2H Solid-State NMR Analysis of the Dynamics and Organization of Water in Hydrated Chitosan
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Chitosan Films
2.3. Tensile Measurements
2.4. X-ray Diffraction (XRD)
2.5. Solid-State NMR Experiments
3. Results and Discussion
3.1. Crystallinity and Mechanical Properties of Hydrated Chitosan Films Based on XRD and Tensile Experiments
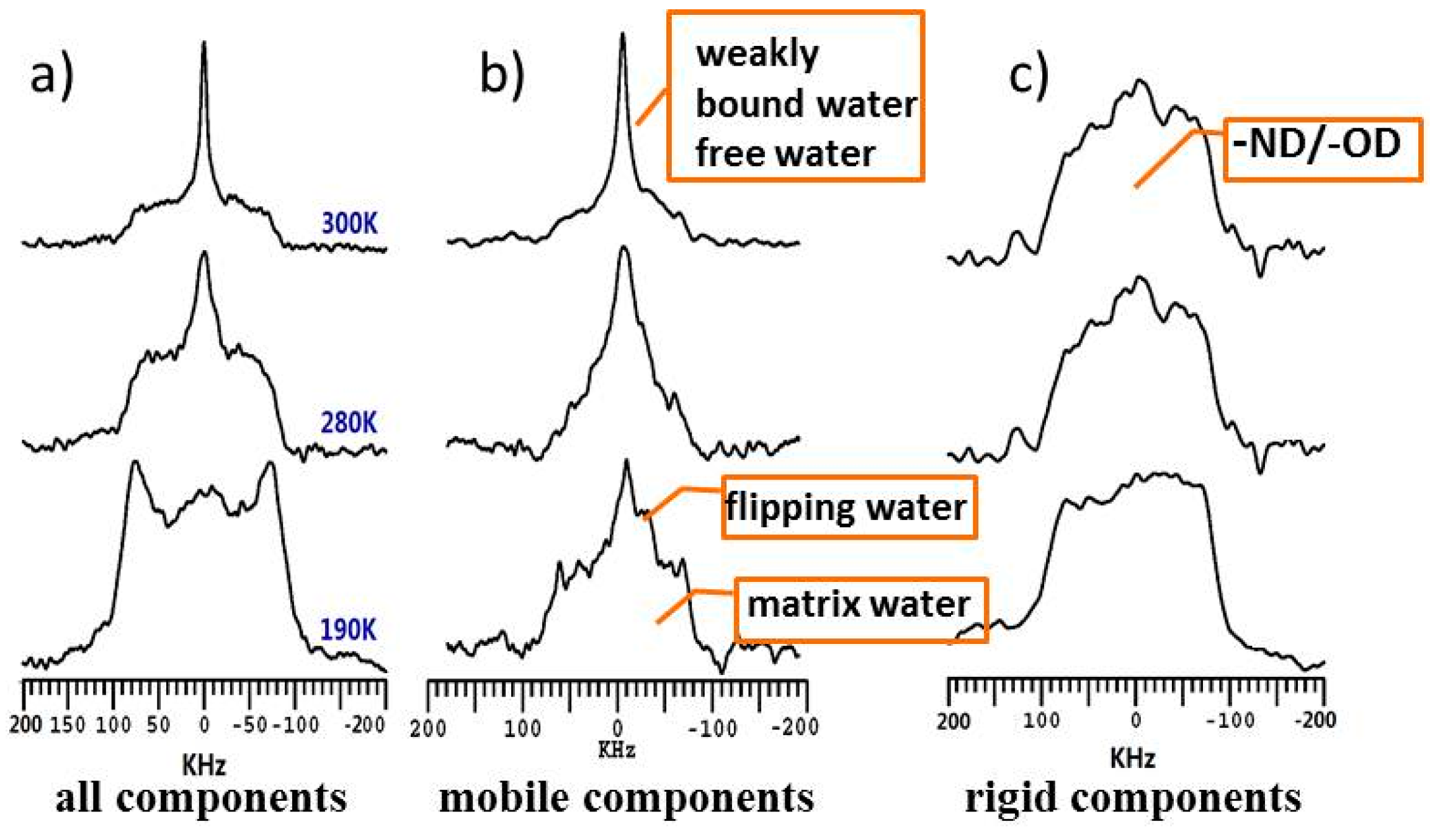
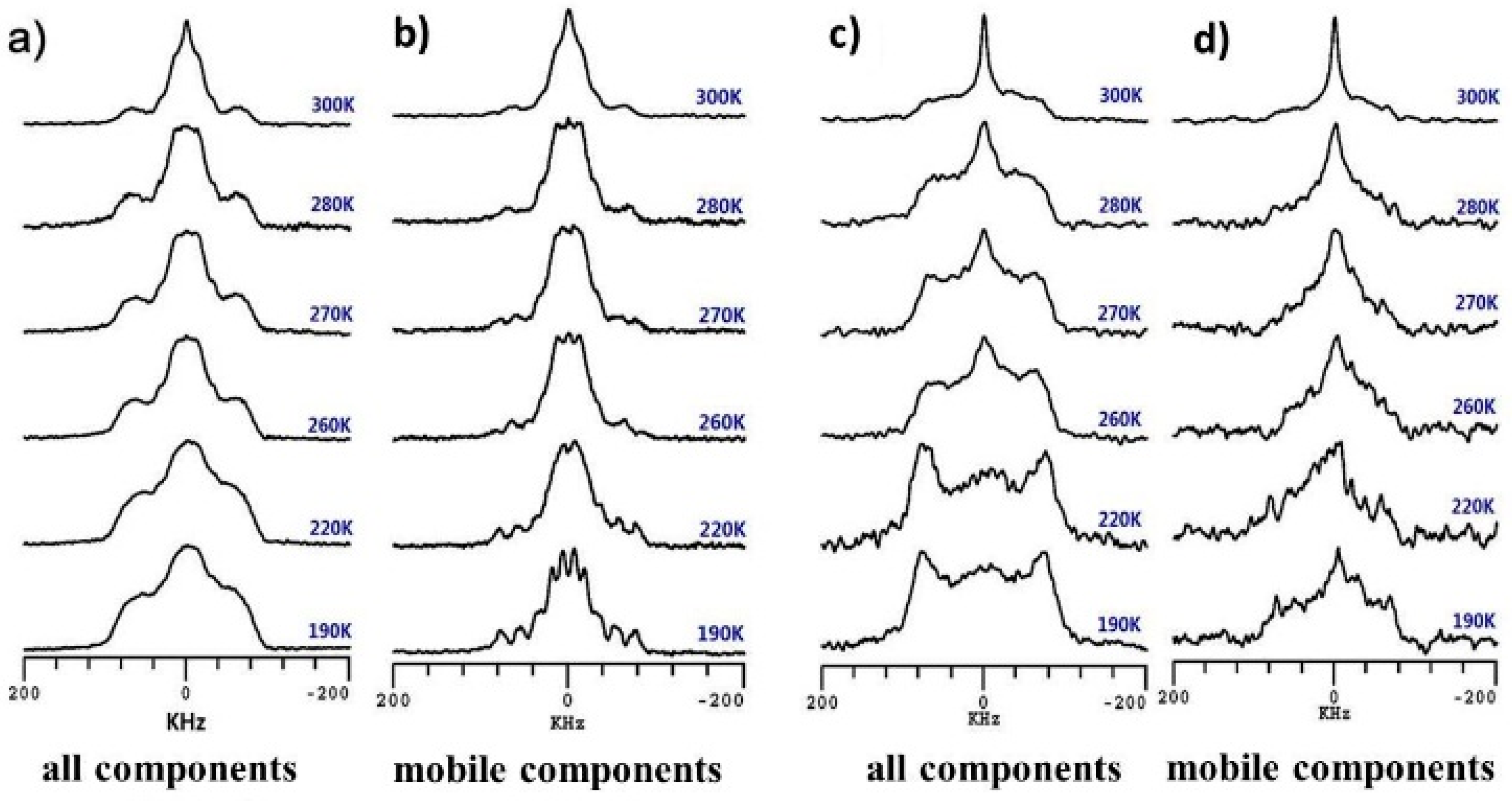
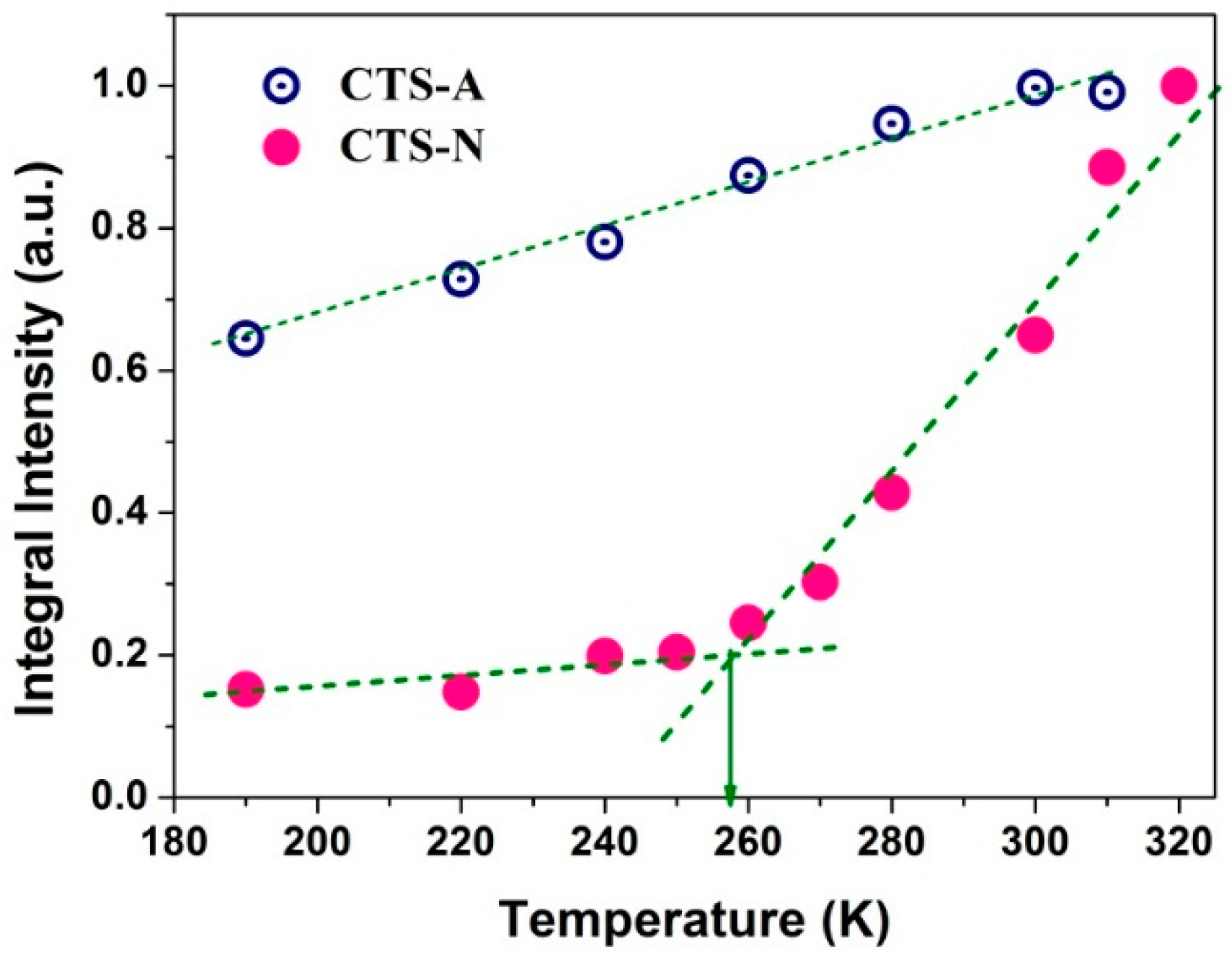
3.2. Dynamics and Different States of Water in Hydrated Chitosan before and after Neutralization Using Dynamic-Editing VT 2H NMR Experiments
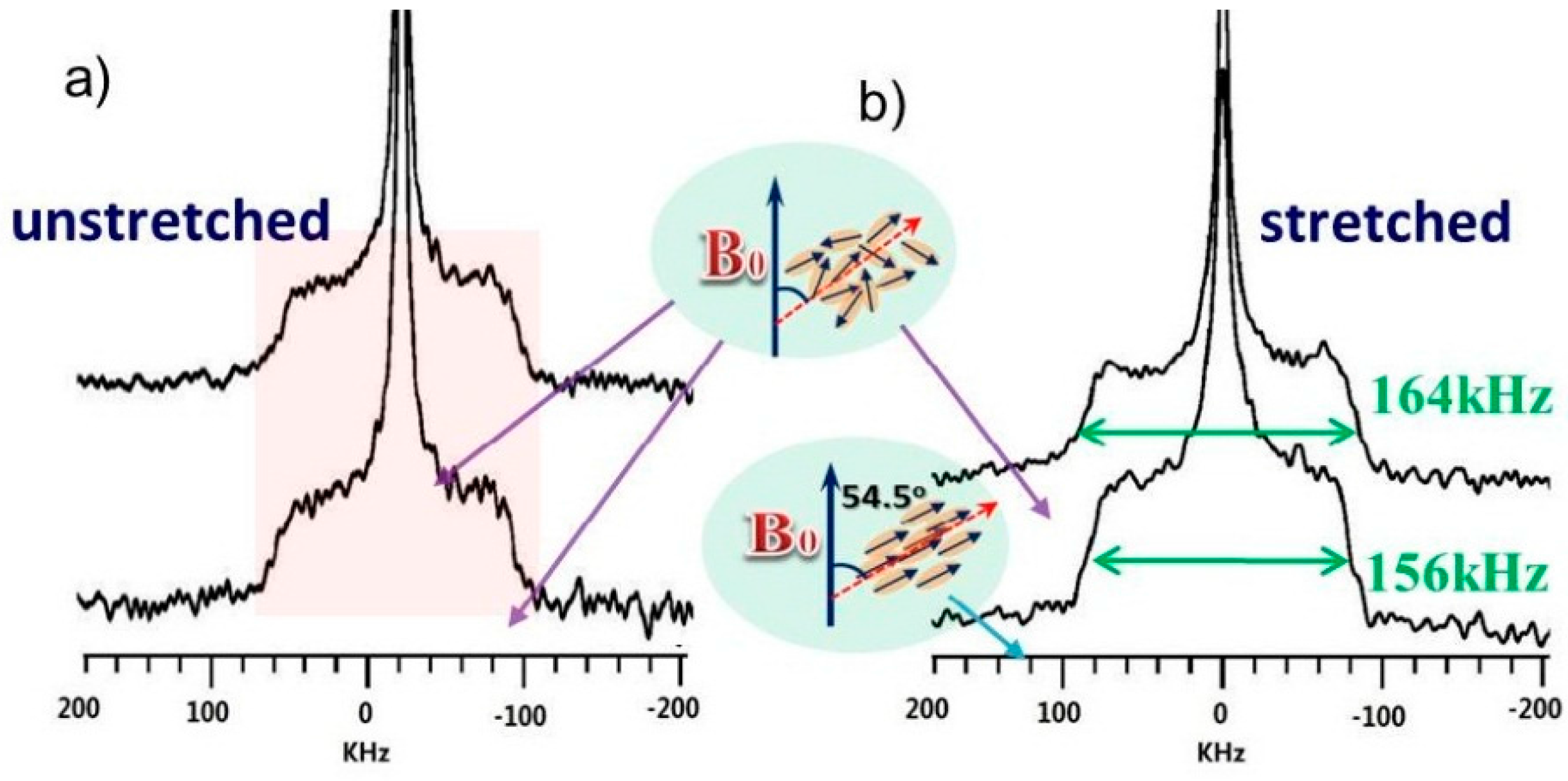
3.3. Effect of Stretching on the Hydrated and Neutralized Chitosan by 2H Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Levy, Y.; Onuchic, J.N. Water and proteins: A love-hate relationship. Proc. Natl. Acad. Sci. USA 2004, 101, 3325–3326. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hassanali, A.A.; Kao, Y.-T.; Zhong, D.; Singer, S.J. Hydration dynamics and time scales of coupled water−protein fluctuations. J. Am. Chem. Soc. 2007, 129, 3376–3382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, Z.; Vollrath, F. Relationships between supercontraction and mechanical properties of spider silk. Nat. Mater. 2005, 4, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, R.; Jin, H.-J.; Kaplan, D.L. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules 2004, 5, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [PubMed]
- Wang, F.; Zhang, R.; Wu, Q.; Chen, T.; Sun, P.; Shi, A.-C. Probing the nanostructure, interfacial interaction, and dynamics of chitosan-based nanoparticles by multiscale solid-state NMR. ACS Appl. Mater. Interfaces 2014, 6, 21397–21407. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.-Z.; Hu, X.; Yang, G.-H.; Zhang, Q.; Chen, F.; Fu, Q. Mechanically reinforced chitosan/cellulose nanocrystals composites with good transparency and biocompatibility. Chin. J. Polym. Sci. 2014, 33, 61–69. [Google Scholar] [CrossRef]
- Petridis, L.; O’Neill, H.M.; Johnsen, M.; Fan, B.; Schulz, R.; Mamontov, E.; Maranas, J.; Langan, P.; Smith, J.C. Hydration control of the mechanical and dynamical properties of cellulose. Biomacromolecules 2014, 15, 4152–4159. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.; Quinn, F.X.; McBrierty, V.J. Water in hydrogels. 2. A study of water in poly(hydroxyethyl methacrylate). Macromolecules 1988, 21, 3198–3204. [Google Scholar] [CrossRef]
- Pasqui, D.; de Cagna, M.; Barbucci, R. Polysaccharide-based hydrogels: The key role of water in affecting mechanical properties. Polymers 2012, 4, 1517–1534. [Google Scholar] [CrossRef]
- Ma, J.; Wang, H.; He, B.; Chen, J. A preliminary in vitro study on the fabrication and tissue engineering applications of a novel chitosan bilayer material as a scaffold of human neofetal dermal fibroblasts. Biomaterials 2001, 22, 331–336. [Google Scholar] [CrossRef]
- Rajabi-Siahboomi, A.R.; Bowtell, R.W.; Mansfield, P.; Davies, M.C.; Melia, C.D. Structure and behavior in hydrophilic matrix sustained release dosage forms: 4. Studies of water mobility and diffusion coefficients in the gel layer of HPMC tablets using NMR imaging. Pharm. Res. 1996, 13, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Kamata, Y.; Parnell, A.J.; Gutfreund, P.; Skoda, M.W.A.; Dennison, A.J.C.; Barker, R.; Mai, S.; Howse, J.R.; Ryan, A.J.; Torikai, N.; et al. Hydration and ordering of lamellar block copolymer films under controlled water vapor. Macromolecules 2014, 47, 8682–8690. [Google Scholar] [CrossRef]
- González-Campos, J.B.; Prokhorov, E.; Luna-Bárcenas, G.; Fonseca-García, A.; Sanchez, I.C. Dielectric relaxations of chitosan: The effect of water on the α-relaxation and the glass transition temperature. J. Polym. Sci. Part B: Polym. Phys. 2009, 47, 2259–2271. [Google Scholar] [CrossRef]
- Huang, W.; Krishnaji, S.; Tokareva, O.R.; Kaplan, D.; Cebe, P. Influence of water on protein transitions: Thermal analysis. Macromolecules 2014, 47, 8098–8106. [Google Scholar] [CrossRef]
- Lin, C.; Gitsov, I. Synthesis and physical properties of reactive amphiphilic hydrogels based on poly(p-chloromethylstyrene) and poly(ethylene glycol): Effects of composition and molecular architecture. Macromolecules 2010, 43, 3256–3267. [Google Scholar] [CrossRef]
- Li, B.; Xu, L.; Wu, Q.; Chen, T.; Sun, P.; Jin, Q.; Ding, D.; Wang, X.; Xue, G.; Shi, A.C. Various types of hydrogen bonds, their temperature dependence and water-polymer interaction in hydrated poly(acrylic acid) as revealed by H-1 solid-state NMR spectroscopy. Macromolecules 2007, 40, 5776–5786. [Google Scholar] [CrossRef]
- Vogel, A.; Katzka, C.P.; Waldmann, H.; Arnold, K.; Brown, M.F.; Huster, D. Lipid modifications of a Ras peptide exhibit altered packing and mobility versus host membrane as detected by 2H solid-state NMR. J. Am. Chem. Soc. 2005, 127, 12263–12272. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.Q.; Wang, X.S.; Li, C.G.; Santiago-Miranda, A.N.; Pielak, G.J.; Tian, F. In situ structural characterization of a recombinant protein in native escherichia coli membranes with solid-state magic-angle-spinning NMR. J. Am. Chem. Soc. 2011, 133, 12370–12373. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, V.K.; Keeler, E.G.; Ong, T.-C.; Craigen, K.N.; Penzel, S.; Wren, J.E.C.; Kroeker, S.; Griffin, R.G. Structural insights into bound water in crystalline amino acids: Experimental and theoretical 17O NMR. J. Phys. Chem. B 2015, 119, 8024–8036. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.C.; Sue, H.-J.; Bremner, T.; Blümel, J. High-temperature steam-treatment of PBI, PEEK, and PEKK polymers with H2O and D2O: A solid-state NMR study. Polymer 2014, 55, 4577–4585. [Google Scholar] [CrossRef]
- Fei, Z.; Zhao, D.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J.; Antonijevic, S.; Bodenhausen, G. A synthetic zwitterionic water channel: Characterization in the solid state by X-ray crystallography and NMR spectroscopy. Angew. Chemie 2005, 117, 5866–5871. [Google Scholar] [CrossRef]
- Radloff, D.; Boeffel, C.; Spiess, H.W. Cellulose and cellulose/poly(vinyl alcohol) blends. 2. Water organization revealed by solid-state NMR spectroscopy. Macromolecules 1996, 29, 1528–1534. [Google Scholar] [CrossRef]
- VanderSchee, C.R.; Ooms, K.J. Investigating water interactions with collagen using H-2 multiple quantum filtered NMR spectroscopy to provide insights into the source of double quantum filtered signal in tissue. J. Phys. Chem. B 2014, 118, 3491–3497. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, J.L.; Murthy, N.S.; Samulski, E.T. Deuterium NMR studies of water in oriented nylon 6 fibers. Macromolecules 1996, 29, 5551–5557. [Google Scholar] [CrossRef]
- Schartel, B.; Wendling, J.; Wendorff, J.H. Cellulose/poly(vinyl alcohol) blends. 1. Influence of miscibility and water content on relaxations. Macromolecules 1996, 29, 1521–1527. [Google Scholar] [CrossRef]
- Maghami, G.G.; Roberts, G.A.F. Evaluation of the viscometric constants for chitosan. Die Makromol. Chemie 1988, 189, 195–200. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Poly. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Torchia, D.A. The measurement of proton-enhanced carbon-13 T1 values by a method which suppresses artifacts. J. Magn. Resonance (1969) 1978, 30, 613–616. [Google Scholar] [CrossRef]
- Saito, H.; Tabeta, R.; Ogawa, K. High-resolution solid-state carbon-13 NMR study of chitosan and its salts with acids: Conformational characterization of polymorphs and helical structures as viewed from the conformation-dependent carbon-13 chemical shifts. Macromolecules 1987, 20, 2424–2430. [Google Scholar] [CrossRef]
- Chowdhury, S.; Madsen, L.A.; Frazier, C.E. Probing alignment and phase behavior in intact wood cell walls using 2H NMR spectroscopy. Biomacromolecules 2012, 13, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Pake, G.E. Nuclear resonance absorption in hydrated crystals: Fine structure of the proton line. J. Chem. Phys. 1948, 16, 327–336. [Google Scholar] [CrossRef]
- Okuyama, K.; Noguchi, K.; Miyazawa, T.; Yui, T.; Ogawa, K. Molecular and crystal structure of hydrated chitosan. Macromolecules 1997, 30, 5849–5855. [Google Scholar] [CrossRef]
- Quinn, F.X.; Kampff, E.; Smyth, G.; McBrierty, V.J. Water in hydrogels. 1. A study of water in poly(N-vinyl-2-pyrrolidone/methyl methacrylate) copolymer. Macromolecules 1988, 21, 3191–3198. [Google Scholar] [CrossRef]
- Cerveny, S.; Colmenero, J.; Alegría, A. Dielectric investigation of the low-temperature water dynamics in the poly(vinyl methyl ether)/H2O system. Macromolecules 2005, 38, 7056–7063. [Google Scholar] [CrossRef]
- Van den Dries, I.J.; van Dusschoten, D.; Hemminga, M.A. Mobility in maltose−water glasses studied with 1H NMR. J. Phys. Chem. B 1998, 102, 10483–10489. [Google Scholar] [CrossRef]
- Simmons, A.H.; Michal, C.A.; Jelinski, L.W. Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science 1996, 271, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Spiess, H.W. Deuteron NMR—A new tool for studying chain mobility and orientation in polymers. In Characterization of Polymers in the Solid State I: Part A: NMR and Other Spectroscopic Methods Part B: Mechanical Methods; Kaush, H.H., Zachman, H.G., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 1985; pp. 23–58. [Google Scholar]



| Samples | Young’s modulus (GPa) | Yield stress (MPa) | Break strength (MPa) | Strain of break % (Elongation) |
|---|---|---|---|---|
| CTS-A | 2.86 ± 0.05 | – | 84.3 ± 2.5 | 6.1 ± 0.8 |
| CTS-N | 2.43 ± 0.08 | 81.4 ± 1.6 | 98.7 ± 1.6 | 35.8 ± 5.3 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhang, R.; Chen, T.; Sun, P. 2H Solid-State NMR Analysis of the Dynamics and Organization of Water in Hydrated Chitosan. Polymers 2016, 8, 149. https://doi.org/10.3390/polym8040149
Wang F, Zhang R, Chen T, Sun P. 2H Solid-State NMR Analysis of the Dynamics and Organization of Water in Hydrated Chitosan. Polymers. 2016; 8(4):149. https://doi.org/10.3390/polym8040149
Chicago/Turabian StyleWang, Fenfen, Rongchun Zhang, Tiehong Chen, and Pingchuan Sun. 2016. "2H Solid-State NMR Analysis of the Dynamics and Organization of Water in Hydrated Chitosan" Polymers 8, no. 4: 149. https://doi.org/10.3390/polym8040149






