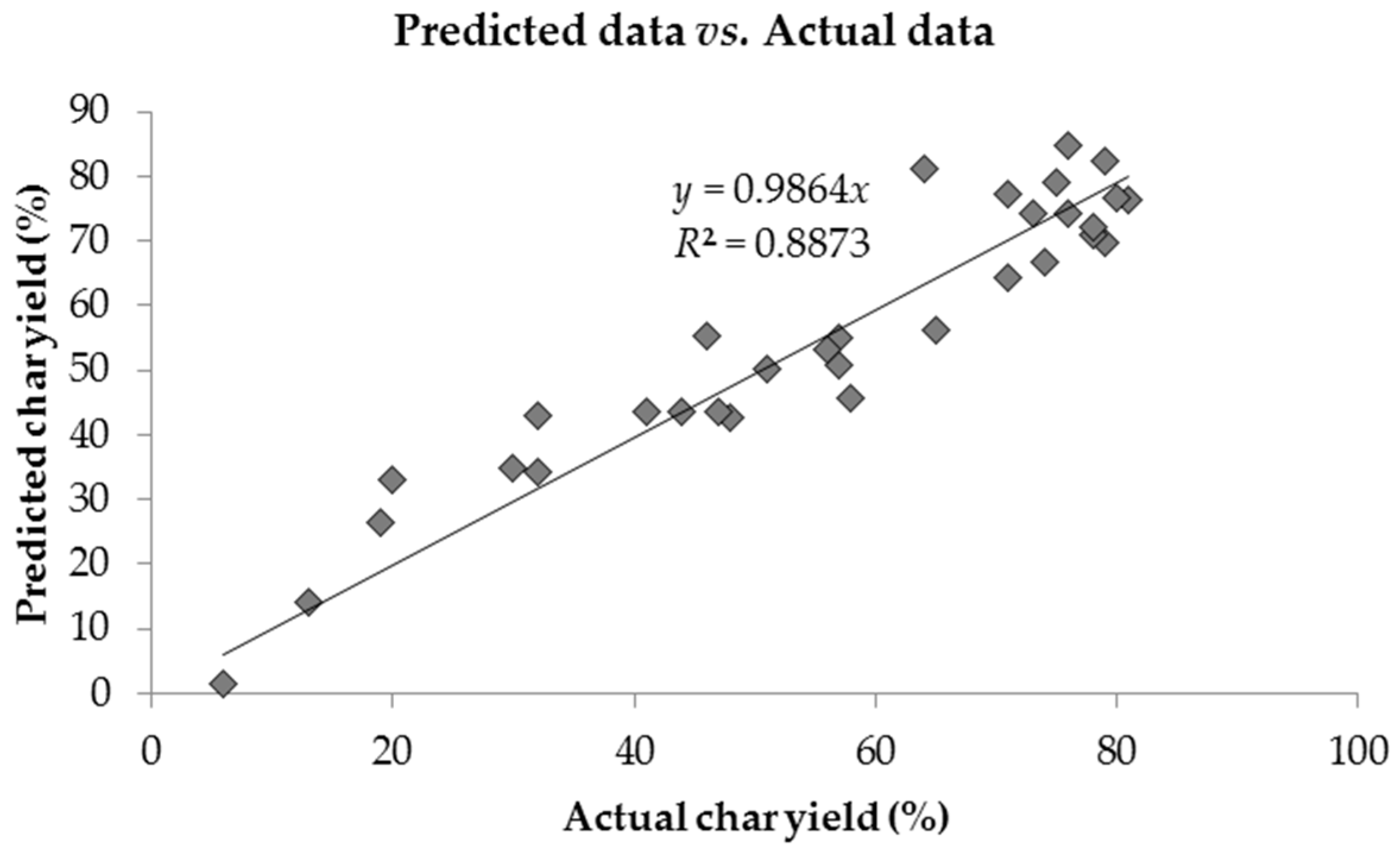
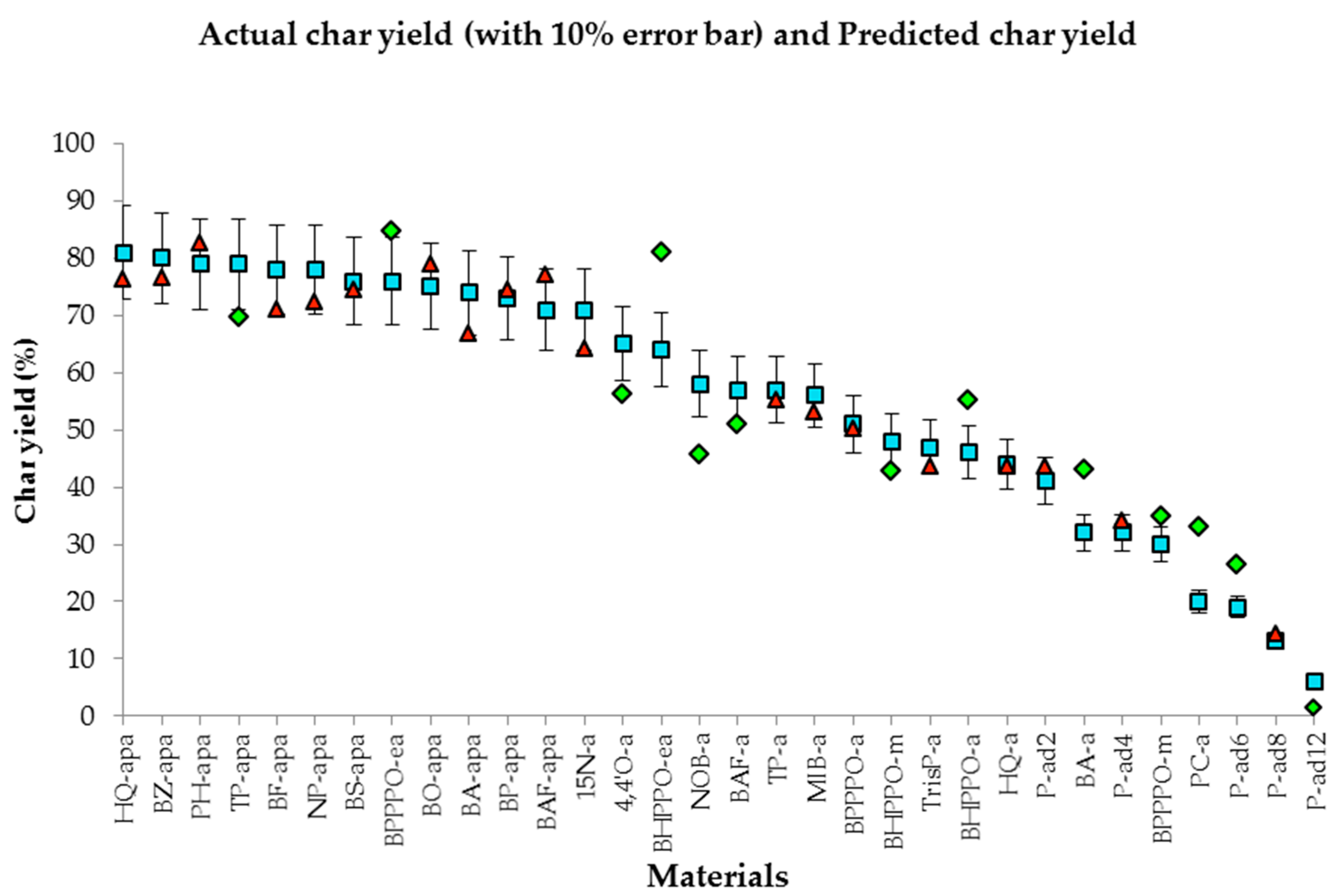
| Materials | Actual data (%) | Predicted data (%) | Difference error | Average error | % error | Average % error | R2 |
|---|---|---|---|---|---|---|---|
| HQ-apa | 81.00 | 74.66 | 6.34 | 5.02 | 7.82 | 12.54 | 91.94% |
| BZ-apa | 80.00 | 82.75 | 2.75 | 3.44 | |||
| PH-apa | 79.00 | 84.69 | 5.69 | 7.20 | |||
| TP-apa | 79.00 | 75.25 | 3.75 | 4.75 | |||
| BF-apa | 78.00 | 77.82 | 0.18 | 0.23 | |||
| BS-apa | 76.00 | 76.55 | 0.55 | 0.72 | |||
| NP-apa | 78.00 | 82.14 | 4.14 | 5.31 | |||
| BPPPO-ea | 76.00 | 72.19 | 3.81 | 5.02 | |||
| BO-apa | 75.00 | 78.04 | 3.04 | 4.05 | |||
| BA-apa | 74.00 | 64.07 | 9.93 | 13.42 | |||
| BP-apa | 73.00 | 69.12 | 3.88 | 5.31 | |||
| BAF-apa | 71.00 | 75.61 | 4.61 | 6.49 | |||
| 15N-a | 71.00 | 65.94 | 5.06 | 7.12 | |||
| 4,4'O-a | 65.00 | 57.64 | 7.36 | 11.32 | |||
| BHPPO-ea | 64.00 | 73.97 | 9.97 | 15.57 | |||
| NOB-a | 58.00 | 43.99 | 14.01 | 24.15 | |||
| BAF-a | 57.00 | 52.39 | 4.61 | 8.08 | |||
| TP-a | 57.00 | 55.74 | 1.26 | 2.22 | |||
| MIB-a | 56.00 | 51.58 | 4.42 | 7.89 | |||
| BPPPO-a | 51.00 | 52.74 | 1.74 | 3.41 | |||
| BHPPO-m | 48.00 | 41.58 | 6.42 | 13.37 | |||
| TrisP-a | 47.00 | 40.97 | 6.03 | 12.82 | |||
| BHPPO-a | 46.00 | 52.51 | 6.51 | 14.16 | |||
| HQ-a | 44.00 | 45.46 | 1.46 | 3.32 | |||
| P-ad2 | 41.00 | 45.44 | 4.44 | 10.83 | |||
| BA-a | 32.00 | 45.22 | 13.22 | 41.31 | |||
| P-ad4 | 32.00 | 32.35 | 0.35 | 1.09 | |||
| BPPPO-m | 30.00 | 34.51 | 4.51 | 15.05 | |||
| PC-a | 20.00 | 30.69 | 10.69 | 53.43 | |||
| P-ad6 | 19.00 | 25.31 | 6.31 | 33.20 | |||
| P-ad8 | 13.00 | 13.43 | 0.43 | 3.31 | |||
| P-ad12 | 6.00 | 2.65 | 3.35 | 55.84 |
| Material | Actual data (%) | Prediction data (%) | % error | Difference error |
|---|---|---|---|---|
| 22P-a | 45.00 | 45.31 | 0.69 | 0.31 |
| Materials | Structures | Materials | Structures |
|---|---|---|---|
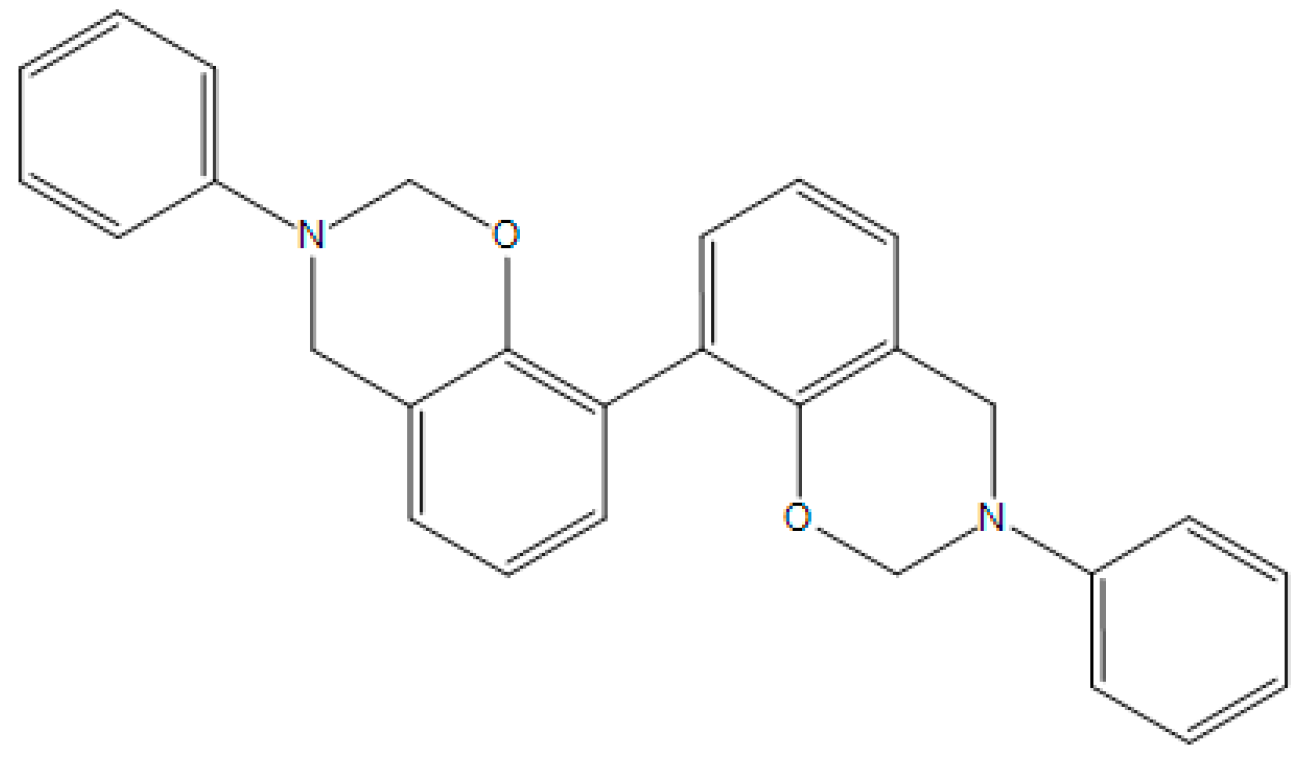
| BA-apa | 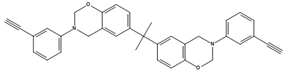 | P-ad6 | 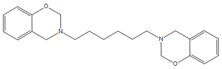 |
| 4,4′O-a | 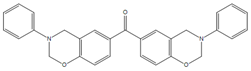 | P-ad12 |  |
| BAF-a |  | NOB-a | 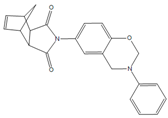 |
| BA-a |  | PC-a |  |
| BHPPO-m | 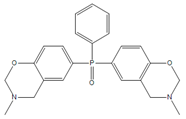 | BHPPO-a | 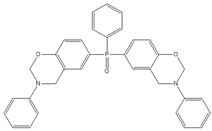 |
| BHPPO-ea |  | BPPPO-m |  |
| BPPPO-ea |  |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sairi, M.; Howlin, B.; Hamerton, I. Developing (Quantitative Structure Property Relationships) QSPR Techniques to Predict the Char Formation of Polybenzoxazines. Polymers 2016, 8, 166. https://doi.org/10.3390/polym8050166
Sairi M, Howlin B, Hamerton I. Developing (Quantitative Structure Property Relationships) QSPR Techniques to Predict the Char Formation of Polybenzoxazines. Polymers. 2016; 8(5):166. https://doi.org/10.3390/polym8050166
Chicago/Turabian StyleSairi, Maryam, Brendan Howlin, and Ian Hamerton. 2016. "Developing (Quantitative Structure Property Relationships) QSPR Techniques to Predict the Char Formation of Polybenzoxazines" Polymers 8, no. 5: 166. https://doi.org/10.3390/polym8050166