Crosslinked Poly(2-oxazoline)s as “Green” Materials for Electronic Applications
Abstract
:1. Introduction
| Parameter | Polymer | ||
|---|---|---|---|
| Polyamides | Polyesters | Epoxy Resins | |
| Density (g·cm−3) | 1.13–1.21 | 1.22–1.26 | 1.10–1.25 |
| Permittivity | 3.6–7 | 3–4.9 | 3.7–4.2 |
| Loss factor | 0.014–0.15 | 0.008–0.06 | 0.007–0.009 |
| Conductivity (S·m−1) | 10−11–10−6 | 10−13–10−12 | 10−15–10−14 |
2. Experimental Section
2.1. Materials
2.2. Instrumentation
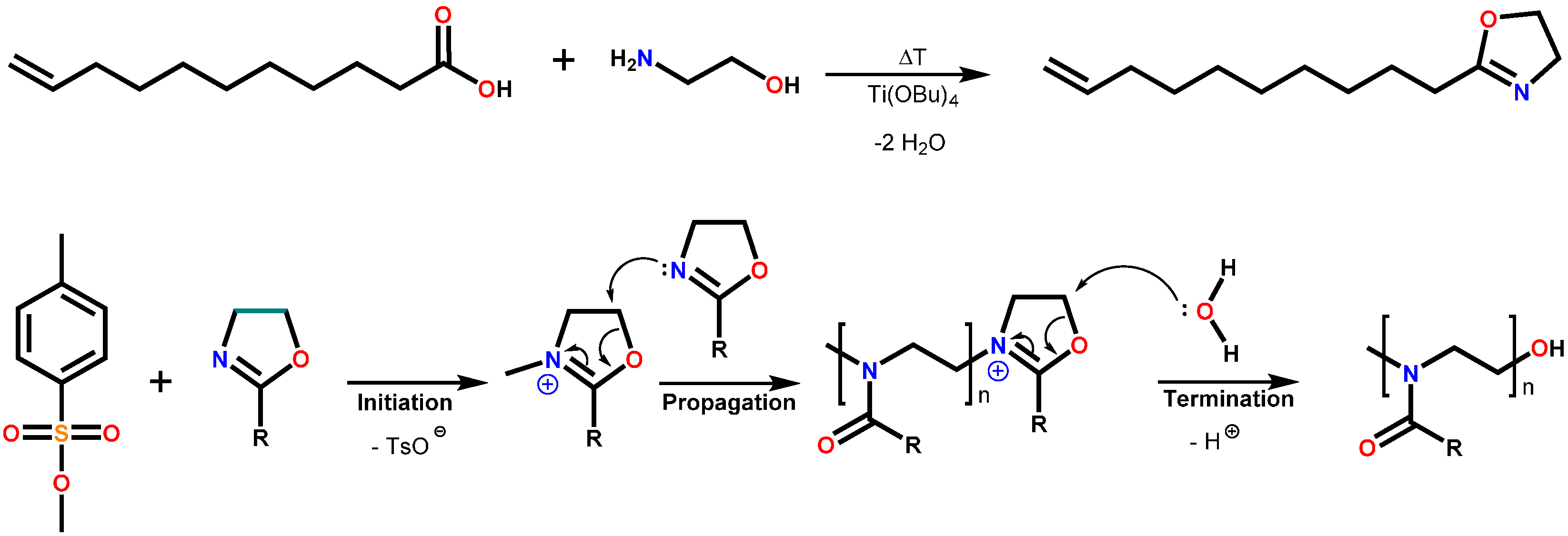
2.3. Polymer Syntheses
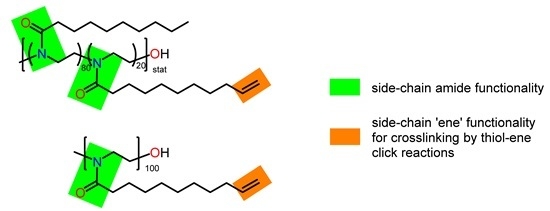
2.3.1. Poly(2-nonyl-2-oxazoline)80-stat-poly(2-dec-9′-enyl-2-oxazoline)20, pNonOx80-stat-pDc=Ox20
2.3.2. Poly(2-dec-9′-enyl-2-oxazoline)100, pDc=Ox100
2.3.3. Preparation of the Test Specimens
3. Results and Discussion
3.1. Choice of Crosslinked (co)poly(2-oxazoline)s
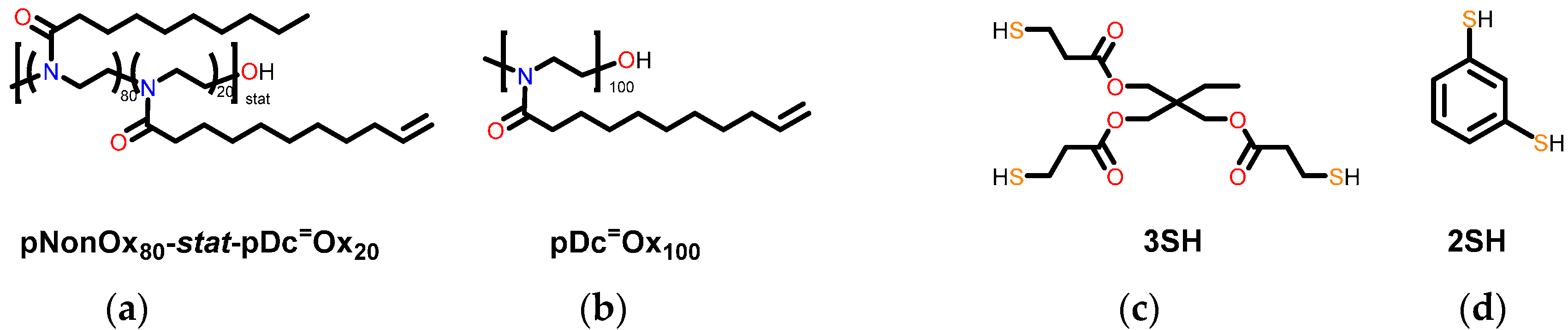
| Type of Network | MBOND (g·moL−1) | ρ (g·cm3) at 25 °C | ρKNOT (mmol·cm−3) |
|---|---|---|---|
| pNonOx80-stat-pDc=Ox20 (CL:3SH) | 1663 | 0.992 | 1.8 |
| pDc=Ox100 (CL:2SH) | 281 | 1.116 | 8.0 |
| pDc=Ox100 (CL:3SH) | 342 | 1.184 | 10.5 |
3.2. Relative Permittivity

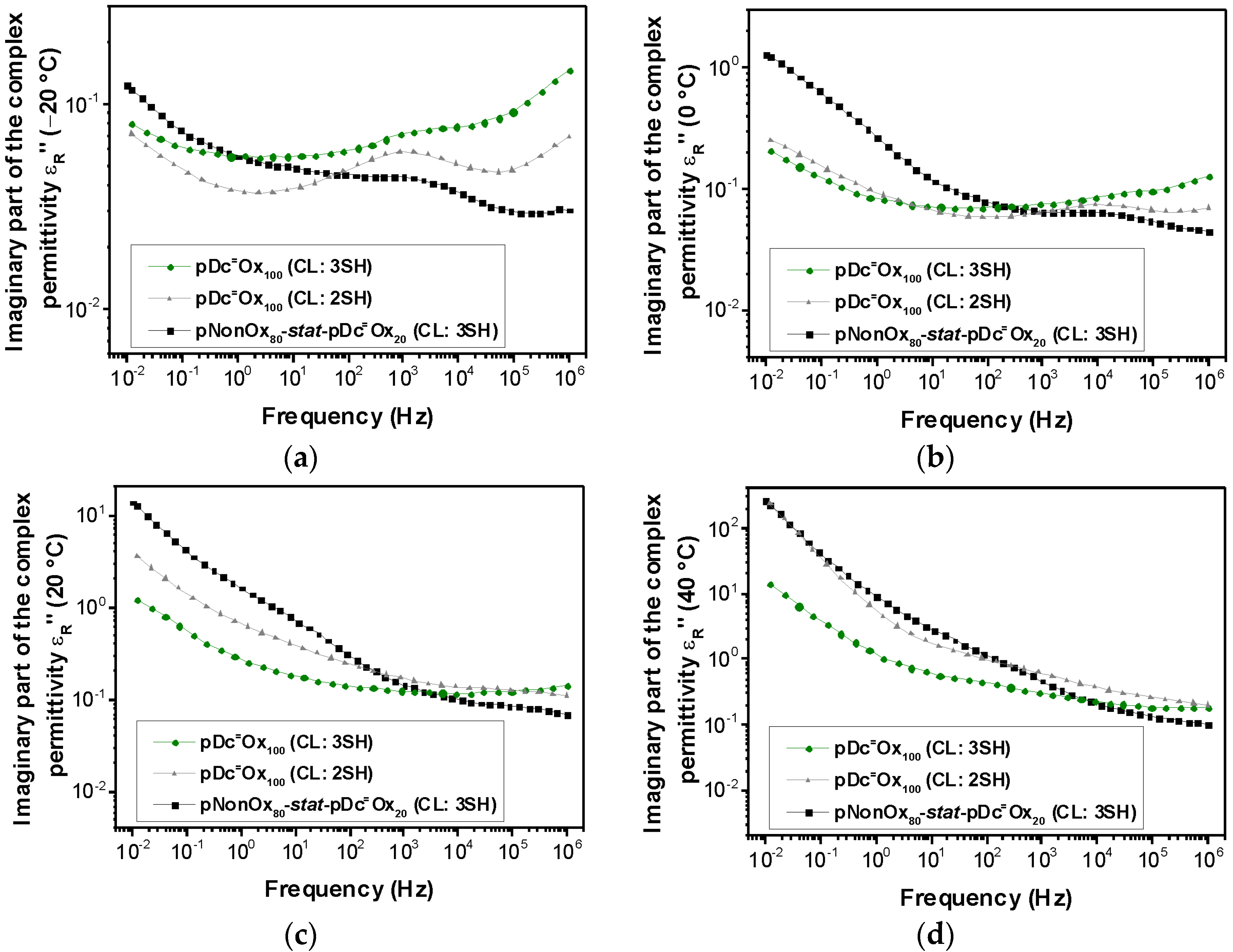
| Polymer Network | Permittivity | |||
|---|---|---|---|---|
| T = −20 °C | T = 0 °C | T = 20 °C | T = 40 °C | |
| pNonOx80-stat-pDc=Ox20 (CL:3SH) | 3.62 | 3.81 | 4.29 | 5.81 |
| pDc=Ox100 (CL:2SH) | 4.00 | 4.19 | 4.97 | 7.28 |
| pDc=Ox100 (CL:3SH) | 4.19 | 4.42 | 4.97 | 6.04 |
3.3. Loss Factor
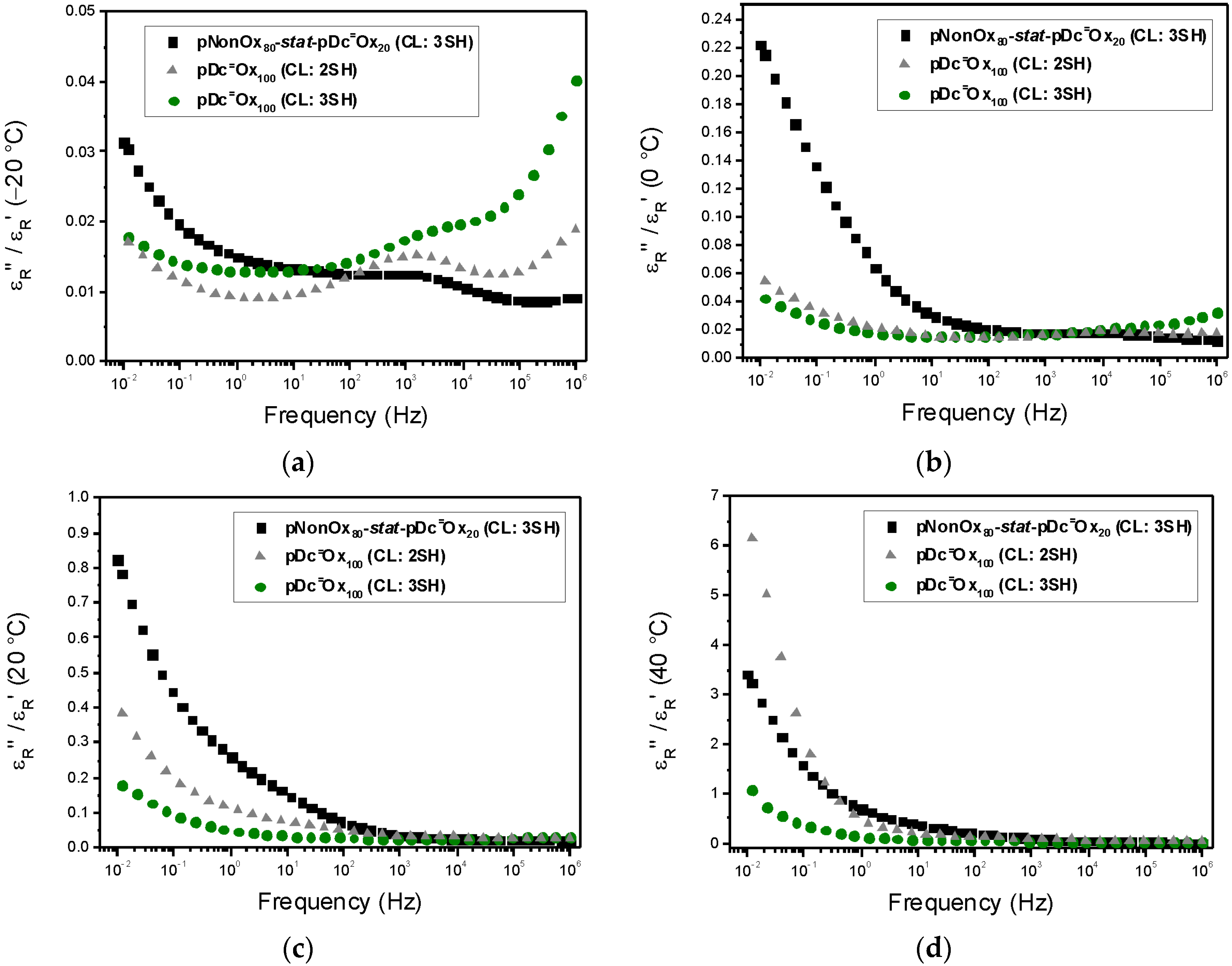
| Polymer Network | Loss Factor | |||
|---|---|---|---|---|
| T = −20 °C | T = 0 °C | T = 20 °C | T = 40 °C | |
| pNonOx80-stat-pDc=Ox20 (CL:3SH) | 0.014 | 0.024 | 0.093 | 0.262 |
| pDc=Ox100 (CL: 2SH) | 0.010 | 0.014 | 0.054 | 0.159 |
| pDc=Ox100 (CL:3SH) | 0.014 | 0.016 | 0.030 | 0.076 |
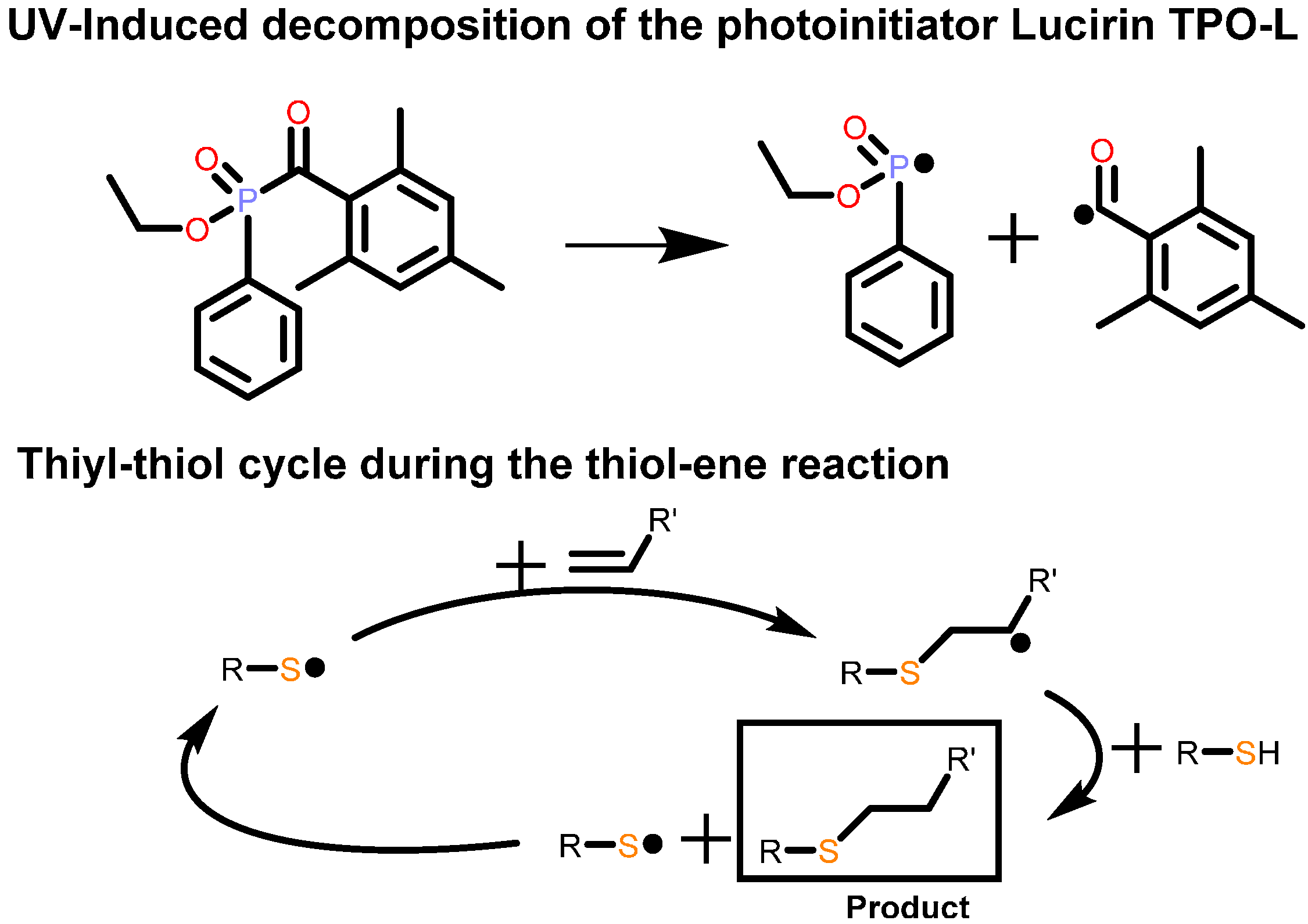
3.4. Electrical Conductivity
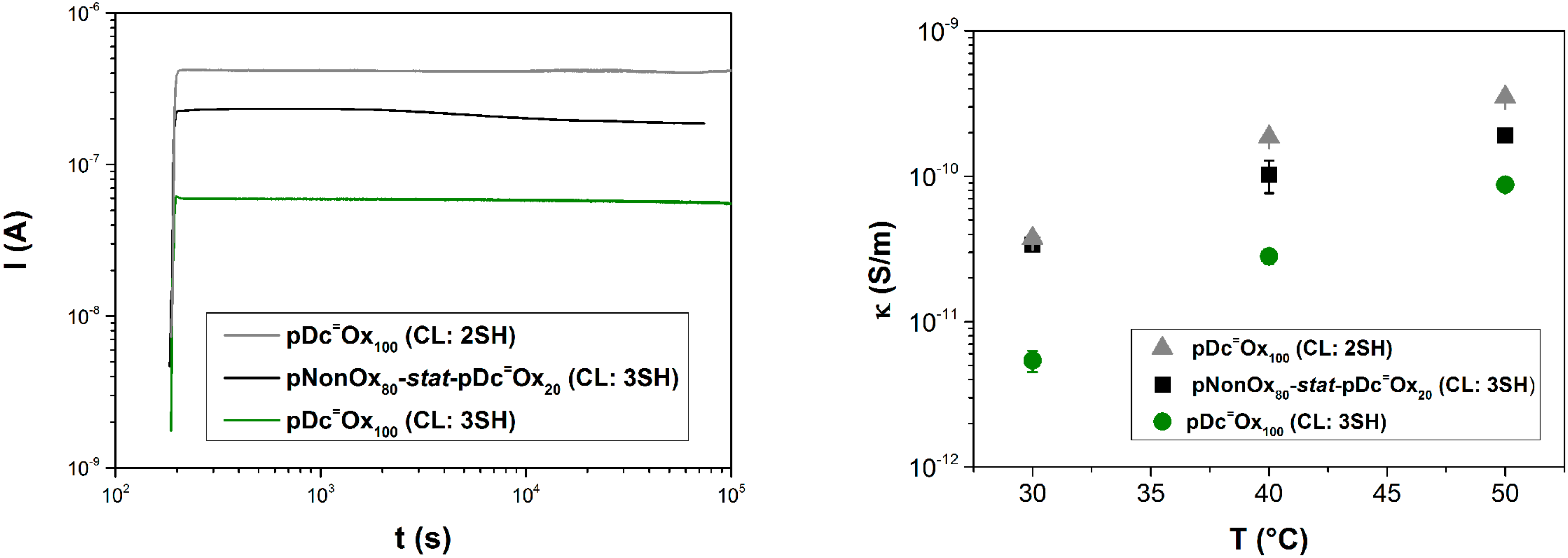
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luef, K.P.; Hoogenboom, R.; Schubert, U.S.; Wiesbrock, F. Microwave-assisted cationic ring-opening polymerization of 2-oxazolines. Adv. Polym. Sci. In press. [CrossRef]
- Rossegger, E.; Schenk, V.; Wiesbrock, F. Design strategies for functionalized poly(2-oxazoline)s and derived materials. Polymers 2013, 5, 956–1011. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Bioinspired poly(2-oxazoline)s. Polymers 2011, 3, 467–488. [Google Scholar] [CrossRef]
- Kelly, A.M.; Hecke, A.; Wirnsberger, B.; Wiesbrock, F. Synthesis of poly(2-oxazoline)-based hydrogels with tailor-made swelling degrees capable of stimuli-triggered compound release. Macromol. Rapid Commun. 2011, 32, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Schenk, V.; Rossegger, E.; Ebner, C.; Bangerl, F.; Reichmann, K.; Hoffmann, B.; Höpfner, M.; Wiesbrock, F. RGD-functionalization of poly(2-oxazoline)-based networks for enhanced adhesion to cancer cells. Polymers 2014, 6, 264–279. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Thijs, H.M.L.; Fijten, M.W.M.; Schubert, U.S. Synthesis, characterization, and cross-linking of a library of statistical copolymers based on 2-“soy alkyl”-2-oxazoline and 2-ethyl-2-oxazoline. J. Polym. Sci. Polym. Chem. 2007, 45, 5371–5379. [Google Scholar] [CrossRef]
- Dargaville, T.R.; Forster, R.; Farrugia, B.L.; Kempe, K.; Voorhaar, L.; Schubert, U.S.; Hoogenboom, R. Poly(2-oxazoline) hydrogel monoliths via thiol-ene coupling. Macromol. Rapid Commun. 2012, 33, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M.; Wiesbrock, F. Strategies for the synthesis of poly(2-oxazoline)-based hydrogels. Macromol. Rapid Commun. 2012, 33, 1632–1647. [Google Scholar] [CrossRef] [PubMed]
- Kempe, K.; Becer, C.R.; Schubert, U.S. Microwave-assisted polymerizations: Recent status and future perspectives. Macromolecules 2011, 44, 5825–5842. [Google Scholar] [CrossRef]
- Ebner, C.; Bodner, T.; Stelzer, F.; Wiesbrock, F. One Decade of microwave-assisted polymerizations: Quo vadis? Macromol. Rapid Commun. 2011, 32, 254–288. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, V.R. Poly(2-oxazoline)s as materials for biomedical applications. J. Mater. Sci. Mater. Med. 2014, 25, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-oxazoline)s as polymer therapeutics. Macromol. Rapid Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Schenk, V.; Ellmaier, L.; Rossegger, E.; Edler, M.; Griesser, T.; Weidinger, G.; Wiesbrock, F. Water-developable poly(2-oxazoline)-based negative photoresists. Macromol. Rapid Commun. 2012, 33, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Fimberger, M.; Schenk, V.; Rossegger, E.; Wiesbrock, F. UV-Induced crosslinking of poly[2-(2′-norbornenyl)-2-oxazoline]s. Period. Polytech. Chem. Eng. 2014, 58, 69–74. [Google Scholar] [CrossRef]
- Petit, C.; Luef, K.P.; Edler, M.; Griesser, T.; Kremsner, J.M.; Stadler, A.; Grassl, B.; Reynaud, S.; Wiesbrock, F. Microwave-assisted syntheses in recyclable ionic liquids: Photoresists based on renewable resources. ChemSusChem 2015, 8, 3401–3404. [Google Scholar] [CrossRef] [PubMed]
- Slama, M.E.A.; Beroual, A. Behavior of AC High voltage polyamide insulators: Evolution of leakage current in different surface conditions. Power Eng. Electr. Eng. 2015, 13, 74–80. [Google Scholar] [CrossRef]
- Hackam, R. Outdoor HV composite polymeric insulators. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 557–585. [Google Scholar] [CrossRef]
- Notingher, P.V. Materiale Pentru Electrotehnică: Proprietăţi Tehnice Utilizări (2); Editura Politehnica Press: Bucharest, Romanian, 2005; p. 140. [Google Scholar]
- Beck, M.; Birnbrich, P.; Eicken, U.; Fischer, H.; Fristad, W.E.; Hase, B.; Krause, H.-J. Polyoxazoline auf fettchemischer Basis. Angew. Makromol. Chem. 1994, 223, 217–233. [Google Scholar] [CrossRef]
- Krause, H.-J.; Neumann, P. Process for the preparation of 2-alkyl and 2-alkenyl oxazolines. European Patent EP0315856 (B1), 19 April 1995. [Google Scholar]
- Wiesbrock, F.; Hoogenboom, R.; Leenen, M.; van Nispen, S.F.G.M.; van der Loop, M.; Abeln, C.H.; van den Berg, A.M.J.; Schubert, U.S. Microwave-assisted synthesis of a 42-membered library of diblock copoly(2-oxazoline)s and chain-extended homo poly(2-oxazoline)s and their thermal characterization. Macromolecules 2005, 38, 7957–7966. [Google Scholar] [CrossRef]
- Mol, J.C. Application of olefin metathesis in oleochemistry: An example of green chemistry. Green Chem. 2002, 4, 5–13. [Google Scholar] [CrossRef]
- Van der Steen, M.; Stevens, C.V. Undecylenic acid: A valuable and physiologically active renewable building block from castor oil. ChemSusChem 2009, 2, 692–713. [Google Scholar] [CrossRef] [PubMed]
- Wiesbrock, F.; Hoogenboom, R.; Abeln, C.H.; Schubert, U.S. Single-mode microwave ovens as new reaction devices: Accelerating the living polymerization of 2-ethyl-2-oxazoline. Macromol. Rapid Commun. 2004, 25, 1895–1899. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.C.; Liepins, R. Electrical Properties of Polymers; Hanser Publishers: Munich, Germany, 1987. [Google Scholar]
- Elias, H.G. Makromoleküle, Band 4: Anwendungen von Polymeren; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2003; p. 479. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fimberger, M.; Tsekmes, I.-A.; Kochetov, R.; Smit, J.J.; Wiesbrock, F. Crosslinked Poly(2-oxazoline)s as “Green” Materials for Electronic Applications. Polymers 2016, 8, 6. https://doi.org/10.3390/polym8010006
Fimberger M, Tsekmes I-A, Kochetov R, Smit JJ, Wiesbrock F. Crosslinked Poly(2-oxazoline)s as “Green” Materials for Electronic Applications. Polymers. 2016; 8(1):6. https://doi.org/10.3390/polym8010006
Chicago/Turabian StyleFimberger, Martin, Ioannis-Alexandros Tsekmes, Roman Kochetov, Johan J. Smit, and Frank Wiesbrock. 2016. "Crosslinked Poly(2-oxazoline)s as “Green” Materials for Electronic Applications" Polymers 8, no. 1: 6. https://doi.org/10.3390/polym8010006