Crystallization of Poly(ε-caprolactone) in Poly(vinylidene fluoride)/Poly(ε-caprolactone) Blend
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurements
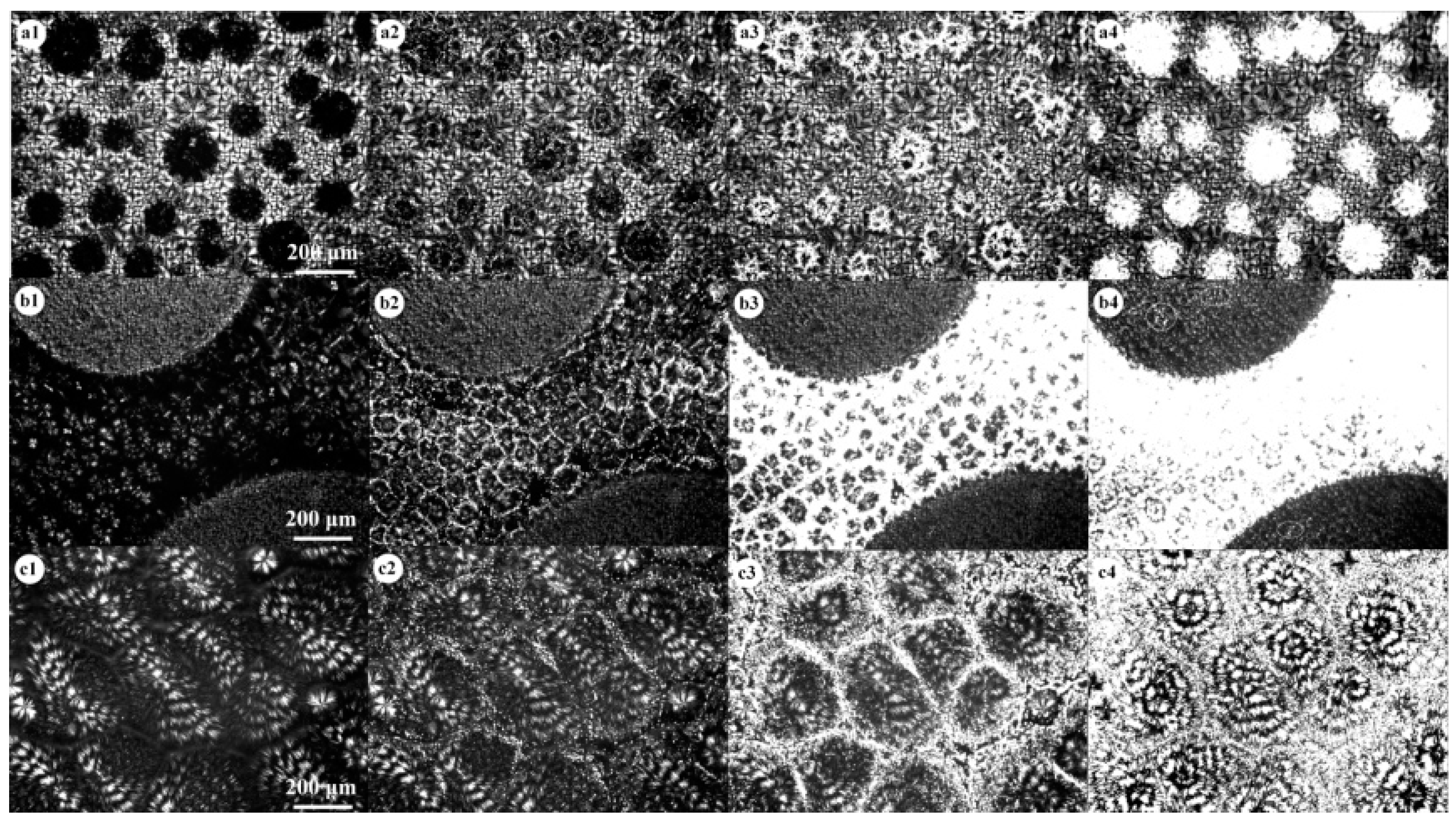
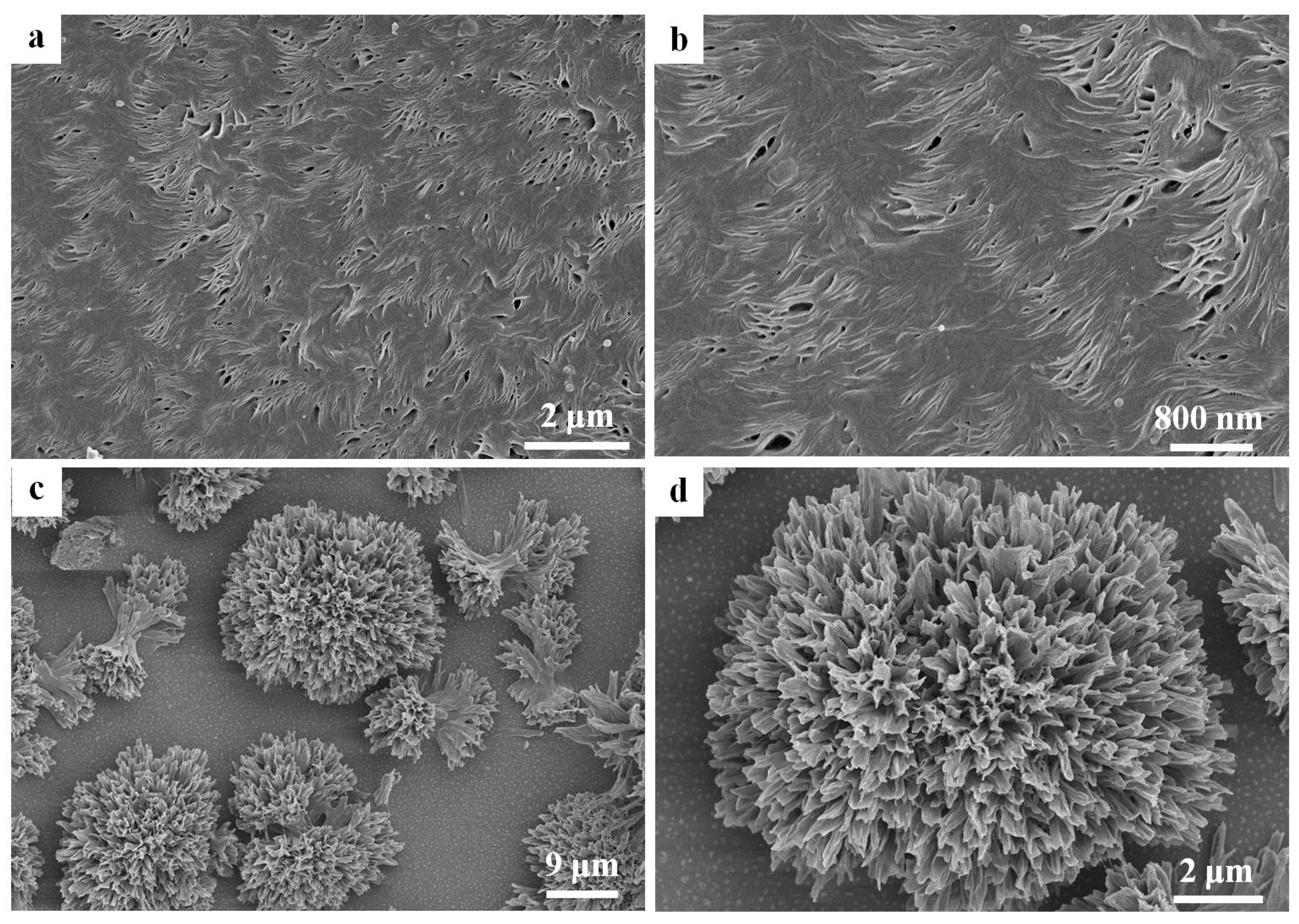
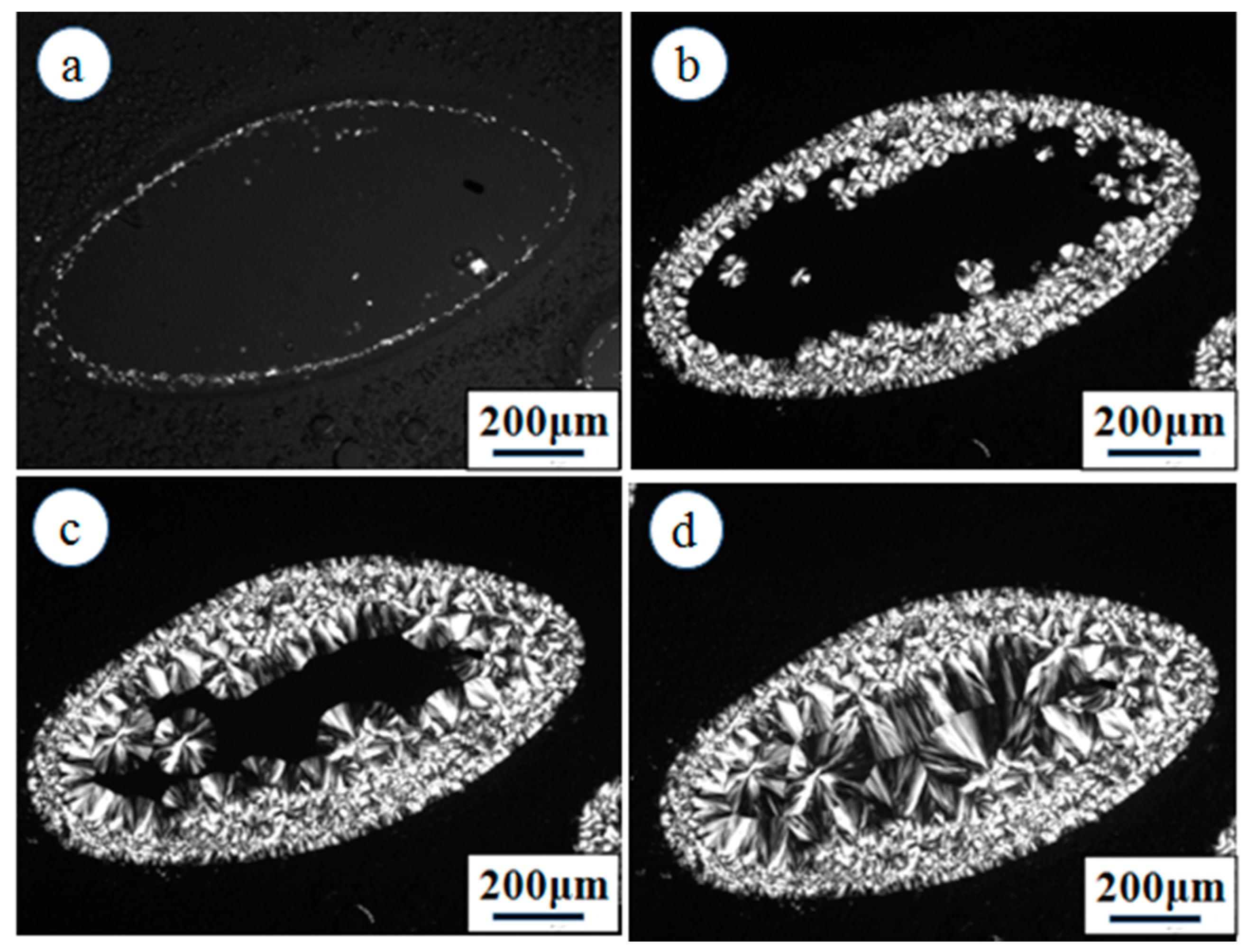
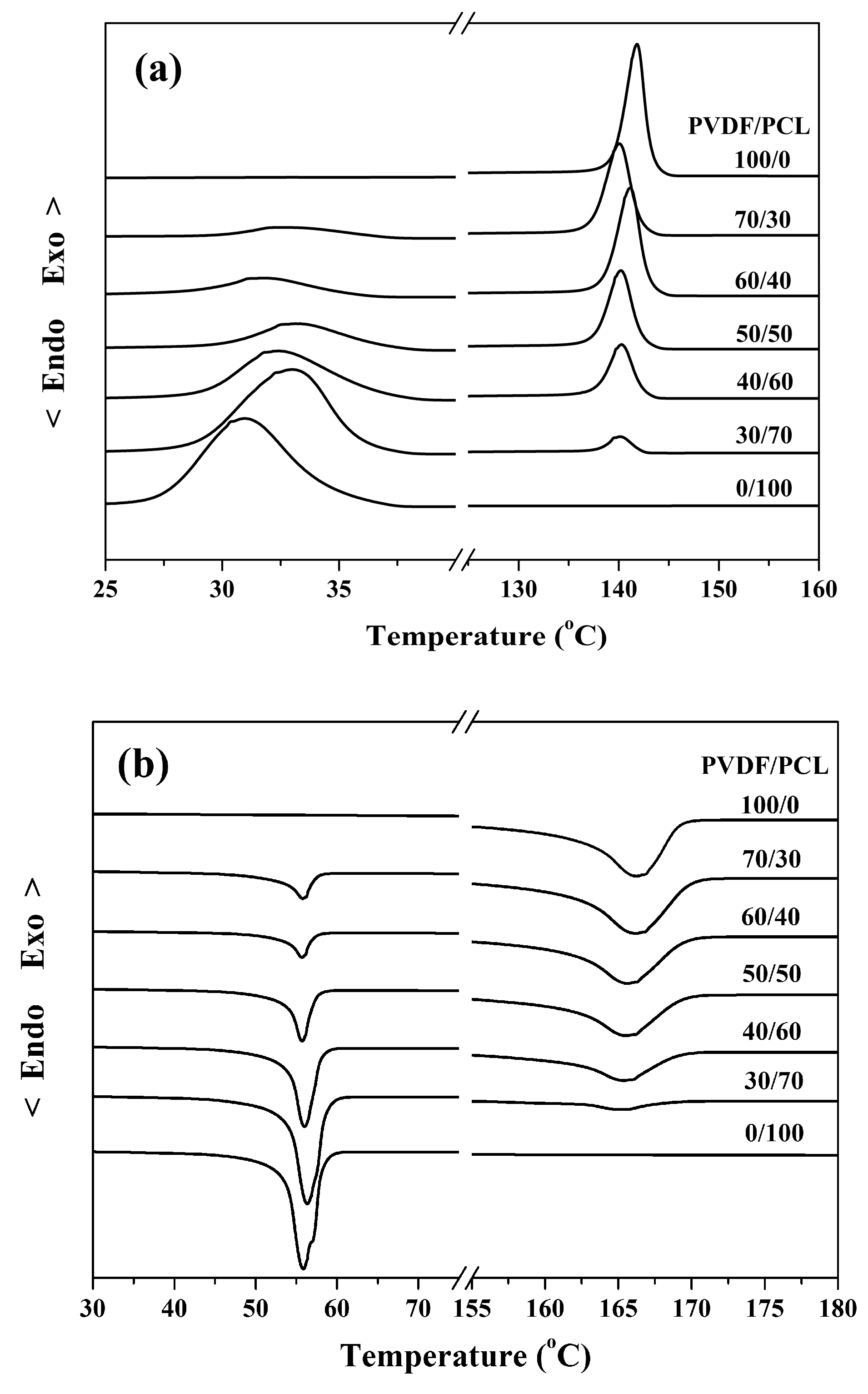
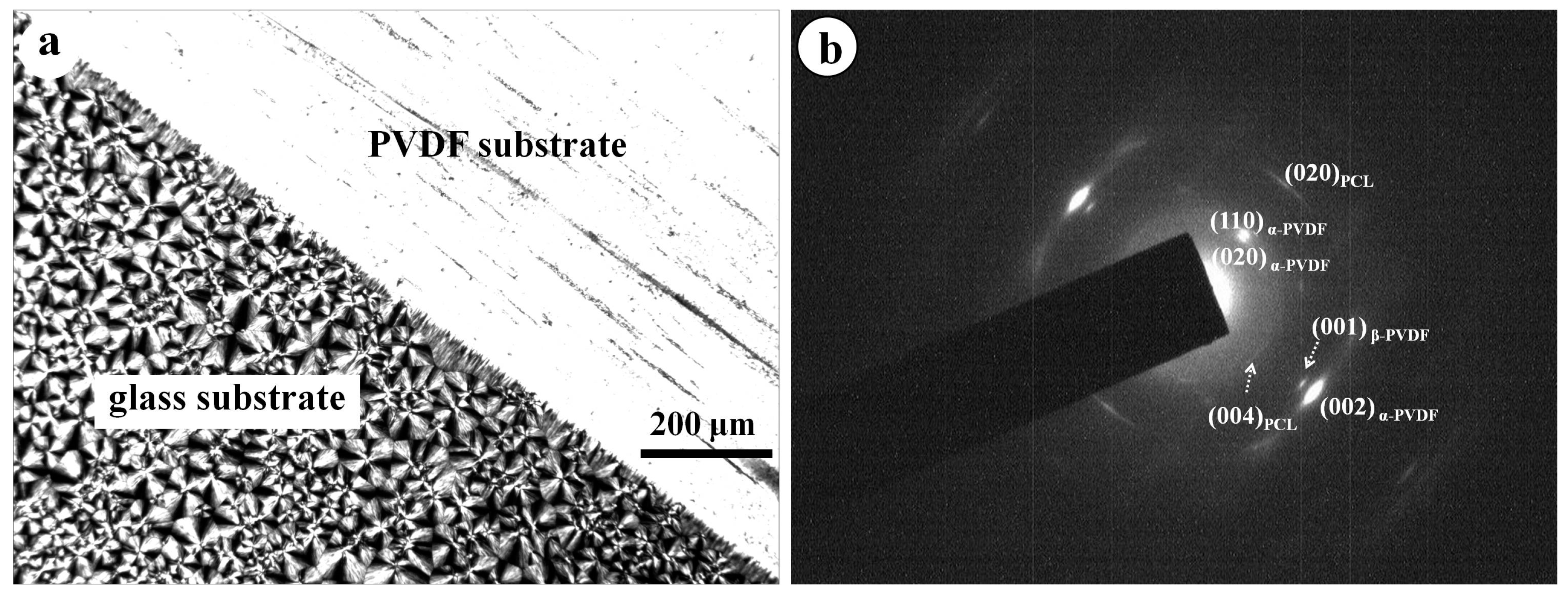
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Si, P.; Luo, F.L.; Luo, F.H. Miscibility, morphology and crystallization behavior of poly(butylene succinate-co-butylene adipate)/poly(vinyl phenol)/poly(l-lactic acid) blends. Polymers 2016, 8, 421. [Google Scholar] [CrossRef]
- Lorenzo, M.L.D. Spherulite growth rates in binary polymer blends. Prog. Polym. Sci. 2003, 28, 663–689. [Google Scholar] [CrossRef]
- Cho, S.J.; Cho, J.H.; Lee, K.H. Phase behavior and its effects on crystallization in a poly(trimethylene terephthalate)/phenoxy resin blend. Polymers 2016, 8, 21. [Google Scholar] [CrossRef]
- Takala, A.; Takala, P.; Seppälä, J.; Levon, K. Interdiffusion and spinodal decomposition in electrically conducting polymer blends. Polymers 2015, 7, 1410–1426. [Google Scholar] [CrossRef]
- Higgins, J.S.; Tambasco, M.; Lipson, J.E.G. Polymer blends: Stretching what we can learn through the combination of experiment and theory. Prog. Polym. Sci. 2005, 30, 832–843. [Google Scholar] [CrossRef]
- Lipatov, Y.S. Polymer blends and interpenetrating polymer networks at the interface with solids. Prog. Polym. Sci. 2002, 27, 1721–1801. [Google Scholar] [CrossRef]
- Ha, C.S.; Cho, W.J. Miscibility, properties, and biodegradability of microbial polyester containing blends. Prog. Polym. Sci. 2002, 27, 759–809. [Google Scholar] [CrossRef]
- Yang, J.J.; Pan, P.J.; Hua, L.; Feng, X.; Yue, J.J.; Ge, Y.H.; Inoue, Y. Effects of crystallization temperature of poly(vinylidene fluoride) on crystal modification and phase transition of poly(butylene adipate) in their blends: A novel approach for polymorphic control. J. Phys. Chem. B 2012, 116, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Pan, P.J.; Hua, L.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphic Crystallization and phase transition of poly(butylene adipate) in its miscible crystalline/crystalline blend with poly(vinylidene fluoride). Macromolecules 2010, 43, 8610–8618. [Google Scholar] [CrossRef]
- Wang, T.C.; Li, H.H.; Wang, F.; Yan, S.K.; Schultz, J.M. Confined growth of poly(butylene succinate) in its miscible blends with poly(vinylidene fluoride): Morphology and growth kinetics. J. Phys. Chem. B 2011, 115, 7814–7822. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.B.; Yan, C.Z.; Lu, J.M.; Yang, W.T.; Ikehara, T.; Nishi, T. Various crystalline morphology of poly(butylene succinate-co-butylene adipate) in its miscible blends with poly(vinylidene fluoride). J. Phys. Chem. B 2007, 111, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Gan, Z.H.; Schultz, J.M.; Yan, S.K. A morphological study of poly(butylene succinate)/poly(butylene adipate) blends with different blend ratios and crystallization processes. Polymer 2008, 49, 2342–2353. [Google Scholar] [CrossRef]
- Wang, H.J.; Feng, H.P.; Wang, X.C.; Du, Q.C.; Yan, C. Crystallization Kinetics and Morphology of Poly(vinylidene fluoride)/Poly(ethylene adipate) Blends. Chin. J. Polym. Sci. 2015, 33, 349–361. [Google Scholar] [CrossRef]
- Madbouly, S.A. Isothermal crystallization kinetics in binary miscible blend of poly(ε-caprolactone)/tetramethyl polycarbonate. J. Appl. Polym. Sci. 2007, 103, 3307–3315. [Google Scholar] [CrossRef]
- Jo, W.H.; Park, S.J.; Kwon, I.H. Phase behavior of poly(ε-caprolactone)/poly(vinylidene fluoride) blends. Polym. Int. 1992, 29, 173–178. [Google Scholar] [CrossRef]
- Petermann, J.; Gohil, R.M. A new method for the preparation of high modulus thermoplastic films. J. Mater. Sci. 1979, 14, 2260. [Google Scholar] [CrossRef]
- Kaito, A.; Iwakura, Y.; Hatakeyama, K.; Li, Y.J. Organization of oriented lamellar structures in a miscible crystalline/crystalline polymer blend under uniaxial compression flow near the melting temperature. Macromolecules 2007, 40, 2751–2759. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Hasegawa, R.; Takahashi, Y.; Chatani, Y.; Tadokoro, H. Crystal structures of three crystalline forms of poly(vinylidene fluoride). Polym. J. 1972, 3, 600–616. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.; Ma, Y.; Lei, L.; Wang, X.; Wang, H. Crystallization of Poly(ε-caprolactone) in Poly(vinylidene fluoride)/Poly(ε-caprolactone) Blend. Polymers 2017, 9, 42. https://doi.org/10.3390/polym9020042
Kong Y, Ma Y, Lei L, Wang X, Wang H. Crystallization of Poly(ε-caprolactone) in Poly(vinylidene fluoride)/Poly(ε-caprolactone) Blend. Polymers. 2017; 9(2):42. https://doi.org/10.3390/polym9020042
Chicago/Turabian StyleKong, Yang, Yangmin Ma, Lele Lei, Xuechuan Wang, and Haijun Wang. 2017. "Crystallization of Poly(ε-caprolactone) in Poly(vinylidene fluoride)/Poly(ε-caprolactone) Blend" Polymers 9, no. 2: 42. https://doi.org/10.3390/polym9020042




