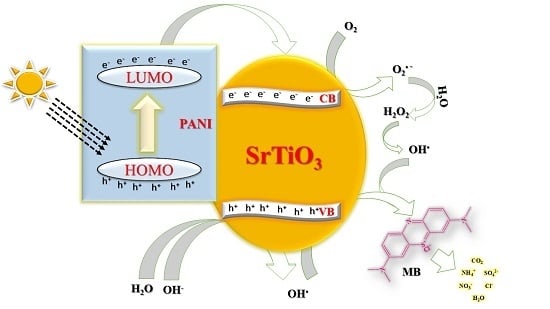
SrTiO3 Nanocube-Doped Polyaniline Nanocomposites with Enhanced Photocatalytic Degradation of Methylene Blue under Visible Light
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of SrTiO3 Nanocubes
2.3. Preparation of Polyaniline
2.4. Preparation of PANI-SrTiO3 Nanocomposite
2.5. Characterization Techniques
2.6. Measurement of Photocatalytic Activities
3. Result and Discussion
3.1. Morphological Analysis of Nanocomposites
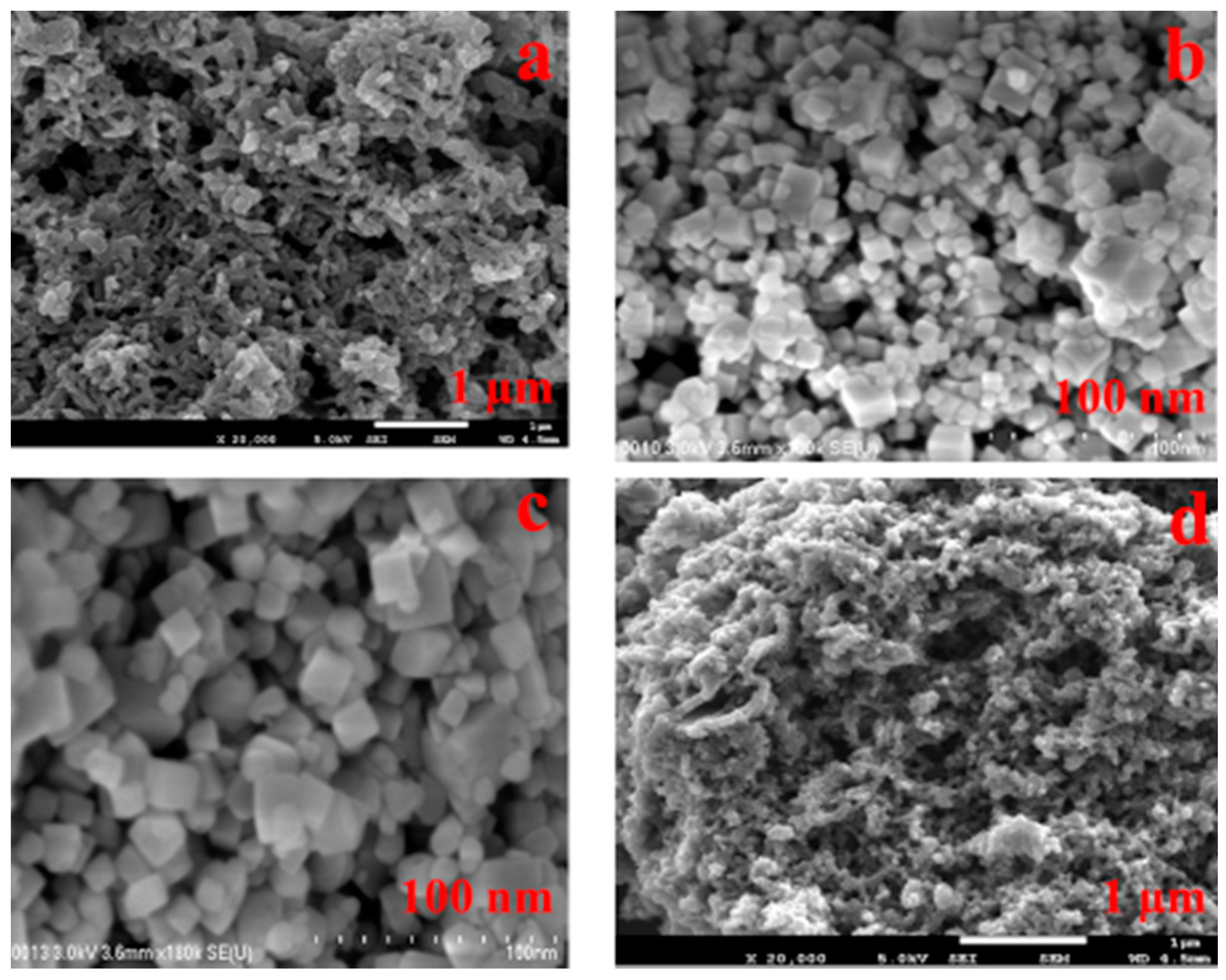
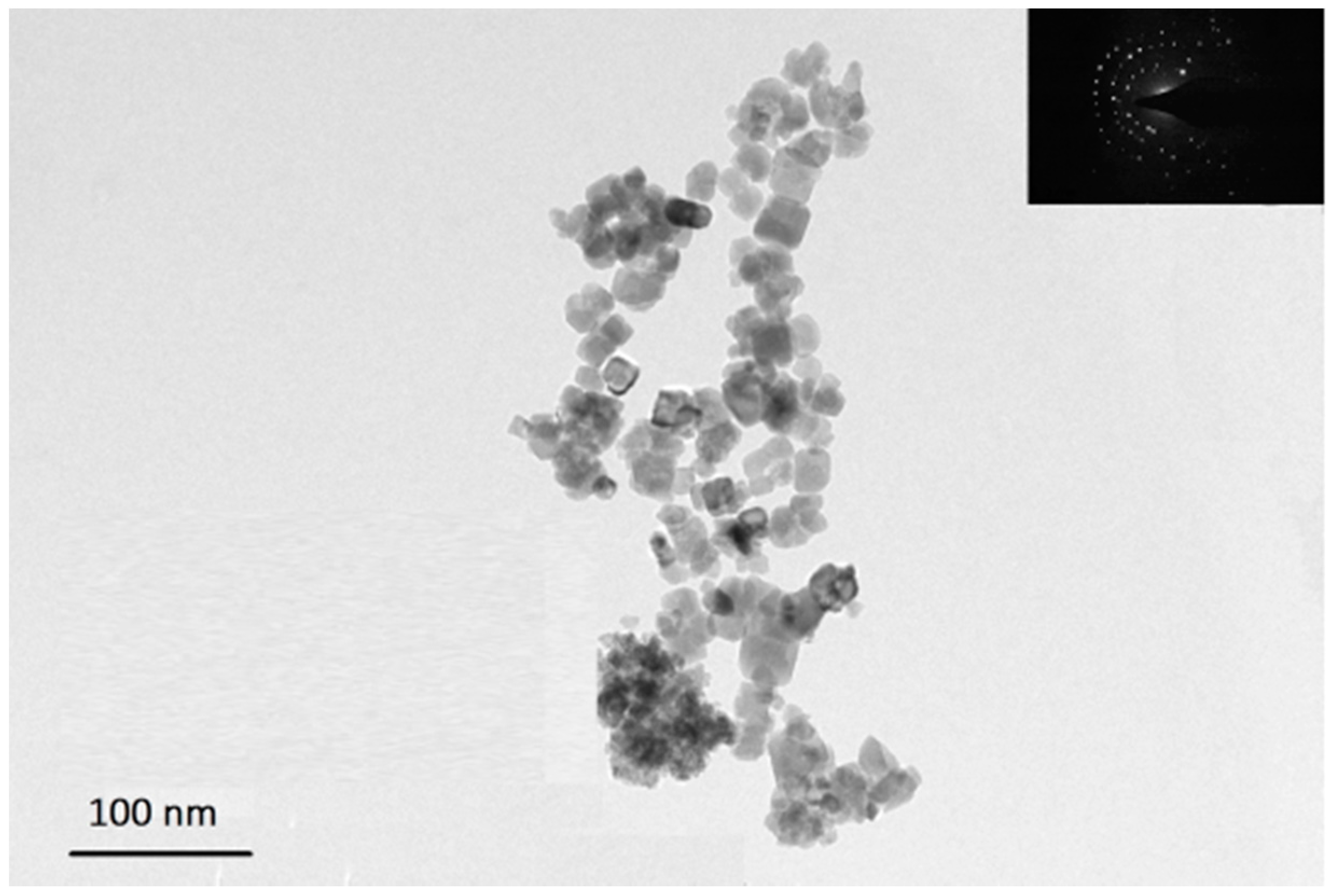
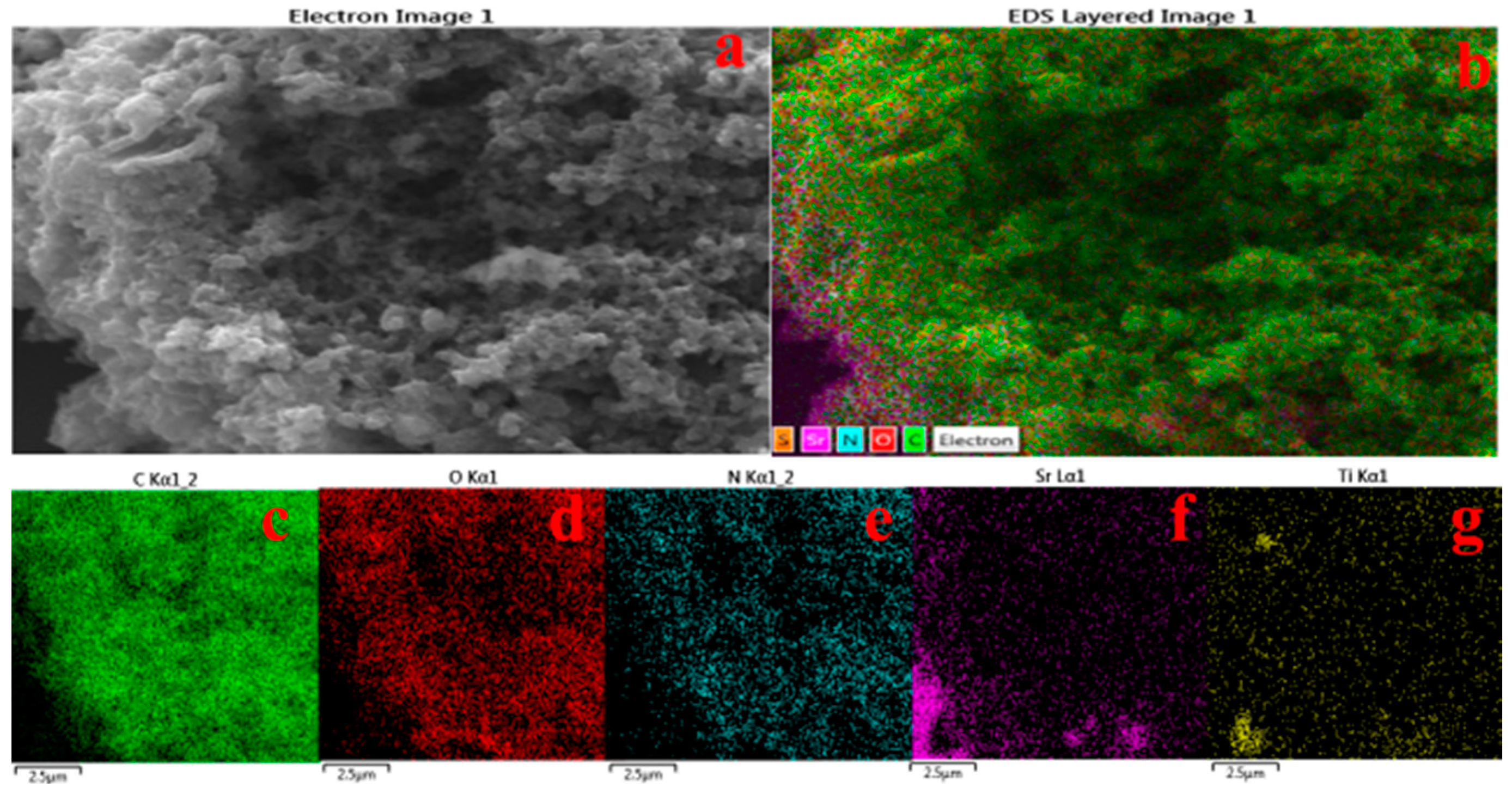
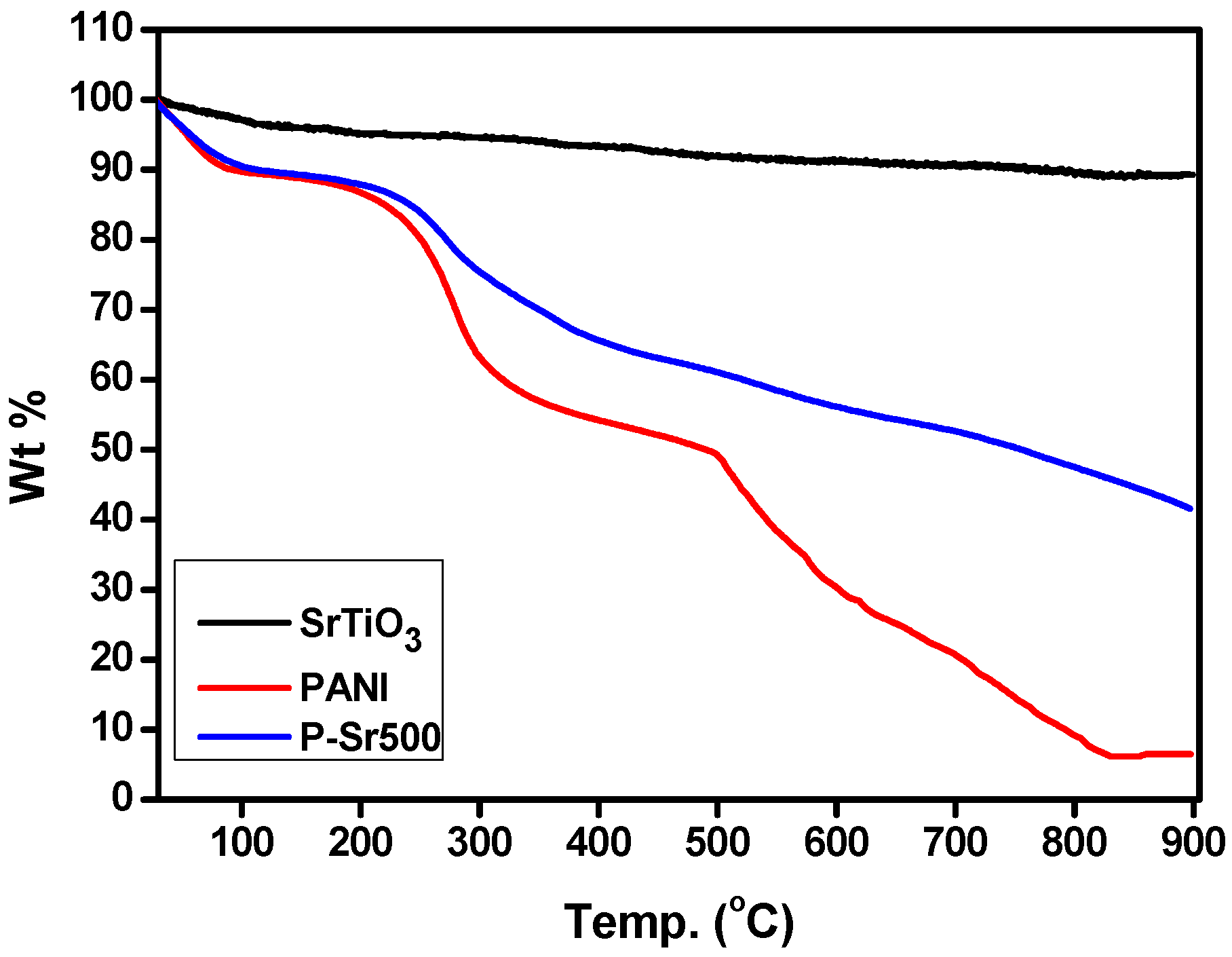
3.2. Thermal Analysis
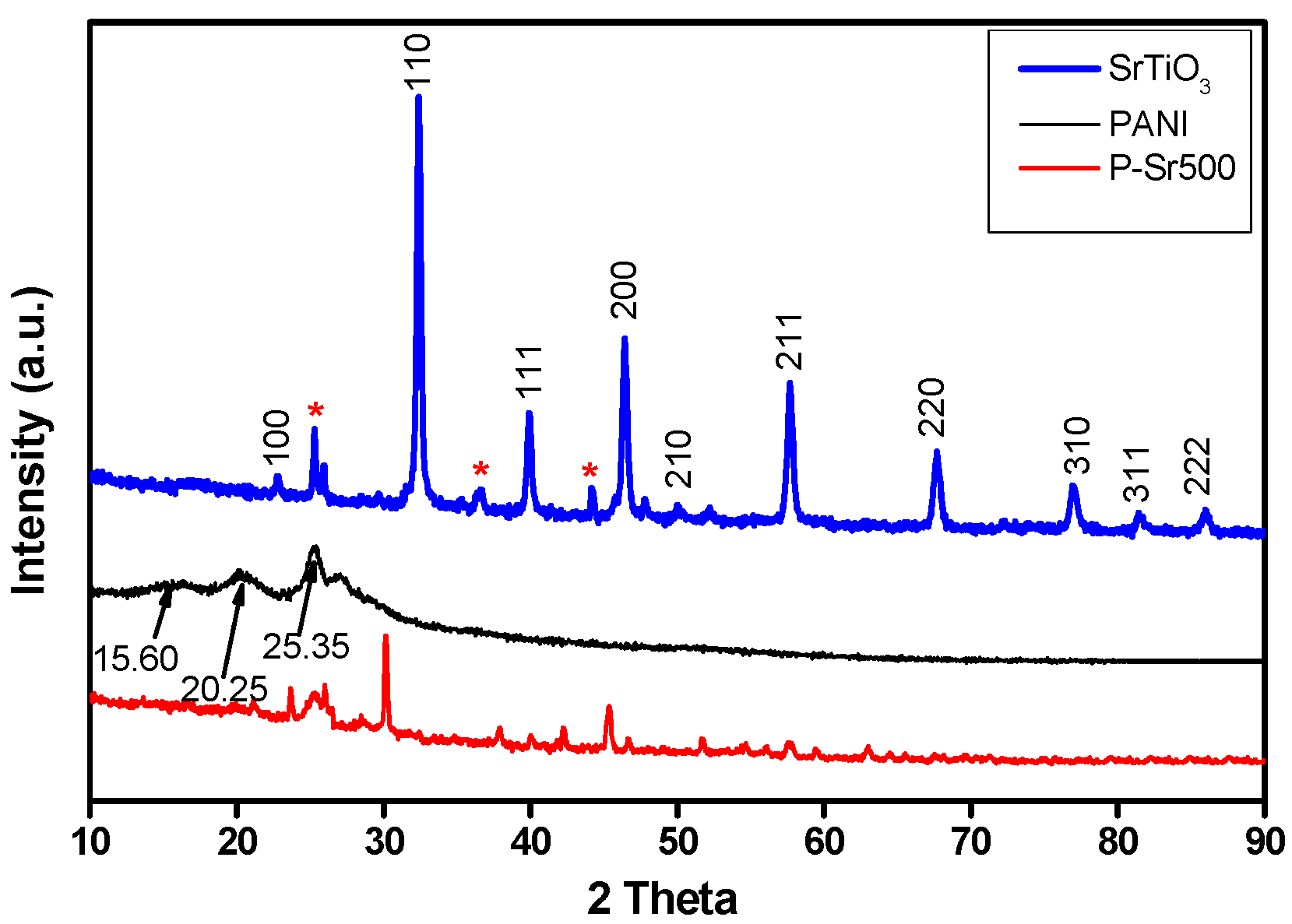
3.3. XRD Analysis
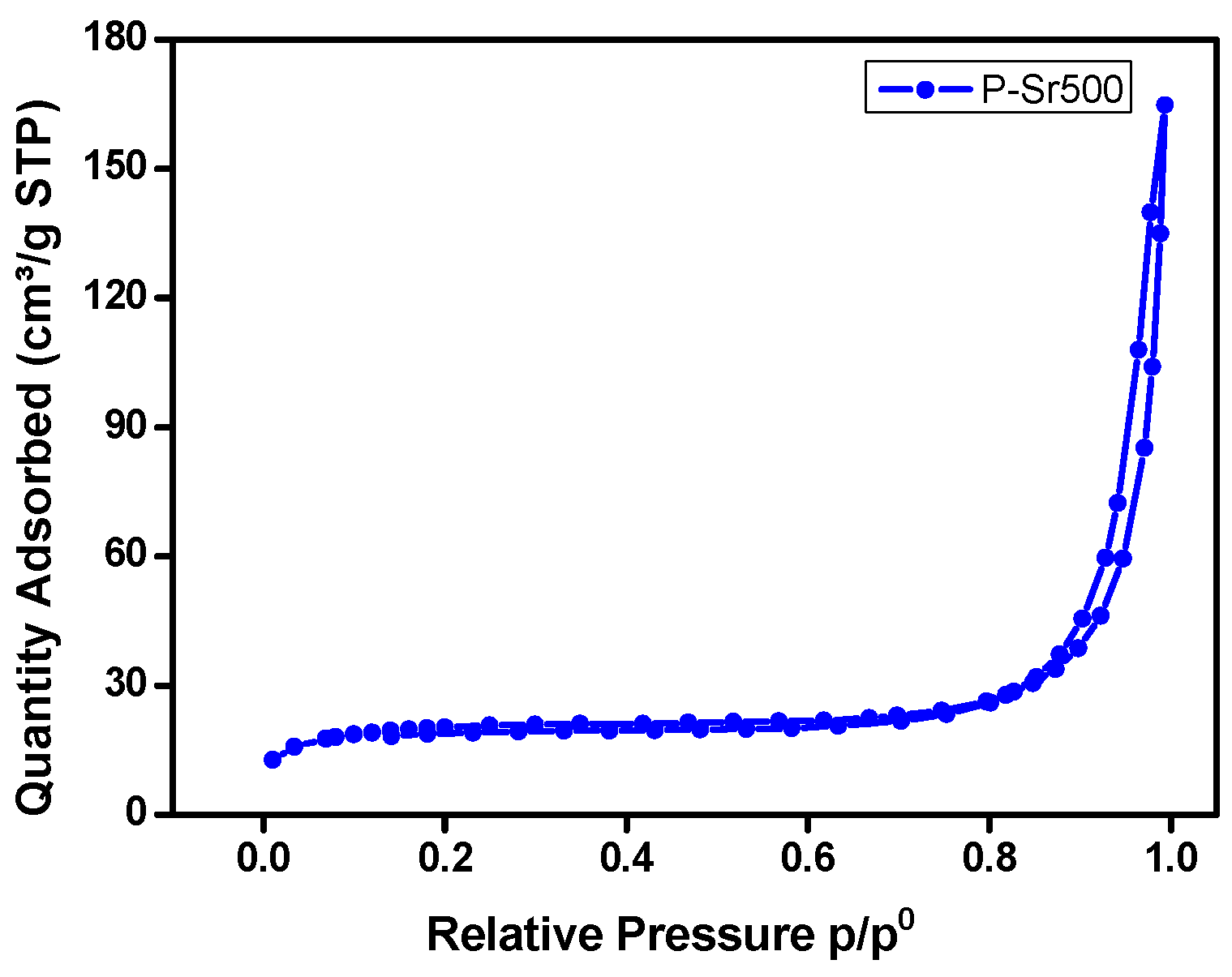
3.4. BET Analysis
3.5. UV–Vis Analysis

3.6. FTIR Analysis

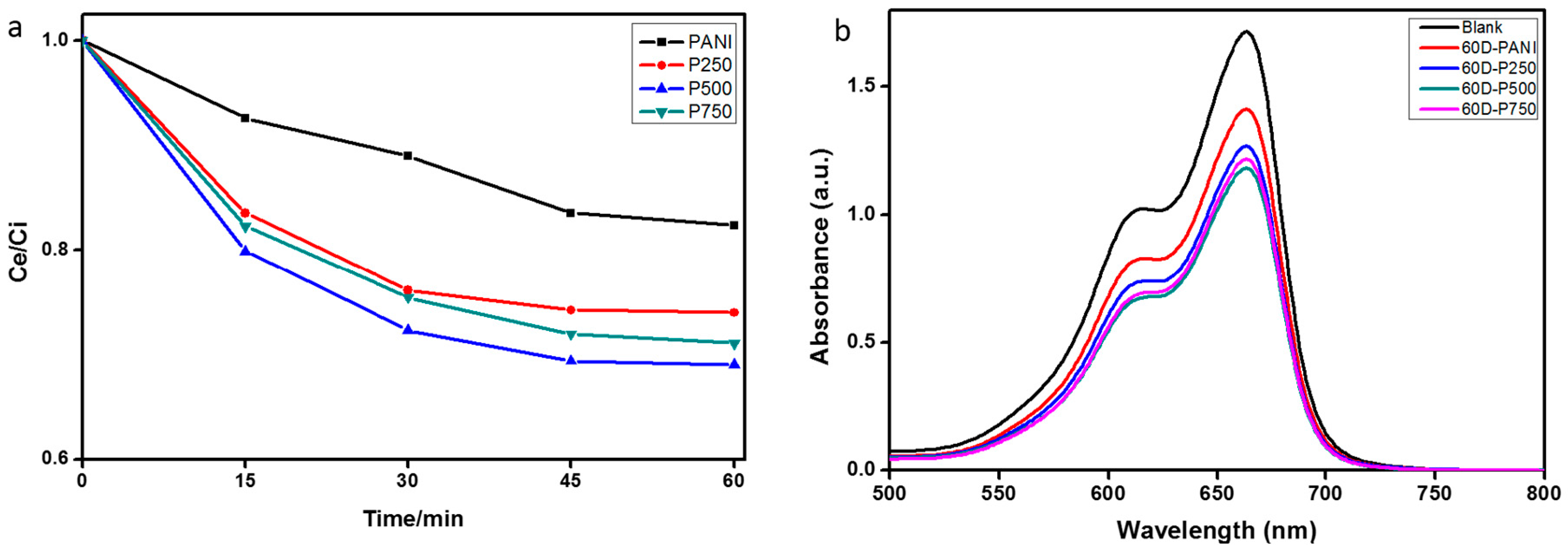
3.7. Photocatalytic Degradation of MB under Sunlight Irradiation
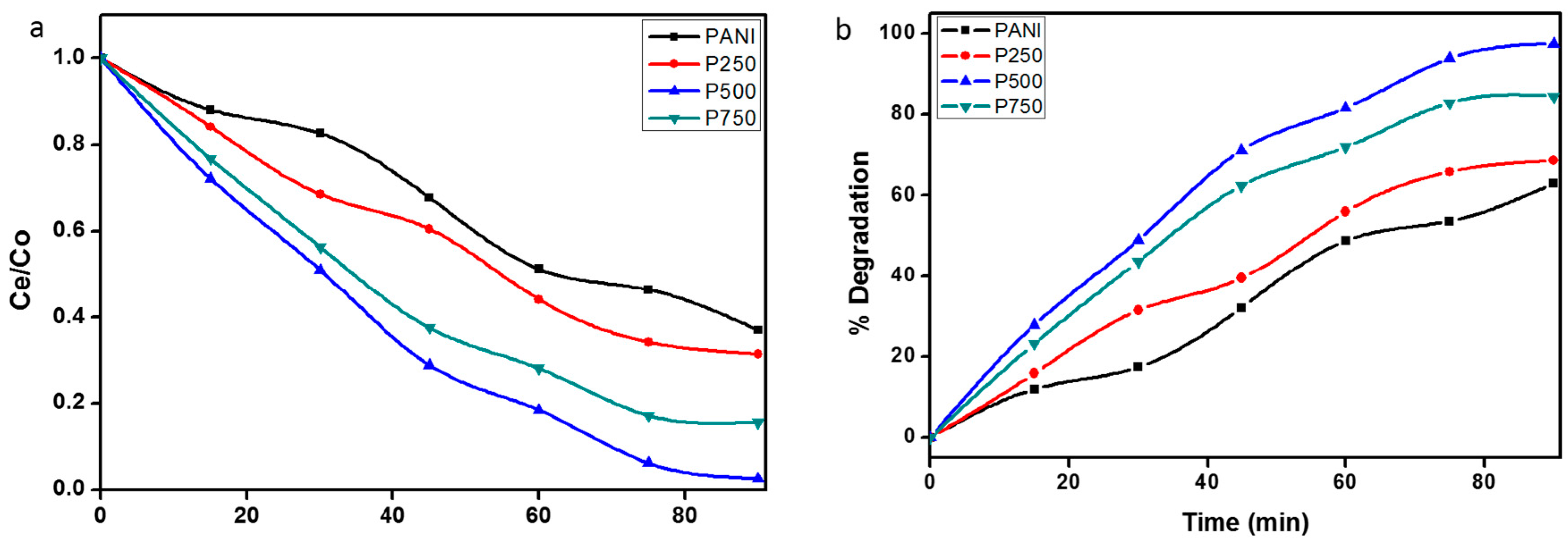
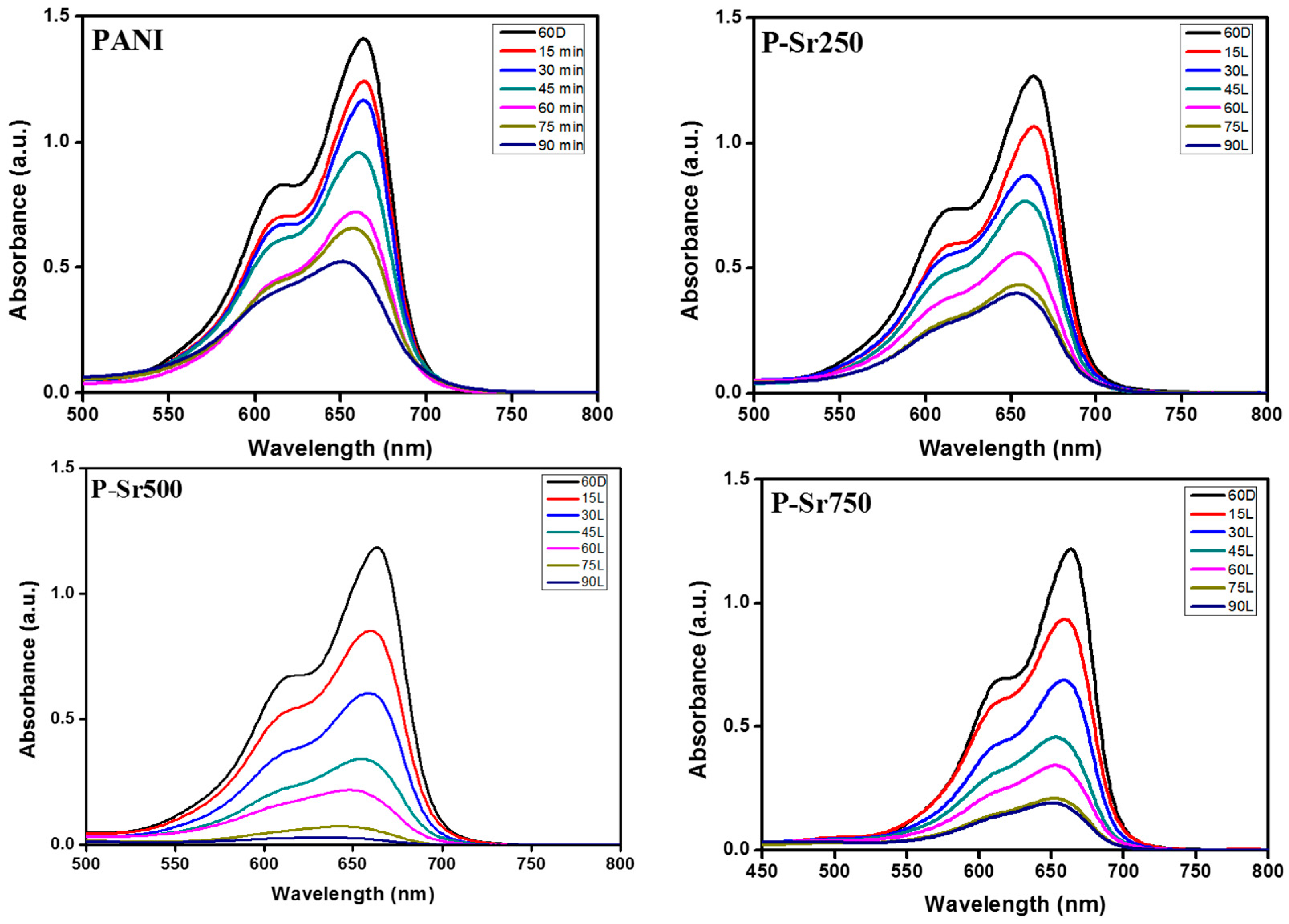
3.8. Comparison of Photocatalytic Efficiencies
| Catalyst | Dye degraded | Conc. of dye (mg/L−1) | Amount of catalyst (mg/mL) | % Degradation | Degradation time (min) | Source of light | Reference |
|---|---|---|---|---|---|---|---|
| Chitosan/PANI/Co3O4 | Methylene Blue | 10 | 0.3 | 88 | 180 | UV | [10] |
| Graphene/ZnS | Methylene Blue | 10 | 0.2 | 95 | 180 | UV | [29] |
| PANI/CdO | Methylene Blue | 4.8 | 0.4 | 97 | 240 | Sunlight | [47] |
| TiO2 | Methylene Blue | 5 | – | 88 | 120 | LED | [27] |
| PANI/ZnO | Methylene Blue | 3.2 | 0.4 | 99 | 300 | Sunlight | [52] |
| PANI/FZNPs | Methylene Blue | 3.2 | 0.25 | 99.47 | 300 | Sunlight | [53] |
| AgBr/ZnO | Methylene Blue | 10 | 1 | 87 | 240 | LED | [54] |
| PANI/ZnO | Methylene Blue | 10 | 300 | 82 | 60 | Visible light | [55] |
| FeOOH-LDO | Methylene Blue | 3 | 35 | 95 | 180 | Visible light | [56] |
| PANI/SrTiO3 | Methylene Blue | 10 | 0.3 | 97 | 90 | Visible light | This work |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chan, S.H.S.; Yeong Wu, T.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Huang, S.T.; Lee, W.W.; Chang, J.L.; Huang, W.S.; Chou, S.Y.; Chen, C.C. Hydrothermal synthesis of SrTiO3 nanocubes: Characterization, photocatalytic activities, and degradation pathway. J. Taiwan Inst. Chem. Eng. 2014, 45, 1927–1936. [Google Scholar] [CrossRef]
- Parida, K.; Sahu, S.; Reddy, K.; Sahoo, P. A kinetic, thermodynamic, and mechanistic approach toward adsorption of methylene blue over water-washed manganese nodule leached residues. Ind. Eng. Chem. Res. 2010, 50, 843–848. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sharma, A. Kinetics and thermodynamics of methylene blue adsorption on neem (azadirachta indica) leaf powder. Dyes Pigment. 2005, 65, 51–59. [Google Scholar] [CrossRef]
- Gouamid, M.; Ouahrani, M.; Bensaci, M. Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using date palm leaves. Energy Proced. 2013, 36, 898–907. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Karthikeyan, C.; Kim, A.R.; Yoo, D.J. One-pot green synthesis of reduced graphene oxide (RGO)/Fe3O4 nanocomposites and its catalytic activity toward methylene blue dye degradation. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 136, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chern, J.M. Kinetics of photocatalytic decomposition of methylene blue. Ind. Eng. Chem. Res. 2006, 45, 6450–6457. [Google Scholar] [CrossRef]
- Adams, V.; Marley, J.; McCarroll, C. Prilocaine induced methaemoglobinaemia in a medically compromised patient. Was this an inevitable consequence of the dose administered? Br. Dent. J. 2007, 203, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Riaz, U.; Ashraf, S.; Aqib, M. Microwave-assisted degradation of acid orange using a conjugated polymer, polyaniline, as catalyst. Arab. J. Chem. 2014, 7, 79–86. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Ismail, F.H.; Shahid, M.M.; Huang, N.M. Synthesis of chitosan grafted-polyaniline/Co3O4 nanocube nanocomposites and their photocatalytic activity toward methylene blue dye degradation. RSC Adv. 2015, 5, 83857–83867. [Google Scholar] [CrossRef]
- Zhou, M.; Han, D.; Liu, X.; Ma, C.; Wang, H.; Tang, Y.; Huo, P.; Shi, W.; Yan, Y.; Yang, J. Enhanced visible light photocatalytic activity of alkaline earth metal ions-doped CdSe/RGO photocatalysts synthesized by hydrothermal method. Appl. Catal. B 2015, 172, 174–184. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2009, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Clifford, J.N.; Martínez-Ferrero, E.; Viterisi, A.; Palomares, E. Sensitizer molecular structure-device efficiency relationship in dye sensitized solar cells. Chem. Soc. Rev. 2011, 40, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Liang, J.; Sumathy, K. Review on dye-sensitized solar cells (DSSCs): Fundamental concepts and novel materials. Renew. Sustain. Energy Rev. 2012, 16, 5848–5860. [Google Scholar] [CrossRef]
- Kazuma, E.; Tatsuma, T. Photoinduced reversible changes in morphology of plasmonic ag nanorods on TiO2 and application to versatile photochromism. Chem. Commun. 2012, 48, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Tatsuma, T. Morphological changes and multicolor photochromism of Ag nanoparticles deposited on single-crystalline TiO2 surfaces. Adv. Mater. 2007, 19, 2802–2806. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Wang, F.; Min, S.; Han, Y.; Feng, L. Visible-light-induced photocatalytic degradation of methylene blue with polyaniline-sensitized composite photocatalysts. Superlattices Microstruct. 2010, 48, 170–180. [Google Scholar] [CrossRef]
- Tang, J.; Durrant, J.R.; Klug, D.R. Mechanism of photocatalytic water splitting in TiO2. Reaction of water with photoholes, importance of charge carrier dynamics, and evidence for four-hole chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. [Google Scholar] [CrossRef] [PubMed]
- Demirors, A.F.; Imhof, A. BaTiO3, SrTiO3, CaTiO3, and BaxSr1−x TiO3 particles: A general approach for monodisperse colloidal perovskites. Chem. Mater. 2009, 21, 3002–3007. [Google Scholar] [CrossRef]
- Nakashima, K.; Kera, M.; Fujii, I.; Wada, S. A new approach for the preparation of SrTiO3 nanocubes. Ceram. Int. 2013, 39, 3231–3234. [Google Scholar] [CrossRef]
- Shen, P.; Lofaro, J.C.; Woerner, W.R.; White, M.G.; Su, D.; Orlov, A. Photocatalytic activity of hydrogen evolution over Rh doped SrTiO3 prepared by polymerizable complex method. Chem. Eng. J. 2013, 223, 200–208. [Google Scholar] [CrossRef]
- Lee, W.W.; Chung, W.-H.; Huang, W.-S.; Lin, W.-C.; Lin, W.-Y.; Jiang, Y.-R.; Chen, C.-C. Photocatalytic activity and mechanism of nano-cubic barium titanate prepared by a hydrothermal method. J. Taiwan Inst. Chem. Eng. 2013, 44, 660–669. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.; Zhou, C.; Hu, Y.; Fu, D.; Liu, J.; Wang, Q. Higher visible photocatalytic activities of nitrogen doped In2TiO5 sensitized by carbon nitride. J. Hazard. Mater. 2011, 190, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Riaz, U.; Ashraf, S.; Kashyap, J. Enhancement of photocatalytic properties of transitional metal oxides using conducting polymers: A mini review. Mater. Res. Bull. 2015, 71, 75–90. [Google Scholar] [CrossRef]
- Chen, S.; Wei, Z.; Qi, X.; Dong, L.; Guo, Y.-G.; Wan, L.; Shao, Z.; Li, L. Nanostructured polyaniline-decorated Pt/C@PANI core–shell catalyst with enhanced durability and activity. J. Am. Chem. Soc. 2012, 134, 13252–13255. [Google Scholar] [CrossRef] [PubMed]
- Autin, O.; Romelot, C.; Rust, L.; Hart, J.; Jarvis, P.; MacAdam, J.; Parsons, S.A.; Jefferson, B. Evaluation of a UV-light emitting diodes unit for the removal of micropollutants in water for low energy advanced oxidation processes. Chemosphere 2013, 92, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Sadek, A.; Wlodarski, W.; Shin, K.; Kaner, R.; Kalantar-Zadeh, K. A polyaniline/WO3 nanofiber composite-based ZnO/64° YX LiNbO3 saw hydrogen gas sensor. Synth. Met. 2008, 158, 29–32. [Google Scholar] [CrossRef]
- Golsheikh, A.M.; Lim, H.N.; Zakaria, R.; Huang, N.M. Sonochemical synthesis of reduced graphene oxide uniformly decorated with hierarchical zns nanospheres and its enhanced photocatalytic activities. RSC Adv. 2015, 5, 12726–12735. [Google Scholar] [CrossRef]
- Sadek, A.; Wlodarski, W.; Shin, K.; Kaner, R.B.; Kalantar-Zadeh, K. A layered surface acoustic wave gas sensor based on a polyaniline/In2O3 nanofibre composite. Nanotechnology 2006, 17, 4488. [Google Scholar] [CrossRef]
- Sengodu, P.; Deshmukh, A.D. Conducting polymers and their inorganic composites for advanced Li-ion batteries: A review. RSC Adv. 2015, 5, 42109–42130. [Google Scholar] [CrossRef]
- Hu, Z.; Zu, L.; Jiang, Y.; Lian, H.; Liu, Y.; Li, Z.; Chen, F.; Wang, X.; Cui, X. High specific capacitance of polyaniline/mesoporous manganese dioxide composite using KI-H2SO4 electrolyte. Polymers 2015, 7, 1939–1953. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Shih, H.-K.; Chen, Y.-H.; Chu, Y.-L.; Cheng, C.-C.; Chang, F.-C.; Zhu, C.-Y.; Kuo, S.-W. Photo-crosslinking of pendent uracil units provides supramolecular hole injection/transport conducting polymers for highly efficient light-emitting diodes. Polymers 2015, 7, 804–818. [Google Scholar] [CrossRef]
- Luzzati, S.; Basso, M.; Catellani, M.; Brabec, C.; Gebeyehu, D.; Sariciftci, N. Photo-induced electron transfer from a dithieno thiophene-based polymer to TiO2. Thin Solid Films 2002, 403, 52–56. [Google Scholar] [CrossRef]
- Gustafsson, G.; Cao, Y.; Treacy, G.; Klavetter, F.; Colaneri, N.; Heeger, A. Flexible light-emitting diodes made from soluble conducting polymers. Nature 1992, 357, 477–479. [Google Scholar] [CrossRef]
- Jang, S.; Han, M.; Im, S. Preparation and characterization of conductive polyaniline/silica hybrid composites prepared by sol–gel process. Synth. Metals 2000, 110, 17–23. [Google Scholar] [CrossRef]
- Pron, A.; Rannou, P. Processible conjugated polymers: From organic semiconductors to organic metals and superconductors. Prog. Polym. Sci. 2002, 27, 135–190. [Google Scholar] [CrossRef]
- Guo, L.; Luo, H.; Gao, J.; Guo, L.; Yang, J. Microwave hydrothermal synthesis of barium titanate powders. Mater. Lett. 2006, 60, 3011–3014. [Google Scholar] [CrossRef]
- Rahy, A.; Yang, D.J. Synthesis of highly conductive polyaniline nanofibers. Mater. Lett. 2008, 62, 4311–4314. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Lu, L.; Yang, X.; Wu, X. Preparation of TiO2/polyaniline nanocomposite from a lyotropic liquid crystalline solution. Synth. Metals 2009, 159, 2525–2529. [Google Scholar] [CrossRef]
- Sing, K.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, R.; Rouquerol, J.; Siemieniewska, T. Physical and biophysical chemistry division commission on colloid and surface chemistry including catalysis. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar]
- Yu, L.; Ruan, H.; Zheng, Y.; Li, D. A facile solvothermal method to produce ZnS quantum dots-decorated graphene nanosheets with superior photoactivity. Nanotechnology 2013, 24, 375601. [Google Scholar] [CrossRef] [PubMed]
- Xian, T.; Yang, H.; Di, L.; Ma, J.; Zhang, H.; Dai, J. Photocatalytic reduction synthesis of SrTiO3-graphene nanocomposites and their enhanced photocatalytic activity. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhao, Z.; Zhu, W.B.; Coker, E.N.; Li, B.; Zheng, M.; Yu, W.; Fan, H.; Sun, Z. Oxygen vacancy enhanced photocatalytic activity of pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Zhao, Y.; Liu, J.; Tang, S.; Feng, W. Synthesis of PANI nanostructures with various morphologies from fibers to micromats to disks doped with salicylic acid. Synth. Metals 2010, 160, 2008–2014. [Google Scholar] [CrossRef]
- Gülce, H.; Eskizeybek, V.; Haspulat, B.; Sarı, F.; Gülce, A.; Avcı, A. Preparation of a new polyaniline/CdO nanocomposite and investigation of its photocatalytic activity: Comparative study under UV light and natural sunlight irradiation. Ind. Eng. Chem. Res. 2013, 52, 10924–10934. [Google Scholar] [CrossRef]
- Adireddy, S.; Lin, C.; Cao, B.; Zhou, W.; Caruntu, G. Solution-based growth of monodisperse cube-like BaTiO3 colloidal nanocrystals. Chemi. Mater. 2010, 22, 1946–1948. [Google Scholar] [CrossRef]
- Gopalakrishnamurthy, H.; Rao, M.S.; Kutty, T.N. Thermal decomposition of titanyl oxalates—I: Barium titanyl oxalate. J. Inorg. Nucl. Chem. 1975, 37, 891–898. [Google Scholar] [CrossRef]
- Last, J.T. Infrared-absorption studies on barium titanate and related materials. Phys. Rev. 1957, 105, 1740. [Google Scholar] [CrossRef]
- Subramanian, E.; Subbulakshmi, S.; Murugan, C. Inter-relationship between nanostructures of conducting polyaniline and the photocatalytic methylene blue dye degradation efficiencies of its hybrid composites with anatase TiO2. Mater. Res. Bull. 2014, 51, 128–135. [Google Scholar] [CrossRef]
- Eskizeybek, V.; Sarı, F.; Gülce, H.; Gülce, A.; Avcı, A. Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under uv and natural sun lights irradiations. Appl. Catal. B 2012, 119, 197–206. [Google Scholar] [CrossRef]
- Kant, S.; Kalia, S.; Kumar, A. A novel nanocomposite of polyaniline and a novel nanocomposite of polyaniline and Fe0.01Ni0.01Zn0.98O: Photocatalytic, electrical and antibacterial properties. J. Alloy. Compd. 2013, 578, 249–256. [Google Scholar] [CrossRef]
- Dai, K.; Lv, J.; Lu, L.; Liu, Q.; Zhu, G.; Li, D. Synthesis of micro-nano heterostructure AgBr/ZnO composite for advanced visible light photocatalysis. Mater. Lett. 2014, 130, 5–8. [Google Scholar] [CrossRef]
- Olad, A.; Nosrati, R. Preparation, characterization, and photocatalytic activity of polyaniline/ZnO nanocomposite. Res. Chem. Intermed. 2012, 38, 323–336. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Pan, G.; Qian, P.; Ni, Z. Photocatalytic degradation of methylene blue with a nanocomposite system: Synthesis, photocatalysis and degradation pathways. Phys. Chem. Chem. Phys. 2015, 17, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahabuddin, S.; Muhamad Sarih, N.; Mohamad, S.; Joon Ching, J. SrTiO3 Nanocube-Doped Polyaniline Nanocomposites with Enhanced Photocatalytic Degradation of Methylene Blue under Visible Light. Polymers 2016, 8, 27. https://doi.org/10.3390/polym8020027
Shahabuddin S, Muhamad Sarih N, Mohamad S, Joon Ching J. SrTiO3 Nanocube-Doped Polyaniline Nanocomposites with Enhanced Photocatalytic Degradation of Methylene Blue under Visible Light. Polymers. 2016; 8(2):27. https://doi.org/10.3390/polym8020027
Chicago/Turabian StyleShahabuddin, Syed, Norazilawati Muhamad Sarih, Sharifah Mohamad, and Juan Joon Ching. 2016. "SrTiO3 Nanocube-Doped Polyaniline Nanocomposites with Enhanced Photocatalytic Degradation of Methylene Blue under Visible Light" Polymers 8, no. 2: 27. https://doi.org/10.3390/polym8020027
APA StyleShahabuddin, S., Muhamad Sarih, N., Mohamad, S., & Joon Ching, J. (2016). SrTiO3 Nanocube-Doped Polyaniline Nanocomposites with Enhanced Photocatalytic Degradation of Methylene Blue under Visible Light. Polymers, 8(2), 27. https://doi.org/10.3390/polym8020027