Poly-Lactide/Exfoliated C30B Interactions and Influence on Thermo-Mechanical Properties Due to Artificial Weathering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PLA Nanocomposite and Blank
2.2. Obtaining Materials with Different Molecular Weights
2.3. Characterization
3. Results
3.1. Molecular Weight
3.2. Thermo-Mechanical Properties
3.2.1. Thermogravimetric Analysis (TGA)
3.2.2. Differential Scanning Calorimetry (DSC)
3.2.3. X-ray Diffraction (XRD)
3.2.4. Dynamic Thermo-Mechanical Analysis (DTMA)
3.2.5. Fourier Transform Infrared (FTIR) Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ATR-FTIR | Attenuated Total Reflectance-Fourier Transform Infrared |
| C30B | Cloisite30B |
| DMTA | Dynamic Mechanical Thermal Analysis |
| DSC | Differential Scanning Calorimetry |
| GPC | Gel Permeation Chromatography |
| HPLC | High Performance Liquid Chromatography |
| MMT | Montmorillonite |
| MW | Molecular Weight |
| MW D | Molecular Weight Distribution |
| MW | Weight Average Molecular Weight |
| Mn | Number Average Molecular Weight |
| PLA | Poly-Lactide |
| PLA/B | Poly-Lactide blank |
| PLA/C30B | Poly-Lactide nanocomposite |
| TGA | Thermogravimetric Analysis |
| THF | Tetrahydrofuran |
| Tg | Glass Transition Temperature |
| Tc | Cold Crystallization Temperature |
| Tm | Melting Temperature |
| Xc | Degree of Crystallinity |
| XRD | X-ray Diffraction |
References
- Pongtanayuta, K.; Thongpina, C.; Santawiteeb, O. The effect of rubber on morphology, thermal properties and mechanical properties of PLA/NR and PLA/ENR blends. Energy Proced. 2013, 34, 888–897. [Google Scholar] [CrossRef]
- Woo, J.; Taik, Y.; Park, O. Thermal characteristics of organoclay and their effects upon the formation of polypropylene/organoclay nanocomposites. Polym. Bull. 2000, 45, 191–198. [Google Scholar]
- McLauchlin, A.R.; Thomas, N.L. Preparation and thermal characterisation of poly(lactic acid) nanocomposites prepared from organoclays based on an amphoteric surfactant. Polym. Degrad. Stab. 2009, 94, 868–872. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Mohanty, S.; Nayak, S.K.; Rahail, M. Effect of glycidyl methacrylate (GMA) on the thermal, mechanical and morphological property of biodegradable PLA/PBAT blends and its nanocomposites. Bioresour. Technol. 2010, 101, 8406–8415. [Google Scholar] [CrossRef] [PubMed]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Poly. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Fukushima, K.; Murariu, M.; Camino, G.; Dubois, P. Effect of expanded graphite/layered-silicate clay on thermal, mechanical and fire retardant properties of poly(lactic acid). Polym. Degrad. Stab. 2010, 95, 1063–1076. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Richert, J.; Rózanski, A. Effect of blow moulding ratio non barrier properties of polylactide nanocomposite films. Polym. Test. 2010, 29, 251–257. [Google Scholar] [CrossRef]
- Picard, E.; Espuche, E.; Fulchiron, R. Effect of an organo-modified montmorillonite on PLA crystallization and gas barrier properties. Appl. Clay Sci. 2011, 53, 58–65. [Google Scholar] [CrossRef]
- Bandera, D.; Meyer, V.R.; Prevost, D.; Zimmermann, T.; Boesel, L.F. Polylactide/montmorillonite hybrid latex as a barrier coating for paper applications. Polymers 2016, 8, 75. [Google Scholar] [CrossRef]
- Petinakis, E.; Liu, X.; Yu, L.; Way, C.; Sangwan, P.; Dean, K.; Bateman, S.; Edward, G. Biodegradation and termal decomposition of poly(lactic acid)-based materials reinforced by hydrophilic fillers. Polym. Degrad. Stab. 2010, 95, 1704–1707. [Google Scholar] [CrossRef]
- Manavietehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Vázquez-Torres, H.; Garfias-Mesías, L.F.; Paul, D.R. Thermal degradation of commercialy available organoclays studied by TGA-FTIR. Thermochem. Acta 2007, 457, 92–102. [Google Scholar] [CrossRef]
- Khankrua, R.; Pivsa-Art, S.; Hiroyuki, H.; Suttiruengwong, S. Effect of chain extenders on thermal and mechanical properties of poly(lactic acid) at high processing temperatures: Potential application in PLA/Polyamide 6 blend. Polym. Degrad. Stab. 2014, 108, 232–240. [Google Scholar] [CrossRef]
- Badía, J.D.; Santonja-Blasco, L.; Moriana, R.; Ribes-Greus, A. Thermal analysis applied to the characterization of degradation in soil of polylactide: II. On the thermal stability and thermal decomposition kinetics. Polym. Degrad. Stab. 2010, 95, 2192–2199. [Google Scholar] [CrossRef]
- Chang-Hong, H.; Chau-Hui, W.; Chin-I, L.; Yu-Der, L. Synthesis and characterization of TPO-PLA copolymer and its behavior. Polymer 2008, 49, 3902–3910. [Google Scholar]
- Nieddu, E.; Mazzucco, L.; Gentile, P.; Benko, T.; Balbo, V.; Mandrile, R.; Ciardelli, G. Preparation and biodegradation of clay composites of PLA. React. Func. Polym. 2009, 69, 371–379. [Google Scholar] [CrossRef]
- Zaidi, L.; Kaci, M.; Bruzaud, S.; Bourmaud, A.; Grohens, Y. Effect of natural weather on the structure and properties of polylactide/Cloisite 30B nanocomposites. Polym. Degrad. Stab. 2010, 95, 1751–1758. [Google Scholar] [CrossRef]
- Chávez-Montes, W.; González-Sánchez, G.; López-Martínez, E.; Lira-Gómez, P.; Ballinas-Casarrubias, L.; Flores-Gallardo, S. Effect of artificial weathering on PLA/nanocomposite molecular weight distribution. Polymers 2015, 7, 760–776. [Google Scholar] [CrossRef]
- Stloukal, P.; Verney, V.; Commereuc, S.; Rychly, J.; Matisova-Rychlá, L.; Pis, V.; Koutny, M. Assessment of the interrelation between photooxidation and biodegradation of selected polyesters after artificial weathering. Chemosphere 2012, 88, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Solarski, S.; Ferreira, M.; Devaux, E. Ageing of polylactide and polylactide nanocomposite filaments. Polym. Degrad. Stab. 2008, 93, 707–713. [Google Scholar] [CrossRef]
- Filippi, S.; Paci, M.; Polacco, G.; Dintcheva, N.T.; Magagnini, P. On the interlayer spacing collapse of Cloisite C30B organoclay. Polym. Degrad. Stab. 2011, 96, 823–832. [Google Scholar] [CrossRef]
- Pandey, J.K.; Reddy, K.; Kumar, A.; Singh, R.P. An overview on the degradability of polymer nanocomposites. Polym. Degrad. Stab. 2005, 88, 234–250. [Google Scholar] [CrossRef]
- Rasselet, D.; Ruellan, A.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C.; Fayolle, B. Oxidative degradation of polylactide (PLA) and its effects on physical and mechanical properties. Eur. Polym. J. 2014, 50, 109–116. [Google Scholar] [CrossRef]
- Novikov, V.N.; Rössler, E.A. Correlation between glass transition temperatura and molecular mass in non-polymeric and polymer glass formers. Polymer 2013, 54, 6987–6991. [Google Scholar] [CrossRef]
- Sabu, T.; Kuruvilla, J.; Sant, K.M.; Koichi, G.; Meyyarappallil, S.S. Polymer Composites, 1st ed.; Wiley-VCH Velag GmbH & Co. KGaA: Boschstr, Weinheim, Germany, 2013. [Google Scholar]
- Young, J.; Okamoto, M.; Okamoto, H.; Nakano, M.; Usuki, A.; Matsuda, M. Morphology and crystallization kinetics in a mixture of low-molecular weight aliphatic amide and polylactide. Polymer 2006, 47, 1340–1347. [Google Scholar]
- Dos Santos, F.A.; Bruno Tavares, M.I. Development and characterization of hybrid materials based on biodegradable PLA matrix, microcrystalline cellulose and organophilic silica. Polimeros 2014, 24. [Google Scholar] [CrossRef]
- Liu, C.; Chan, K.W.; Shen, J.; Wong, H.M.; Yeung, K.W.; Tjong, S.C. Melt-compounded polylactid acid composite hybrids with hydroxyapatite nanorods and silver nanoparticles: Biodegradation, antibacterial ability, bioactivity and cytotoxicity. RCS Adv. 2015, 5, 72288–72299. [Google Scholar]
- Yang, S.-L.; Wu, Z.-H.; Yang, W.; Yang, M.-B. Thermal and mechanical properties of chemical crosslinked polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Orozco, V.; Brostow, W.; Chonkaew, W.; Lopez, B. Preparation and characterization of poly(lactic acid)-g-maleic anhydride+ starch blends. Macromol. Symp. 2009, 277, 69–80. [Google Scholar] [CrossRef]
- Zhang, W.; Dehghani-Sanij, A.A.; Blachburn, R.S. IR Study on hydrogen bonding in epoxy resin-silica nanocomposites. Prog. Nat. Sci. 2008, 18, 801–805. [Google Scholar] [CrossRef]
- Mohanty, S.; Nayak, S.K.; Kaith, B.S.; Kalia, S. Nanocomposites based on inorganic nanoparticles. In Polymer Nanocomposites Based on Inorganic and Organic Nanomaterials, 2nd ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 259–341. [Google Scholar]
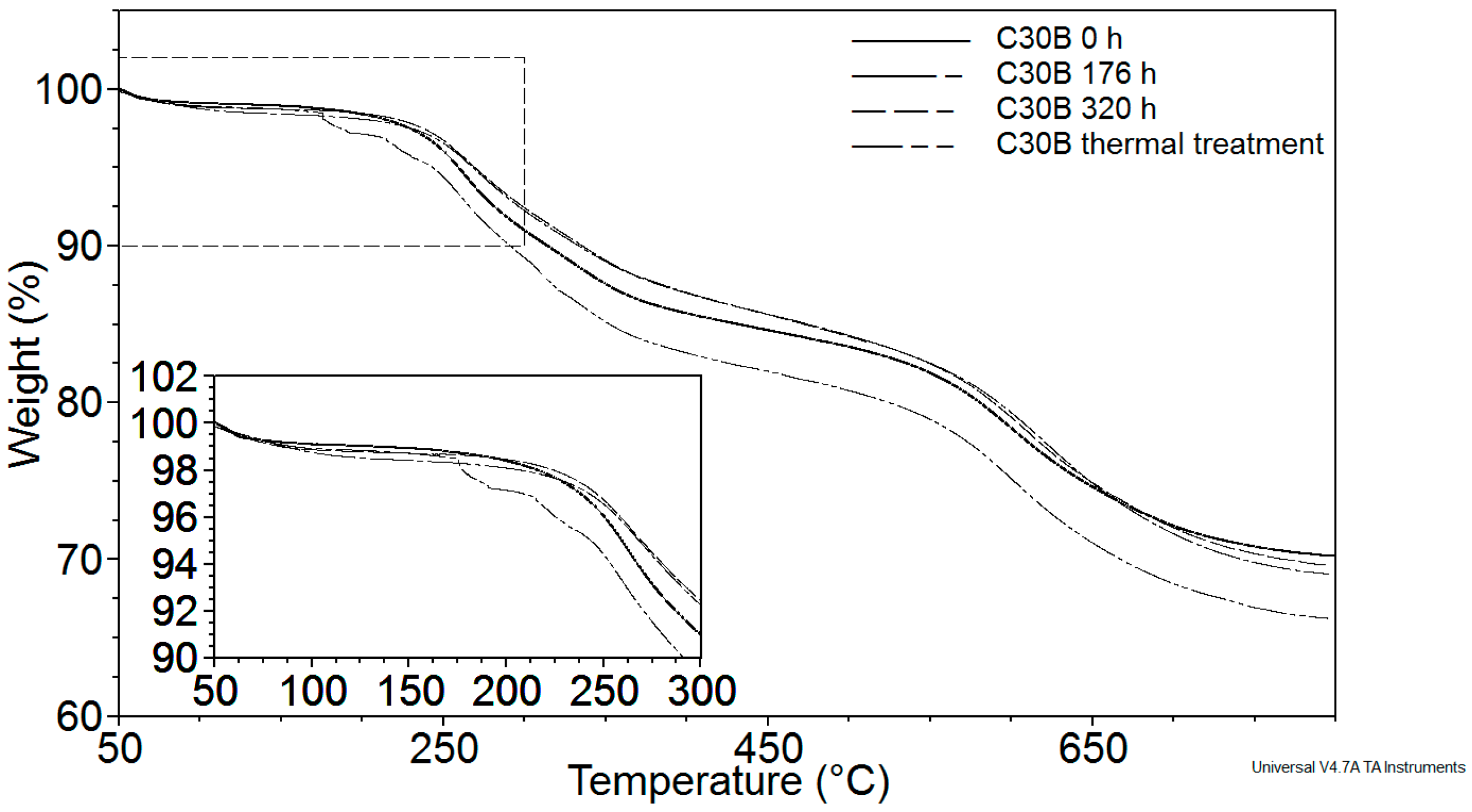




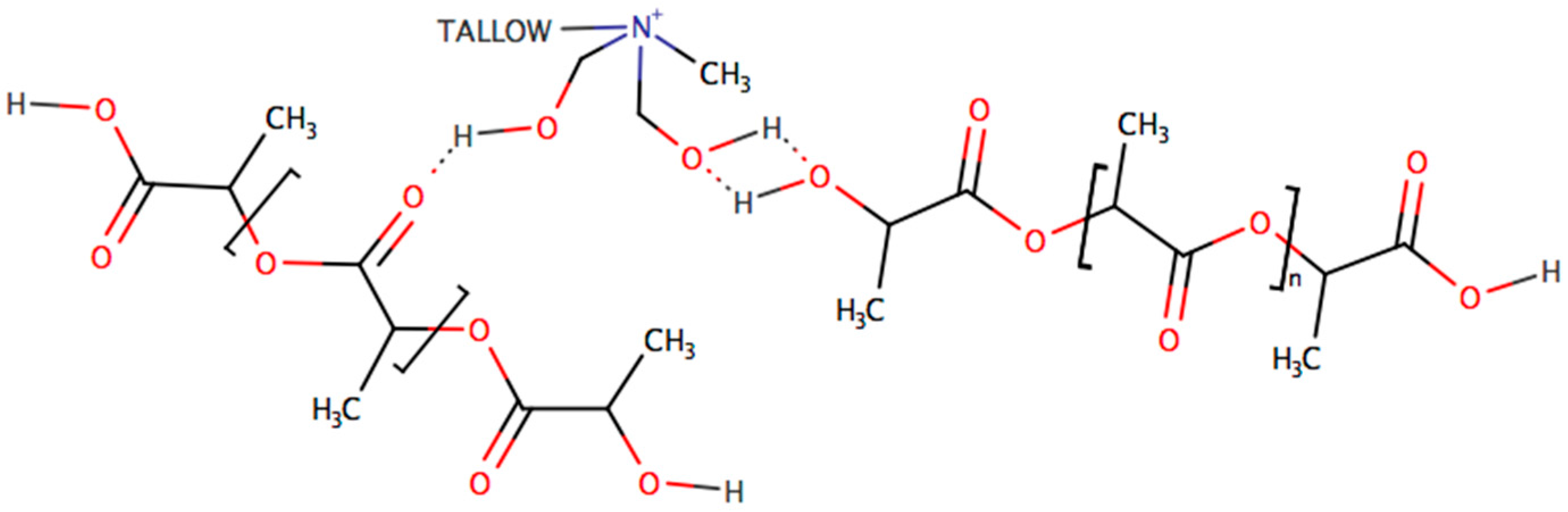
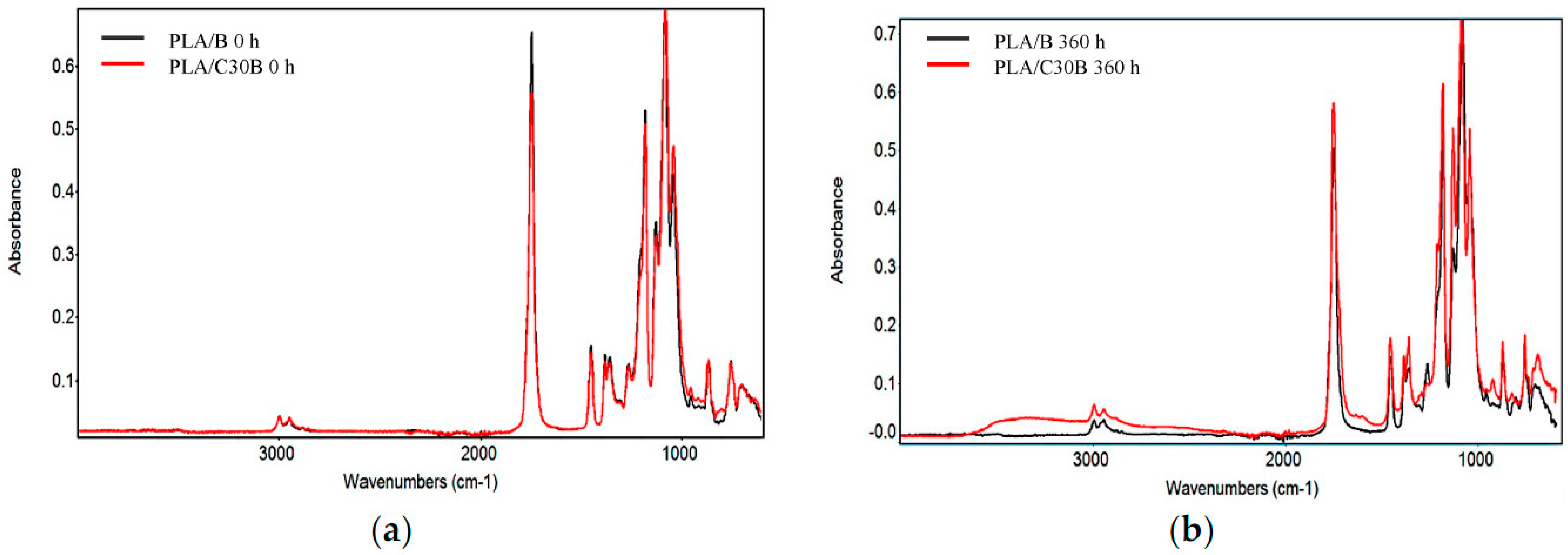
| Sample | Exposure Time (h) | Mn (kg·mol−1) | MW (kg·mol−1) | Mn/MW |
|---|---|---|---|---|
| PLA neat | – | 135.9 | 329.0 | 2.42 |
| PLA/B | 0 | 49.0 | 125.0 | 2.55 |
| 176 | 33.1 | 74.3 | 2.25 | |
| 360 | 16.4 | 33.3 | 2.03 | |
| 0 | 43.5 | 99.0 | 2.27 | |
| PLA/C30B | 176 | 31.9 | 69.7 | 2.18 |
| 360 | 11.3 | 22.8 | 1.72 |
| PLA/B (Temperature °C) | PLA/C30B (Temperature °C) | |||||
|---|---|---|---|---|---|---|
| Weight loss (%) | Mn 49.04 kg·mol−1 | Mn 33.06 kg·mol−1 | Mn 16.40 kg·mol−1 | Mn 43.49 kg·mol−1 | Mn 31.90 kg·mol−1 | Mn 11.32 kg·mol−1 |
| 10 | 334.81 | 329.57 | 318.05 | 328.77 | 313.80 | 307.60 |
| 20 | 344.94 | 341.42 | 336.15 | 340.99 | 332.84 | 323.65 |
| 30 | 350.69 | 348.06 | 344.94 | 347.77 | 341.89 | 334.43 |
| 40 | 355.03 | 352.93 | 350.65 | 352.6 | 347.68 | 342.34 |
| 50 | 358.70 | 357.01 | 355.18 | 356.55 | 352.16 | 348.42 |
| 60 | 361.95 | 360.68 | 359.01 | 360.02 | 355.99 | 353.50 |
| 70 | 364.81 | 364.22 | 362.51 | 363.31 | 359.45 | 358.23 |
| 80 | 367.45 | 367.93 | 366.20 | 366.96 | 363.13 | 363.28 |
| 90 | 371.42 | 372.38 | 371.42 | 372.72 | 369.33 | 370.93 |
| PLA/B | PLA/C30B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn (kg·mol−1) | Tg (°C) | Tc (°C) | Tm (°C) | ΔHc (J·g−1) | Xc (%) | Mn (kg·mol−1) | Tg (°C) | Tc (°C) | Tm (°C) | ΔHc (J·g−1) | Xc (%) |
| 49.0 | 59.86 | 119.13 | 156.83 | 35.96 | 22.75 | 43.5 | 58.27 | 122.77 | 155.85 | 33.14 | 18.92 |
| 33.1 | 59.22 | 112.66 | 157.32 | 37.52 | 23.93 | 31.9 | 58.07 | 115.72 | 155.86 | 38.24 | 23.23 |
| 16.4 | 55.08 | 104.02 | 154.56 | 38.16 | 27.75 | 11.3 | 48.49 | 100.12 | 150.46 | 39.13 | 27.01 |
| Sample | E′ (GPa) | Tg (° C) |
|---|---|---|
| PLA/B (Mn 49.04 kg·mol−1) | 3.43 | 65.95 |
| PLA/B (Mn 33.06 kg·mol−1) | 2.89 | 69.88 |
| PLA/B (Mn 16.40 kg·mol−1) | 2.82 | 65.27 |
| PLA/C30B (Mn 43.49 kg·mol−1) | 3.97 | 58.28 |
| PLA/C30B (Mn 31.90 kg·mol−1) | 3.43 | 66.51 |
| PLA/C30B (Mn 11.32 kg·mol−1) | 2.94 | 69.43 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Montes, W.M.; González-Sánchez, G.; Flores-Gallardo, S.G. Poly-Lactide/Exfoliated C30B Interactions and Influence on Thermo-Mechanical Properties Due to Artificial Weathering. Polymers 2016, 8, 154. https://doi.org/10.3390/polym8040154
Chávez-Montes WM, González-Sánchez G, Flores-Gallardo SG. Poly-Lactide/Exfoliated C30B Interactions and Influence on Thermo-Mechanical Properties Due to Artificial Weathering. Polymers. 2016; 8(4):154. https://doi.org/10.3390/polym8040154
Chicago/Turabian StyleChávez-Montes, Wendy Margarita, Guillermo González-Sánchez, and Sergio Gabriel Flores-Gallardo. 2016. "Poly-Lactide/Exfoliated C30B Interactions and Influence on Thermo-Mechanical Properties Due to Artificial Weathering" Polymers 8, no. 4: 154. https://doi.org/10.3390/polym8040154






