Investigations on Green Blends Comprising Biodegradable Polymer and Ionic Liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Blend Preparation
2.2. Instruments and Experiments
3. Results and Discussion
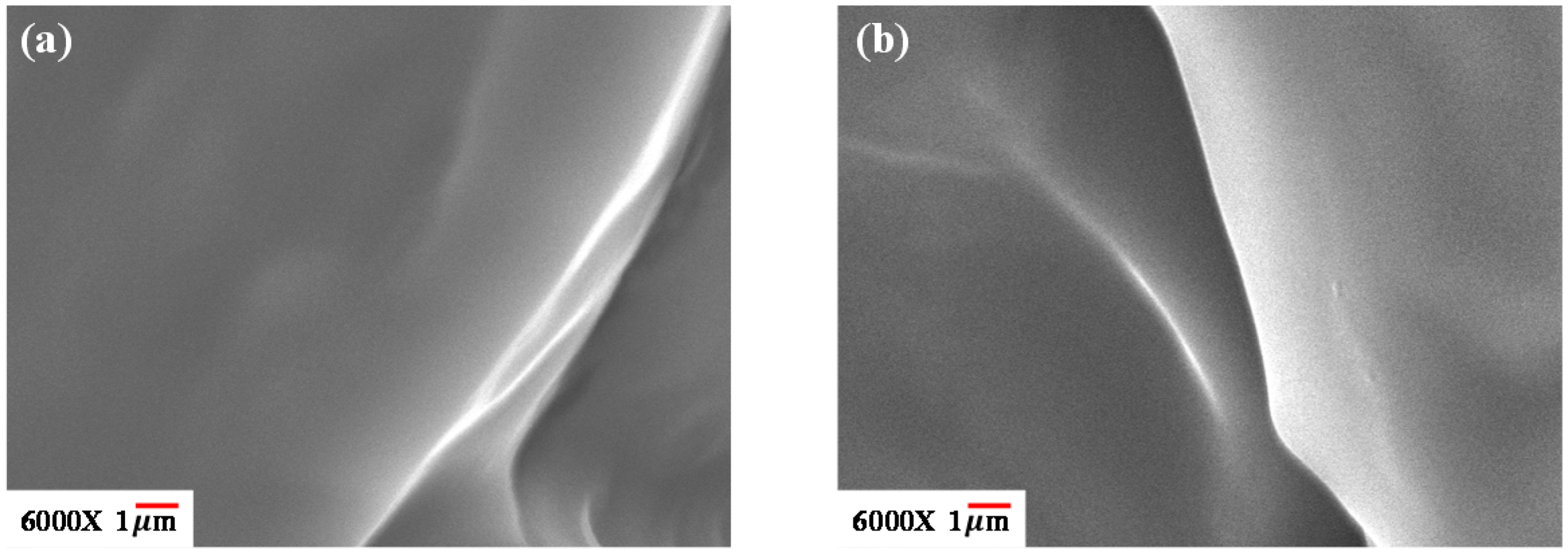
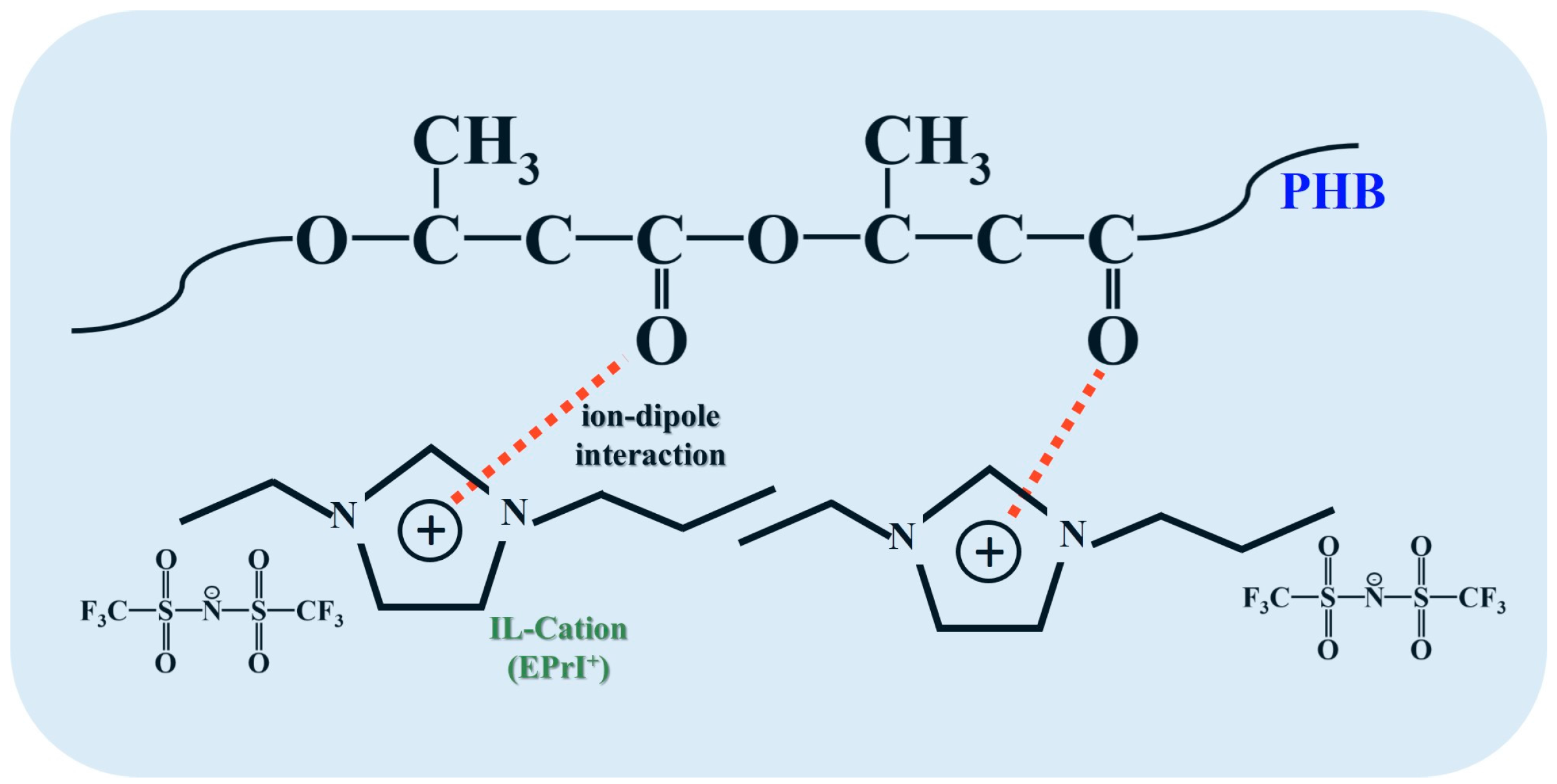
3.1. Phase Morphology and Thermal Properties of [EPrI][TFSI]/PHB Blends
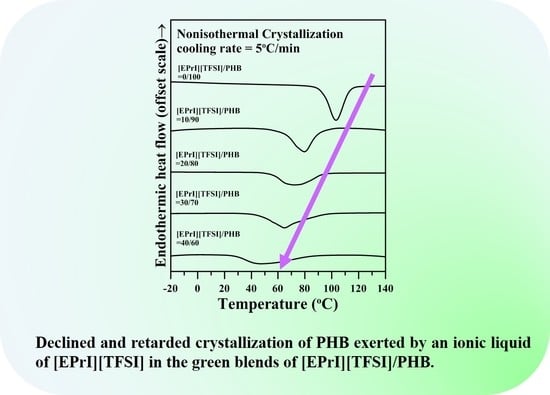
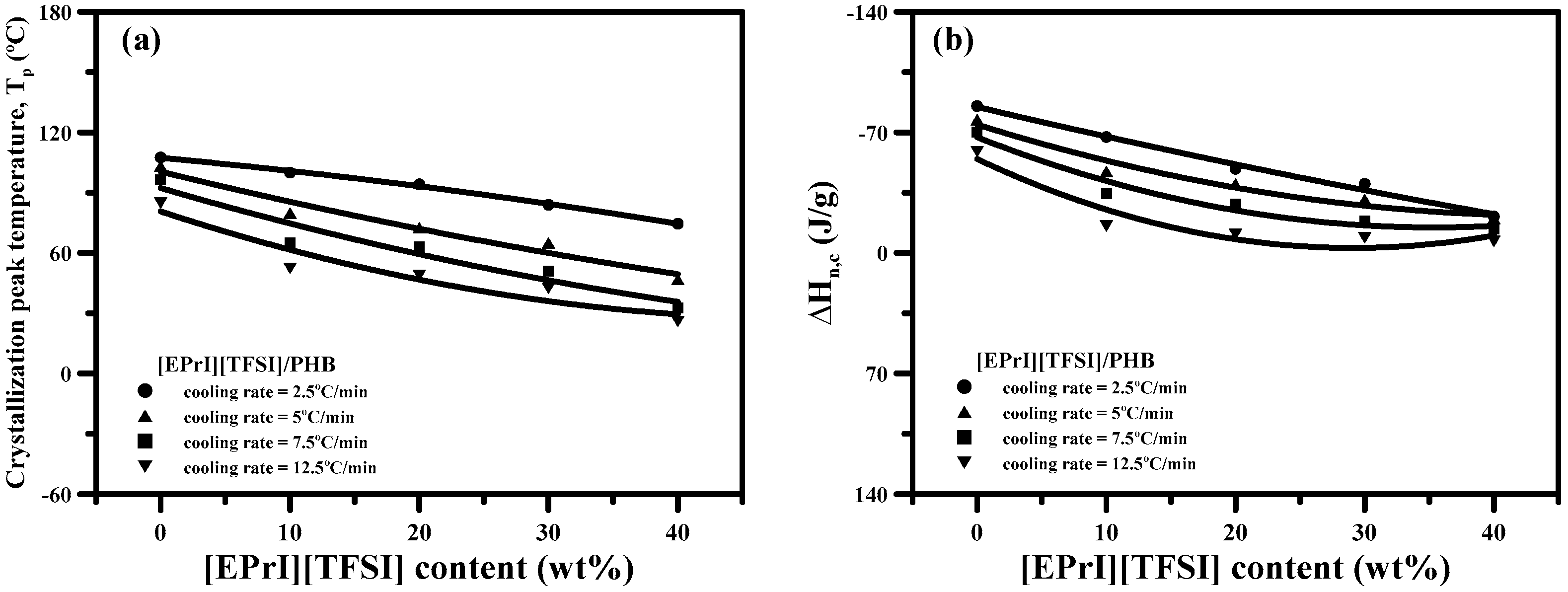
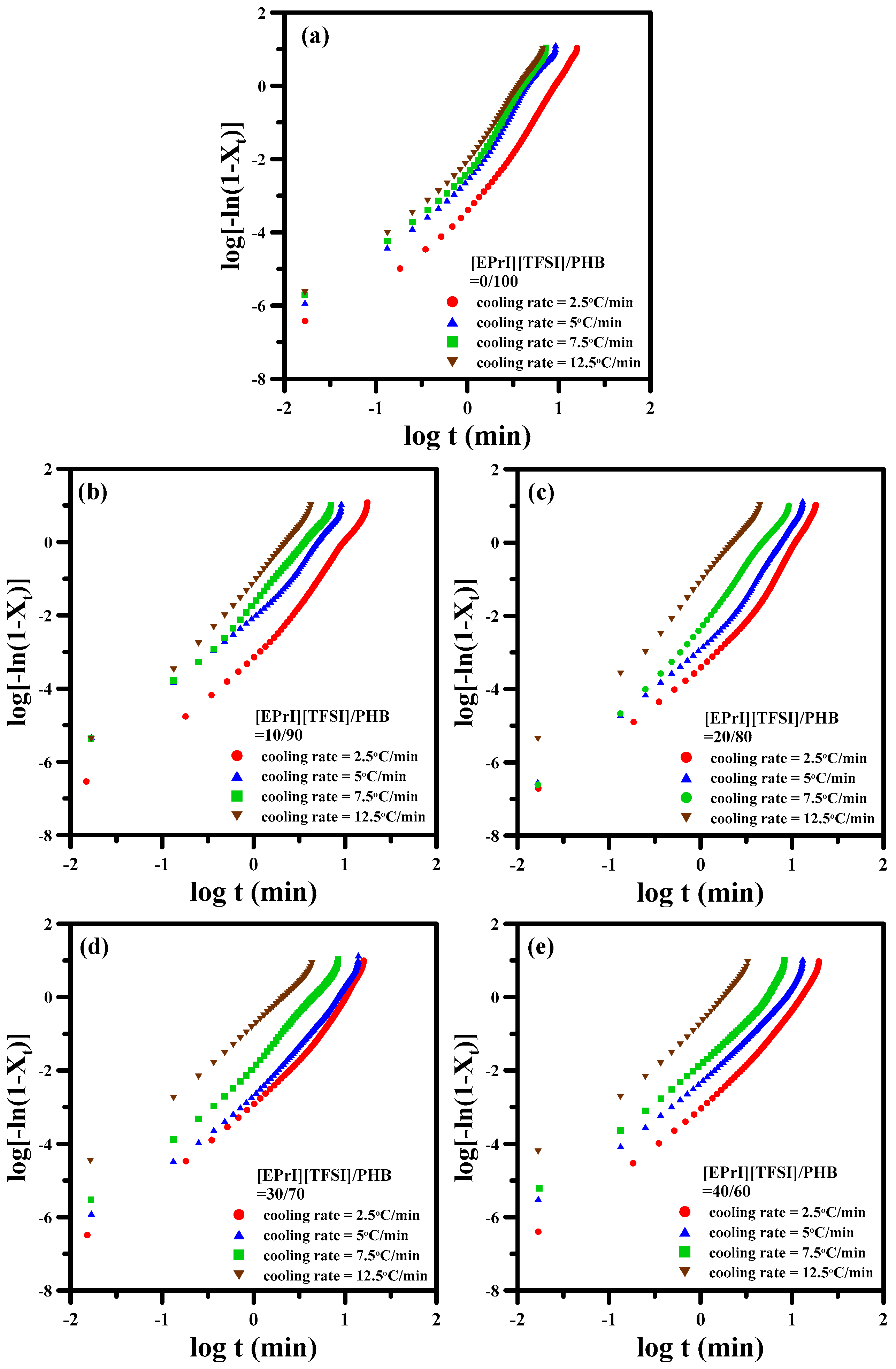
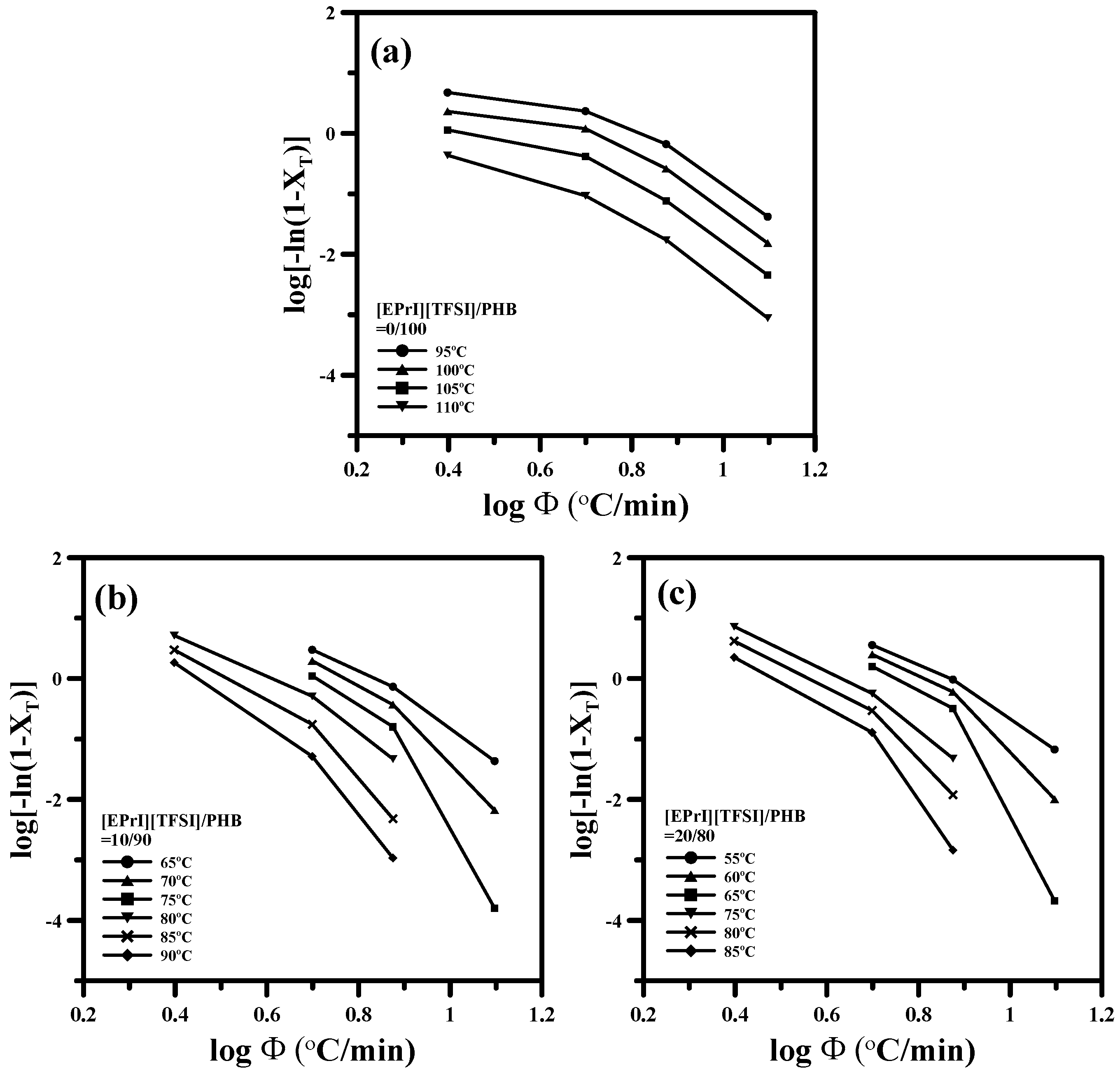
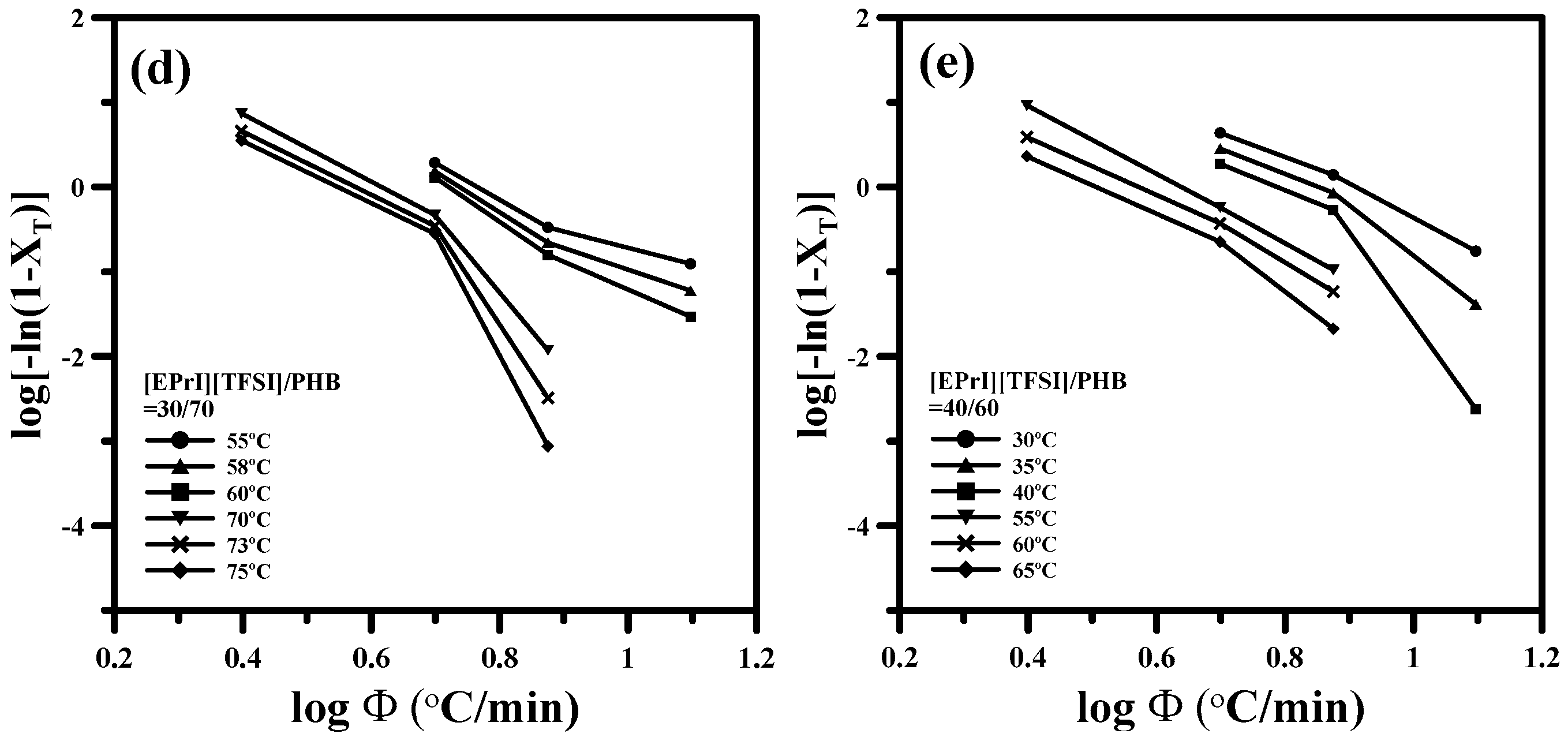
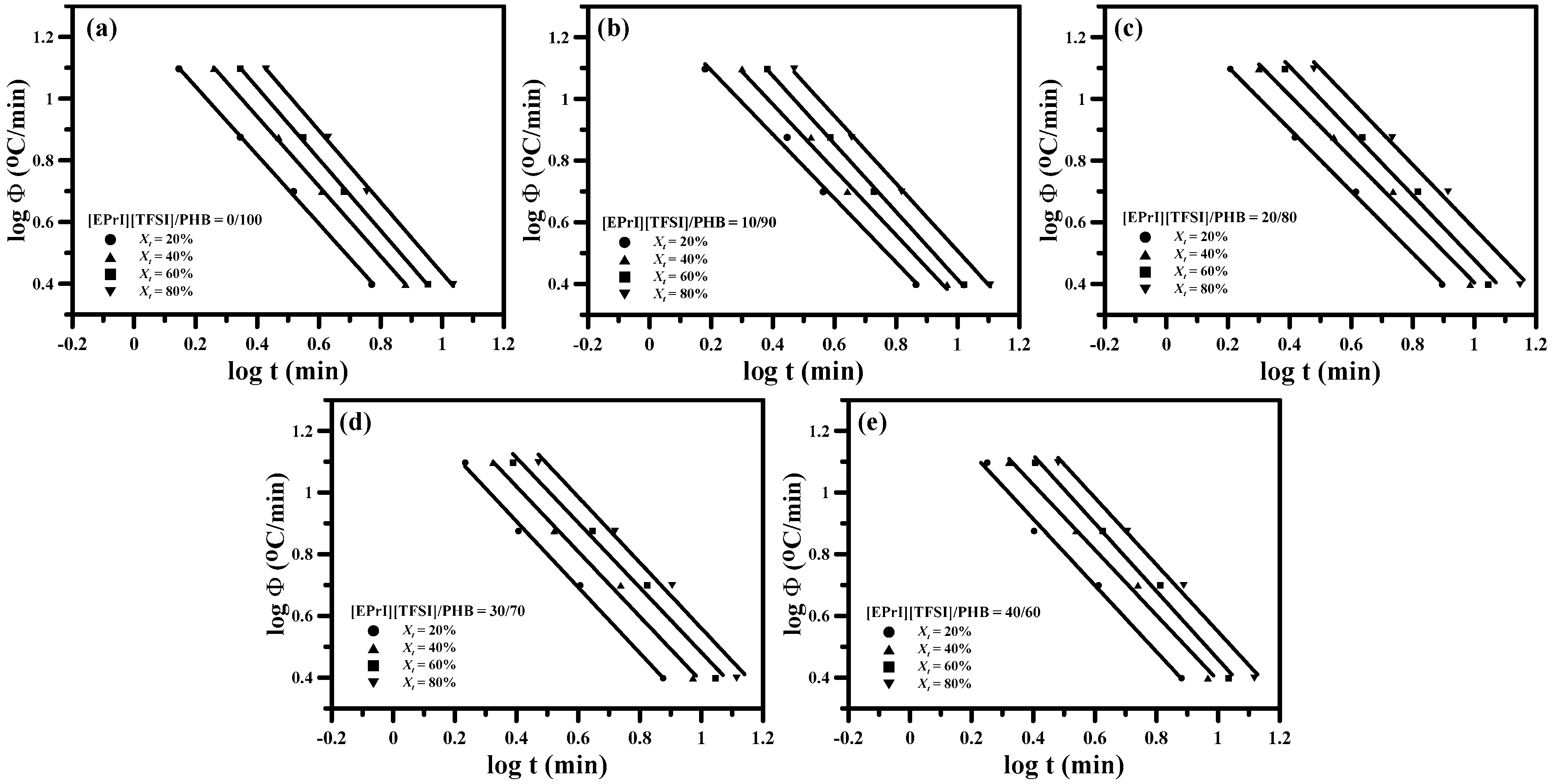
3.2. Study of Isothermal and Non-Isothermal Crystallization Kinetics in [EPrI][TFSI]/PHB Blends
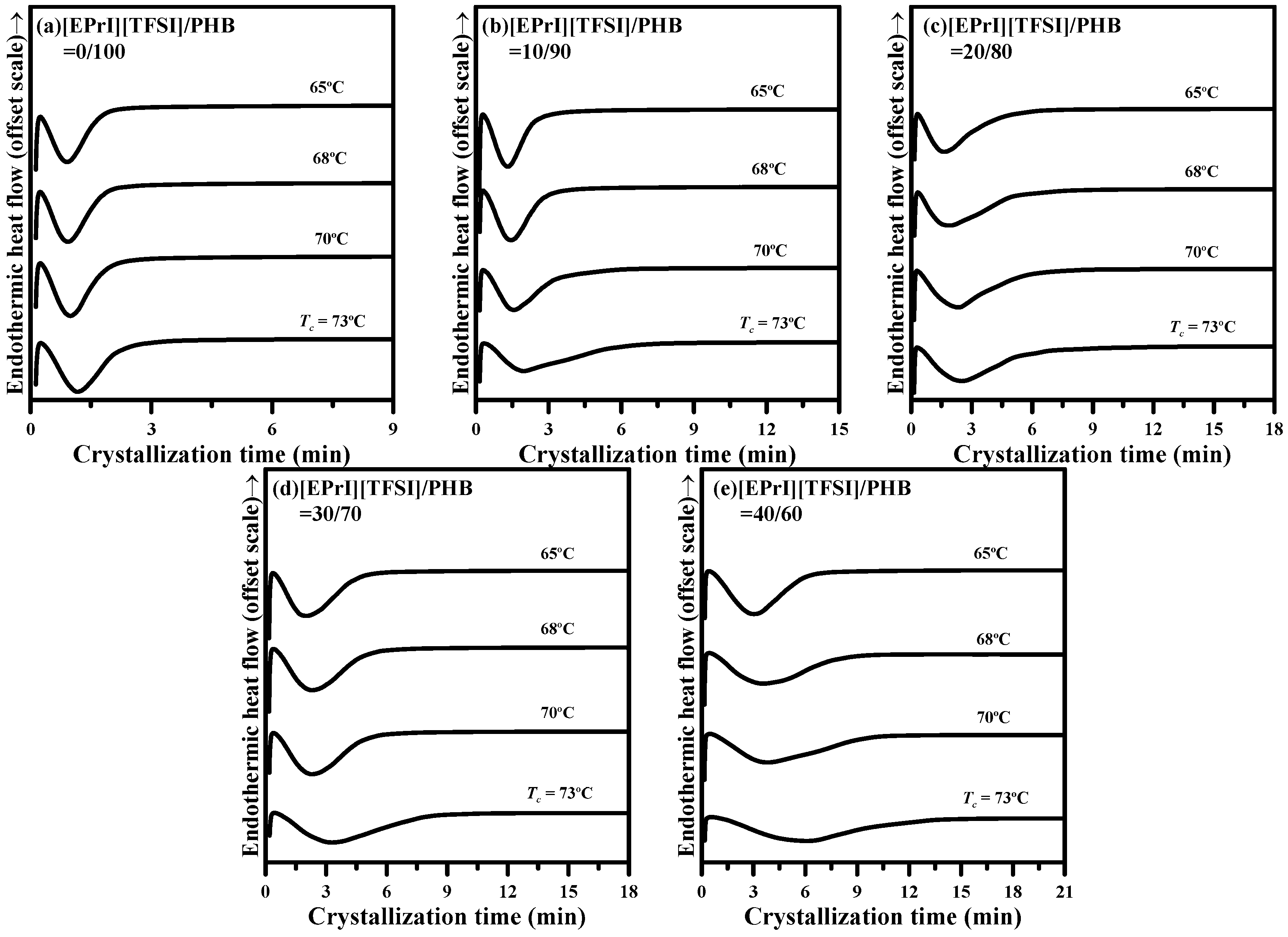
3.2.1. Isothermal Crystallization Kinetics
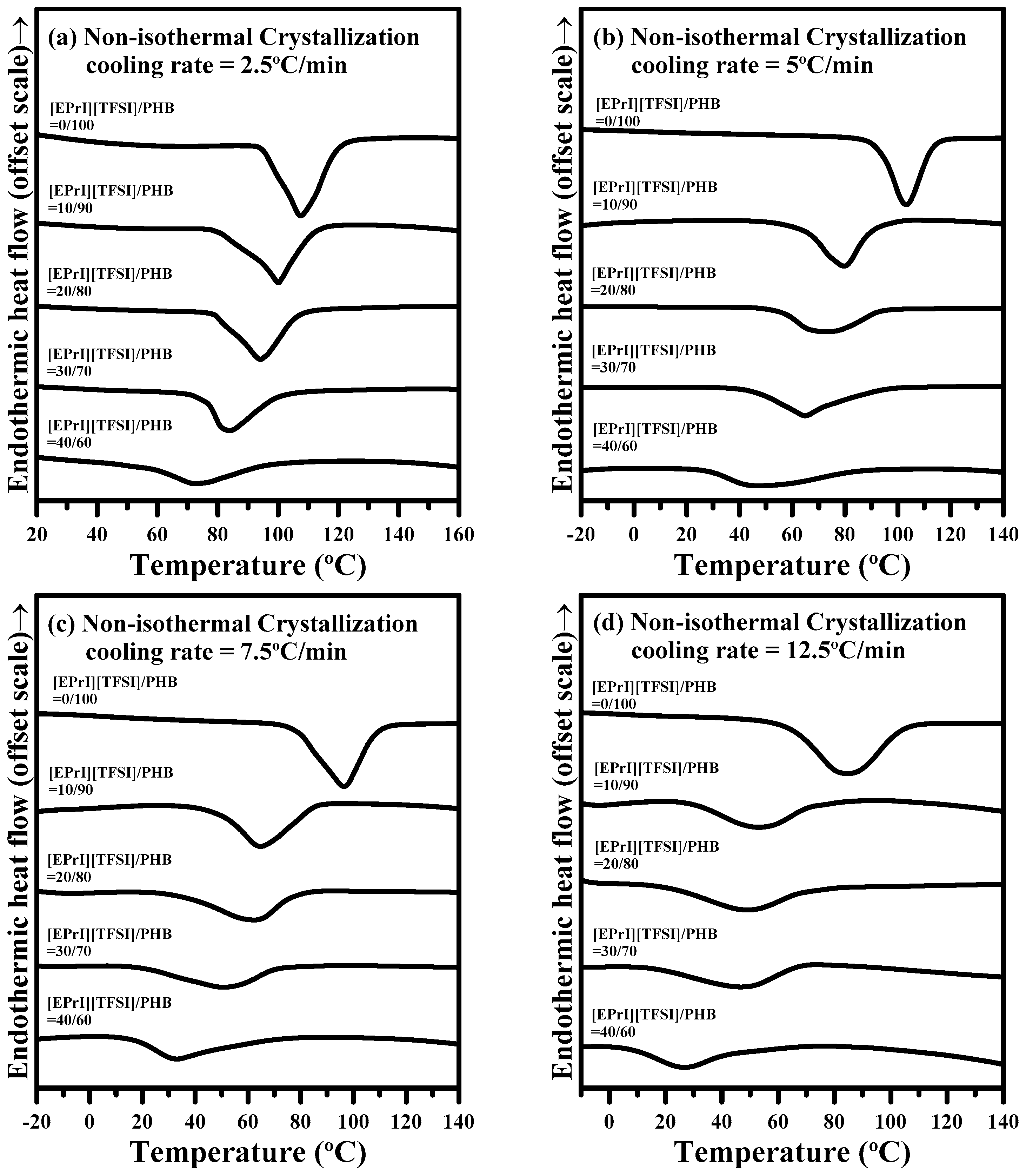
3.2.2. Non-Isothermal Crystallization Kinetics
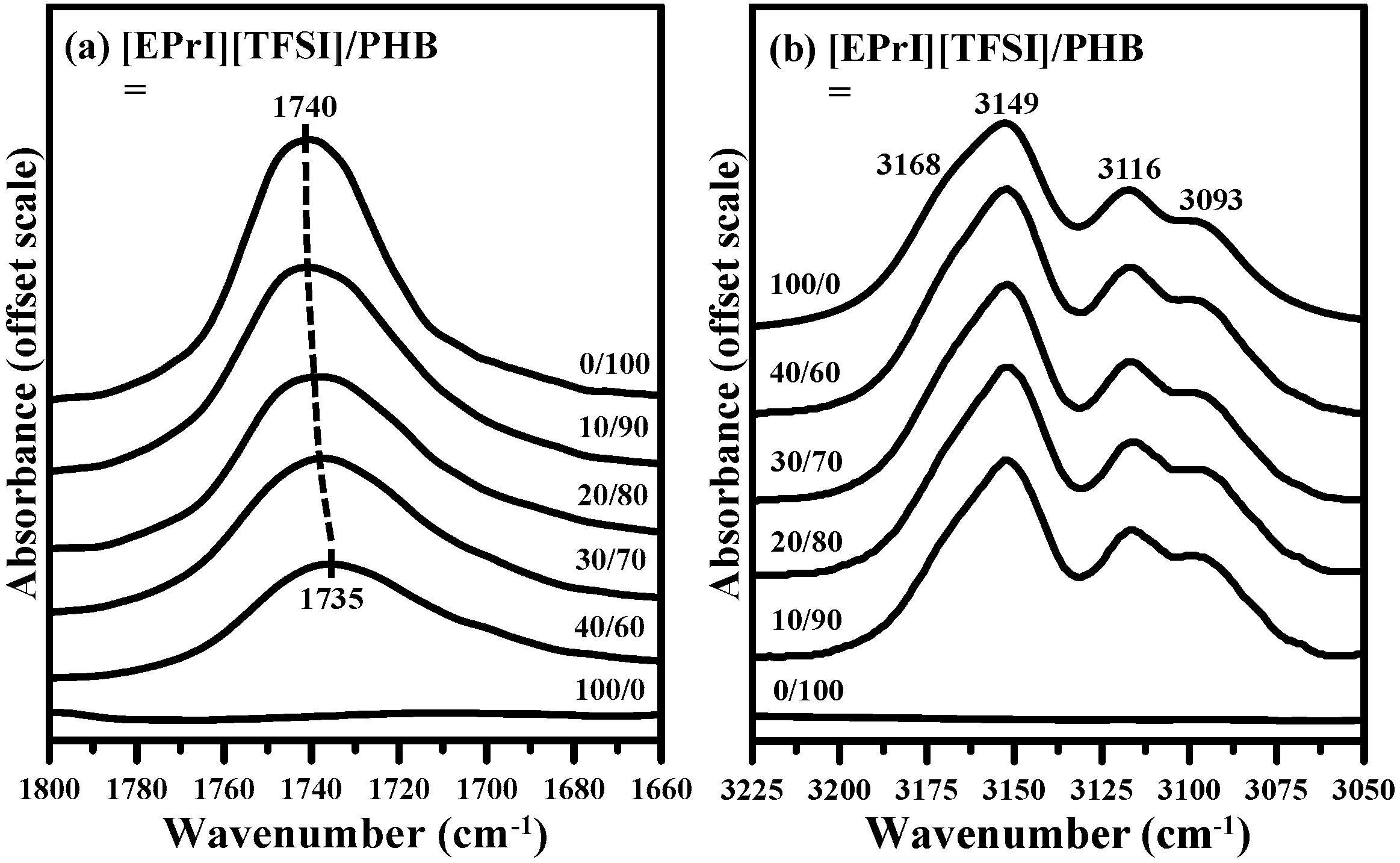
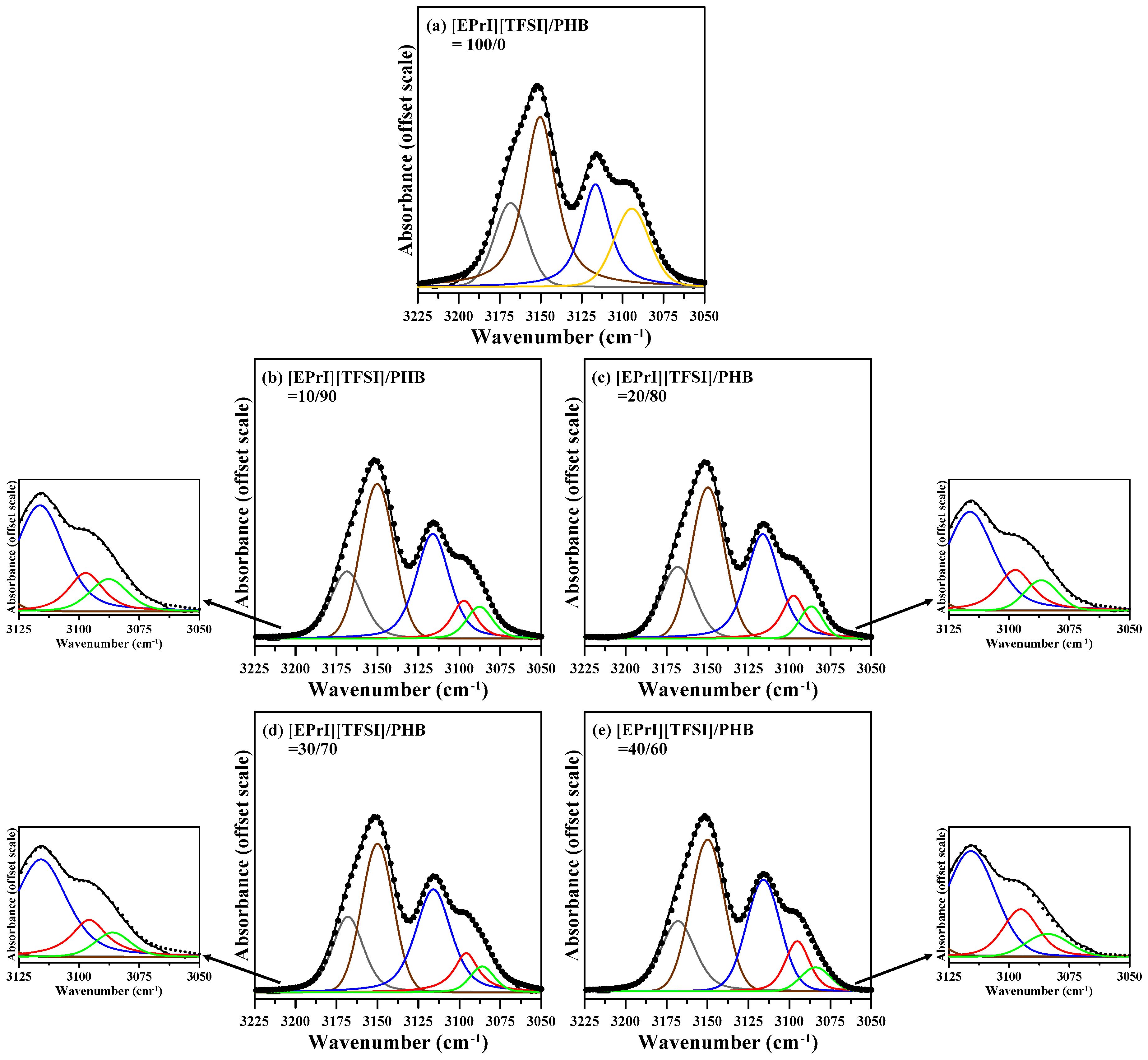
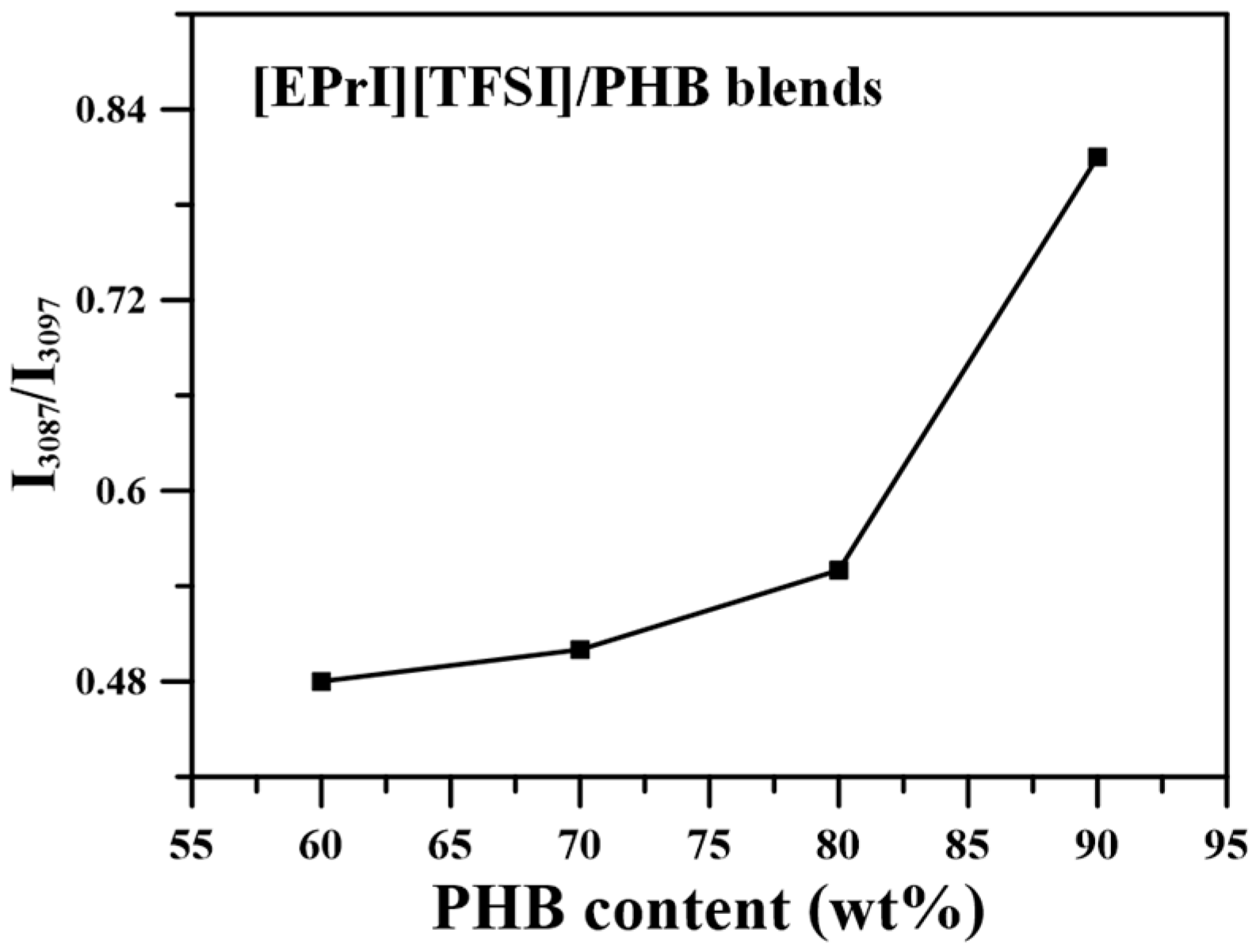
3.3. FTIR Characterization on [EPrI][TFSI]/PHB Blends
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, F.; Qiu, Z.; Yang, W. Miscibility and crystallization of biodegradable poly(butylene succinate-co-butylene adipate)/poly(vinyl phenol) blends. Polymer 2009, 50, 2328–2333. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, H.Y.; Chen, M.; Peng, J.S.; Tsai, C.J.; Yang, C.S. Synthesis and characterization of poly(ethylene succinate) and its copolyesters containing minor amounts of butylene succinate. J. Appl. Polym. Sci. 2009, 111, 1433–1439. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-based polymers with potential for biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Hosoda, N.; Uyama, H. Fabrication of porous poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) monoliths via thermally induced phase separation. Polymers 2016, 8, 66. [Google Scholar] [CrossRef]
- Li, H.Y.; Chang, C.M.; Hsu, K.Y.; Liu, Y.L. Poly(lactide)-functionalized and Fe3O4 nanoparticle-decorated multiwalled carbon nanotubes for preparation of electrically-conductive and magnetic poly(lactide) films and electrospun nanofibers. J. Mater. Chem. 2012, 22, 4855–4860. [Google Scholar] [CrossRef]
- Harmansyah, F.; Woo, E.M.; Lee, L.T.; Chien, H.R. Distorted ring-banded spherulites in poly(l-lactic acid)/poly(epsilon-caprolactone) blends. RSC Adv. 2014, 4, 49006–49015. [Google Scholar] [CrossRef]
- Lu, Y.S.; Bastakoti, B.P.; Pramanik, M.; Malgras, V.; Yamauchi, Y.; Kuo, S.W. Direct assembly of mesoporous silica functionalized with polypeptides for efficient dye adsorption. Chem. Eur. J. 2016, 22, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Zhu, B.; Li, J.C.; He, Y.; Inoue, Y. Studies on binary blends of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) and natural polyphenol catechin: Specific interactions and thermal properties. Macromol. Biosci. 2003, 3, 258–267. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Poly(hydroxybutyrate)/poly(butylene succinate) blends: Miscibility and nonisothermal crystallization. Polymer 2003, 44, 2503–2508. [Google Scholar] [CrossRef]
- Yang, J.M.; Chen, H.L.; You, J.W.; Hwang, J.C. Miscibility and crystallization of poly(l-lactide) poly(ethylene glycol) and poly(l-lactide)/poly(epsilon-caprolactone) blends. Polym. J. 1997, 29, 657–662. [Google Scholar] [CrossRef]
- Chiu, H.J. Miscibility and crystallization behavior of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(vinyl acetate) blends. J. Appl. Polym. Sci. 2006, 100, 980–988. [Google Scholar] [CrossRef]
- Silva, L.; Tognana, S.; Salgueiro, W. Miscibility in crystalline/amorphous blends of poly(3-hydroxybutyrate)/DGEBA. J. Polym. Sci. B Polym. Phys. 2013, 51, 680–686. [Google Scholar] [CrossRef]
- Xing, P.X.; Dong, L.S.; An, Y.X.; Feng, Z.L.; Avella, M.; Martuscelli, E. Miscibility and crystallization of poly(beta-hydroxybutyrate) and poly(p-vinyl phenol) blends. Macromolecules 1997, 30, 2726–2733. [Google Scholar] [CrossRef]
- An, Y.X.; Dong, L.S.; Li, L.X.; Mo, Z.S.; Feng, Z.L. Isothermal crystallization kinetics and melting behavior of poly(β-hydroxybutyrate)/poly(vinyl acetate) blends. Eur. Polym. J. 1999, 35, 365–369. [Google Scholar] [CrossRef]
- Zhang, L.L.; Deng, X.M.; Zhao, S.J.; Huang, Z.T. Biodegradable polymer blends of poly(3-hydroxybutyrate) and starch acetate. Polym. Int. 1997, 44, 104–110. [Google Scholar] [CrossRef]
- Kovalcik, A.; Machovsky, M.; Kozakova, Z.; Koller, M. Designing packaging materials with viscoelastic and gas barrier properties by optimized processing of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with lignin. React. Funct. Polym. 2015, 942, 25–34. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Bucci, D.Z. Application of poly(hydroxyalkanoate) in food packaging: Improvements by nanotechnology. Chem. Biochem. Eng. Q. 2015, 29, 275–285. [Google Scholar] [CrossRef]
- Gunaratne, L.M.W.K.; Shanks, R.A. Multiple melting behaviour of poly(3-hydroxybutyrate-co-hydroxyvalerate) using step-scan DSC. Eur. Polym. J. 2005, 41, 2980–2988. [Google Scholar] [CrossRef]
- Lim, J.S.; Noda, I.; Im, S.S. Miscibility and crystallization behavior of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and methoxy poly(ethylene glycol) blends. J. Polym. Sci. B Polym. Phys. 2006, 44, 2852–2863. [Google Scholar] [CrossRef]
- Gui, H.G.; Li, Y.; Chen, S.Y.; Xu, P.; Zheng, B.; Ding, Y.S. Effects of biodegradable imidazolium-based ionic liquid with ester group on the structure and properties of PLLA. Macromol. Res. 2014, 22, 583–591. [Google Scholar] [CrossRef]
- Cinelli, M.; Coles, S.R.; Nadagouda, M.N.; Blaszczynski, J.; Slowinski, R.; Varma, R.S.; Kirwan, K. A green chemistry-based classification model for the synthesis of silver nanoparticles. Green Chem. 2015, 17, 2825–2839. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J.S. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Qiu, Z.; Fujinami, S.; Komura, M.; Nakajima, K.; Ikehara, T.; Nishi, T. Nonisothermal crystallization kinetics of poly(butylene succinate) and poly(ethylene succinate). Polym. J. 2004, 36, 642–646. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, B.K.; Kuo, C.W.; Hao, L.; Peng, Y.C.; Sun, I.W. Standard entropy, surface excess entropy, surface enthalpy, molar enthalpy of vaporization, and critical temperature of bis(trifluoromethanesulfonyl)imide-based ionic liquids. J. Taiwan Inst. Chem. Eng. 2012, 43, 860–867. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Singh, R.K.; Chandra, S. Ion–polymer and ion–ion interaction in PEO-based polymer electrolytes having complexing salt LiClO4 and/or ionic liquid, [BMIM][PF6]. J. Raman Spectrosc. 2011, 42, 2168–2172. [Google Scholar] [CrossRef]
- Schäfer, T.; Di Paolo, R.E.; Franco, R.; Crespo, J.G. Elucidating interactions of ionic liquids with polymer films using confocal Raman spectroscopy. Chem. Commun. 2005, 50, 2594–2596. [Google Scholar]
- Pizzoli, M.; Scandola, M.; Ceccorulli, G. Crystallization and melting of isotactic poly(3-hydroxy butyrate) in the presence of a low molecular weight diluent. Macromolecules 2002, 35, 3937–3941. [Google Scholar] [CrossRef]
- Lee, J.C.; Nakajima, K.; Ikehara, T.; Nishi, T. Miscibility in blends of poly(3-hydroxybutyrate) and poly(vinylidene chloride-co-acrylonitrile). J. Polym. Sci. B Polym. Phys. 1997, 35, 2645–2652. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change II Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change III Granulation, phase change, and microstructure. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Xu, C.; Qiu, Z. Crystallization behavior and thermal property of biodegradable poly(3-hydroxybutyrate)/multi-walled carbon nanotubes nanocomposite. Polym. Adv. Technol. 2011, 22, 538–544. [Google Scholar] [CrossRef]
- Jing, X.; Qiu, Z. Effect of low thermally reduced graphene loadings on the crystallization kinetics and morphology of biodegradable poly(3-hydroxybutyrate). Ind. Eng. Chem. Res. 2012, 51, 13686–13691. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by DSC. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Liu, T.X.; Mo, Z.S.; Wang, S.G.; Zhang, H.F. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Su, Z.; Guo, W.; Liu, Y.; Li, Q.; Wu, C. Non-isothermal crystallization kinetics of poly(lactic acid)/modified carbon black composite. Polym. Bull. 2009, 62, 629–642. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Memarzadeh, M.R. Characterization and non-isothermal crystallization behavior of biodegradable poly(ethylene sebacate)/SiO2 nanocomposites. Polym. Bull. 2013, 70, 2471–2491. [Google Scholar] [CrossRef]
- Auliawan, A.; Woo, E.M. Crystallization kinetics and degradation of nanocomposites based on ternary blend of poly(l-lactic acid), poly(methyl methacrylate), and poly(ethylene oxide) with two different organoclays. J. Appl. Polym. Sci. 2012, 125, E444–E458. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, R.K. Development of ion conducting polymer gel electrolyte membranes based on polymer PVdF-HFP, BMIMTFSI ionic liquid and the Li-salt with improved electrical, thermal and structural properties. J. Mater. Chem. C 2015, 3, 7305–7318. [Google Scholar]
- Chiu, C.Y.; Yen, Y.J.; Kuo, S.W.; Chen, H.W.; Chang, F.C. Complicated phase behavior and ionic conductivities of PVP-co-PMMA-based polymer electrolytes. Polymer 2007, 48, 1329–1342. [Google Scholar] [CrossRef]


| [EPrI][TFSI]/PHB (wt %) | ΔHm (J/g) | Xc (%) |
|---|---|---|
| 0/100 | 36.08 | 24.63 |
| 10/90 | 32.27 | 22.03 |
| 20/80 | 30.42 | 20.76 |
| 30/70 | 23.67 | 16.16 |
| 40/60 | 20.30 | 13.86 |
| [EPrI][TFSI]/PHB (wt %) | Tc (°C) | n | k (min−n) | t0.5 (min) | 1/t0.5 (min−1) |
|---|---|---|---|---|---|
| 0/100 | 65 | 2.84 | 0.695 | 0.99 | 1.01 |
| 68 | 2.84 | 0.620 | 1.04 | 0.96 | |
| 70 | 2.90 | 0.527 | 1.09 | 0.91 | |
| 73 | 2.93 | 0.314 | 1.31 | 0.76 | |
| 10/90 | 65 | 2.77 | 0.234 | 1.48 | 0.68 |
| 68 | 2.75 | 0.175 | 1.65 | 0.61 | |
| 70 | 2.38 | 0.131 | 2.01 | 0.50 | |
| 73 | 2.12 | 0.076 | 2.84 | 0.35 | |
| 20/80 | 65 | 2.13 | 0.129 | 2.20 | 0.45 |
| 68 | 2.05 | 0.100 | 2.57 | 0.39 | |
| 70 | 2.23 | 0.072 | 2.76 | 0.36 | |
| 73 | 2.27 | 0.055 | 3.05 | 0.33 | |
| 30/70 | 65 | 2.50 | 0.076 | 2.42 | 0.41 |
| 68 | 2.58 | 0.053 | 2.71 | 0.37 | |
| 70 | 2.48 | 0.037 | 3.26 | 0.31 | |
| 73 | 2.47 | 0.023 | 3.97 | 0.25 | |
| 40/60 | 65 | 2.77 | 0.025 | 3.32 | 0.30 |
| 68 | 2.60 | 0.018 | 4.07 | 0.25 | |
| 70 | 2.48 | 0.015 | 4.69 | 0.21 | |
| 73 | 2.76 | 0.004 | 6.47 | 0.15 |
| [EPrI][TFSI]/PHB (wt %) | Xt (%) | a | F(T) |
|---|---|---|---|
| 0/100 | 20 | 1.11 | 18.24 |
| 40 | 1.13 | 24.77 | |
| 60 | 1.15 | 31.48 | |
| 80 | 1.15 | 39.08 | |
| 10/90 | 20 | 1.04 | 19.91 |
| 40 | 1.06 | 25.53 | |
| 60 | 1.10 | 32.73 | |
| 80 | 1.09 | 39.90 | |
| 20/80 | 20 | 1.00 | 20.18 |
| 40 | 1.01 | 26.06 | |
| 60 | 1.04 | 33.19 | |
| 80 | 1.04 | 41.30 | |
| 30/70 | 20 | 1.06 | 21.53 |
| 40 | 1.05 | 27.61 | |
| 60 | 1.05 | 34.20 | |
| 80 | 1.07 | 42.17 | |
| 40/60 | 20 | 1.08 | 22.13 |
| 40 | 1.05 | 28.12 | |
| 60 | 1.10 | 36.48 | |
| 80 | 1.09 | 43.15 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.-T.; Yang, C.-T. Investigations on Green Blends Comprising Biodegradable Polymer and Ionic Liquid. Polymers 2016, 8, 444. https://doi.org/10.3390/polym8120444
Lee L-T, Yang C-T. Investigations on Green Blends Comprising Biodegradable Polymer and Ionic Liquid. Polymers. 2016; 8(12):444. https://doi.org/10.3390/polym8120444
Chicago/Turabian StyleLee, Li-Ting, and Chun-Ting Yang. 2016. "Investigations on Green Blends Comprising Biodegradable Polymer and Ionic Liquid" Polymers 8, no. 12: 444. https://doi.org/10.3390/polym8120444
APA StyleLee, L.-T., & Yang, C.-T. (2016). Investigations on Green Blends Comprising Biodegradable Polymer and Ionic Liquid. Polymers, 8(12), 444. https://doi.org/10.3390/polym8120444