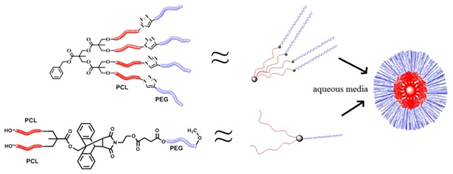
Synthesis and Characterization of Biodegradable Amphiphilic Star and Y-Shaped Block Copolymers as Potential Carriers for Vinorelbine
Abstract
:1. Introduction
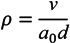

2. Experimental Section
2.1. Materials and Instrumentation
2.2. Syntheses and Polymerizations
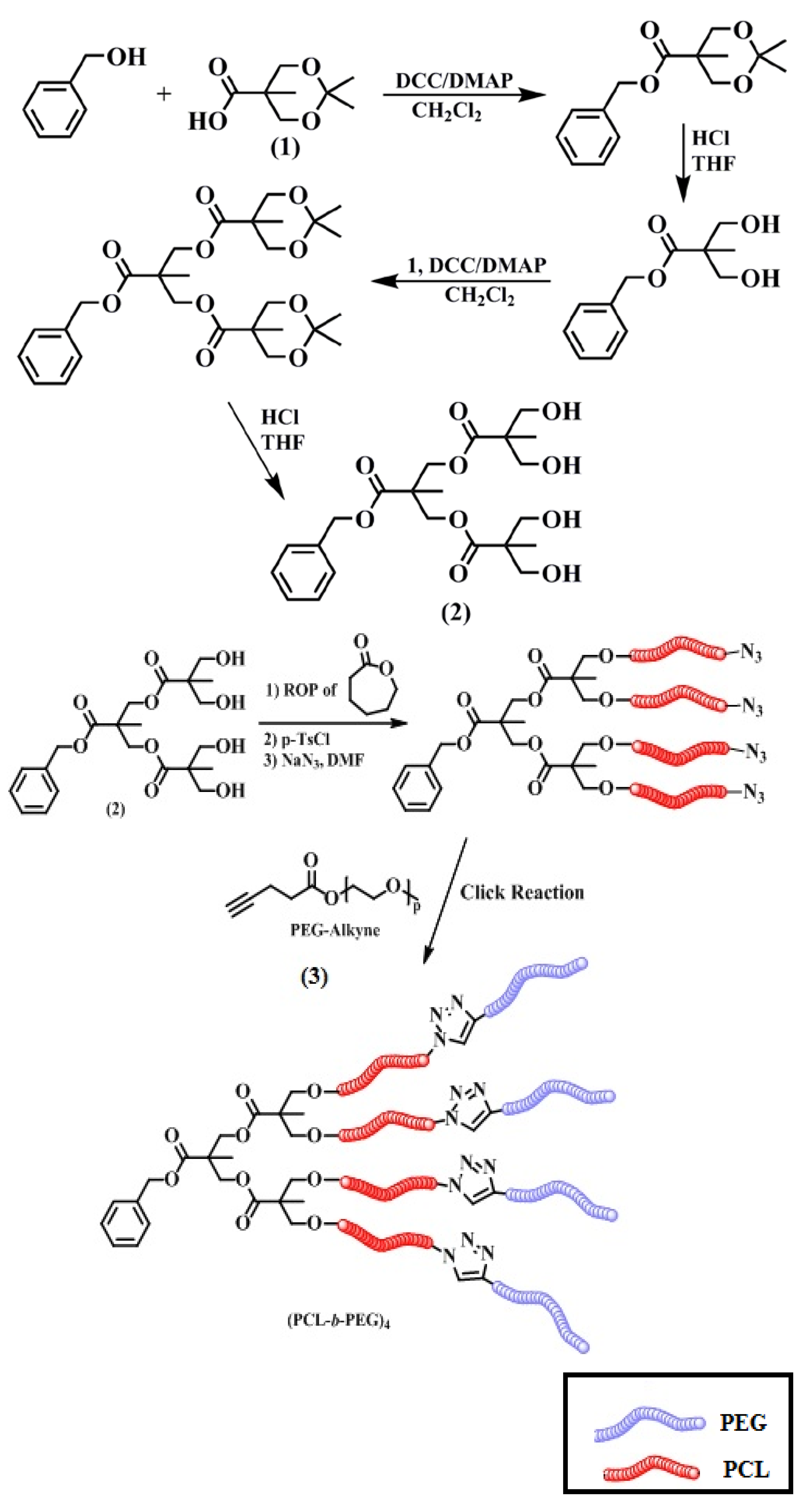
2.2.1. Synthesis of 2-(Benzyloxycarbonyl)-2-methylpropane-1,3-diyl bis[3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate] (2)
2.2.2. Preparation of Azide End-Functionalized PCL Star Polymer (PCL-Azide)4
2.2.3. Synthesis of (A-b-B)4 Type Star Polymer
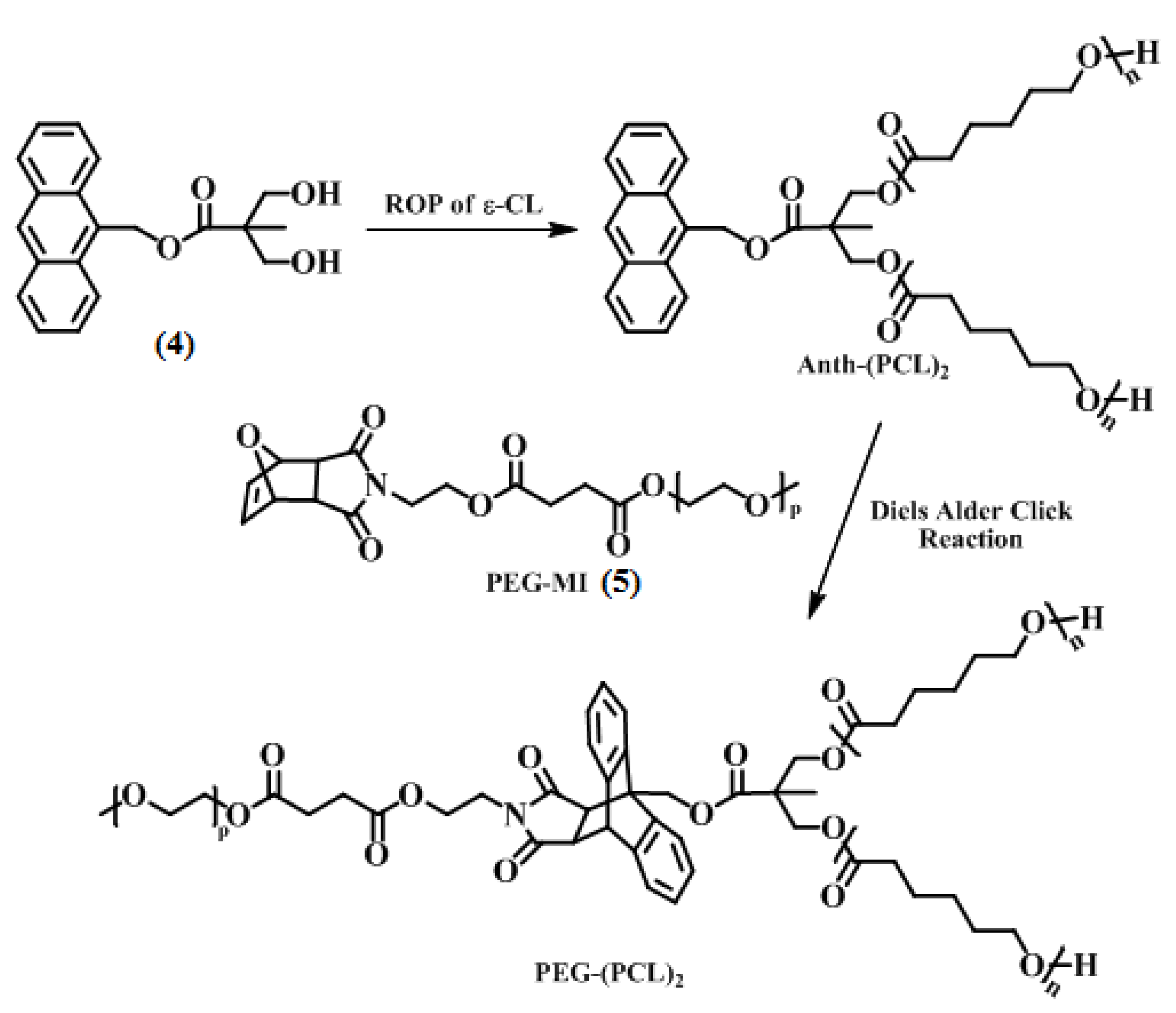
2.2.4. Synthesis of Anthracene End-Functionalized Poly(e-caprolactone) [Anth-(PCL)2]
2.2.5. Synthesis of Y-shaped PEG–(PCL)2 Block Copolymer via Diels-Alder Click Reaction
2.3. Preparation of Micelles
2.3.1. Preparation of Free Polymeric Micelles
2.3.2. Preparation of Drug Loaded Polymeric Micelles and Determination of Vinorelbine Concentration Associated with Polymeric Micelles, Using Elisa Reader Spectrophotometer
2.4. Particle Size Determination
2.5. Determination of the Critical Micelle Concentration (CMC)
2.6. Stability of Vinorelbine Associated with Polymeric Micelles Formulation after Lyophilization
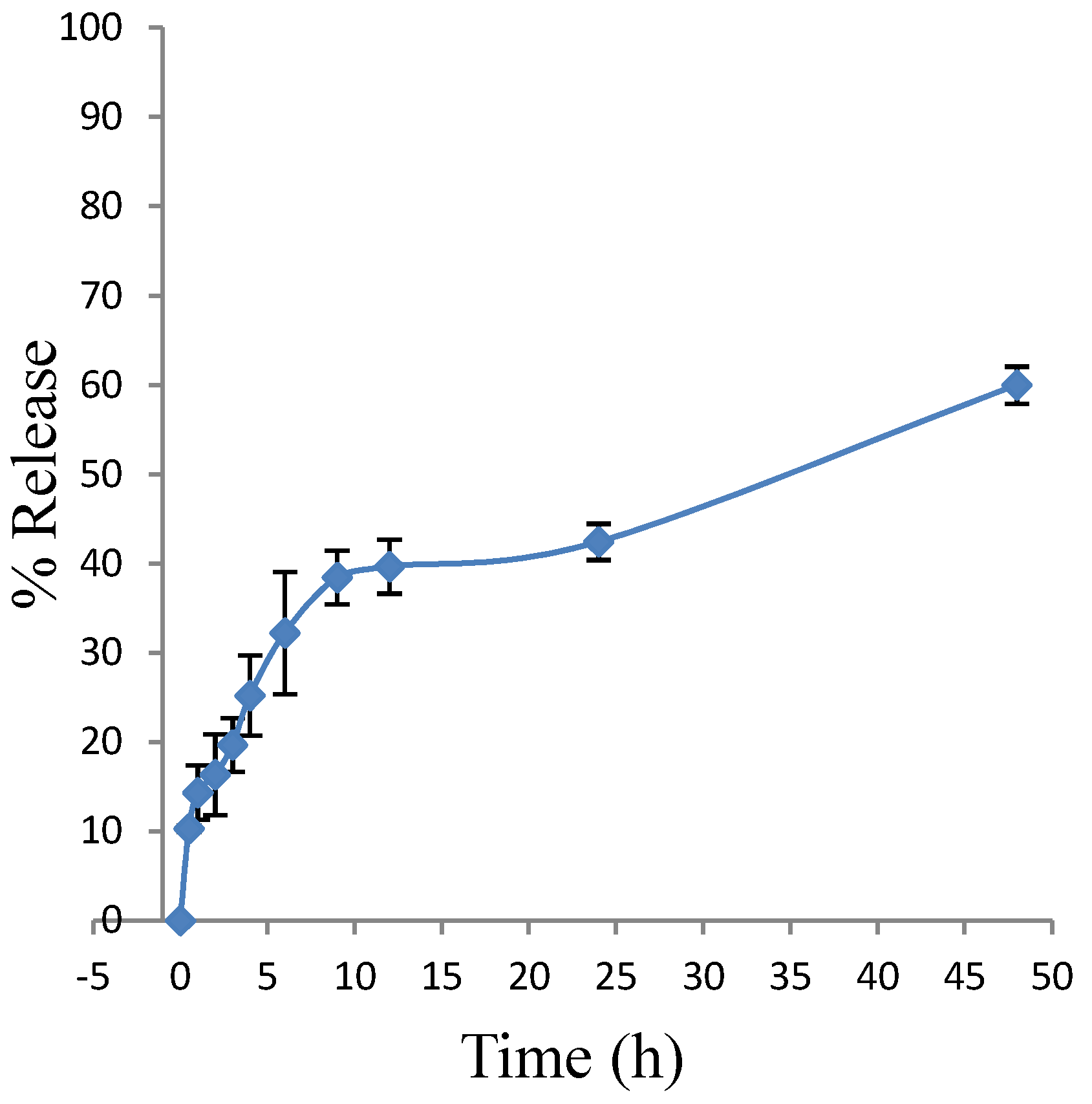
2.7. Drug Release Studies by Dialysis of Polymeric Micelles Containing Vinorelbine (Pol-VLB)
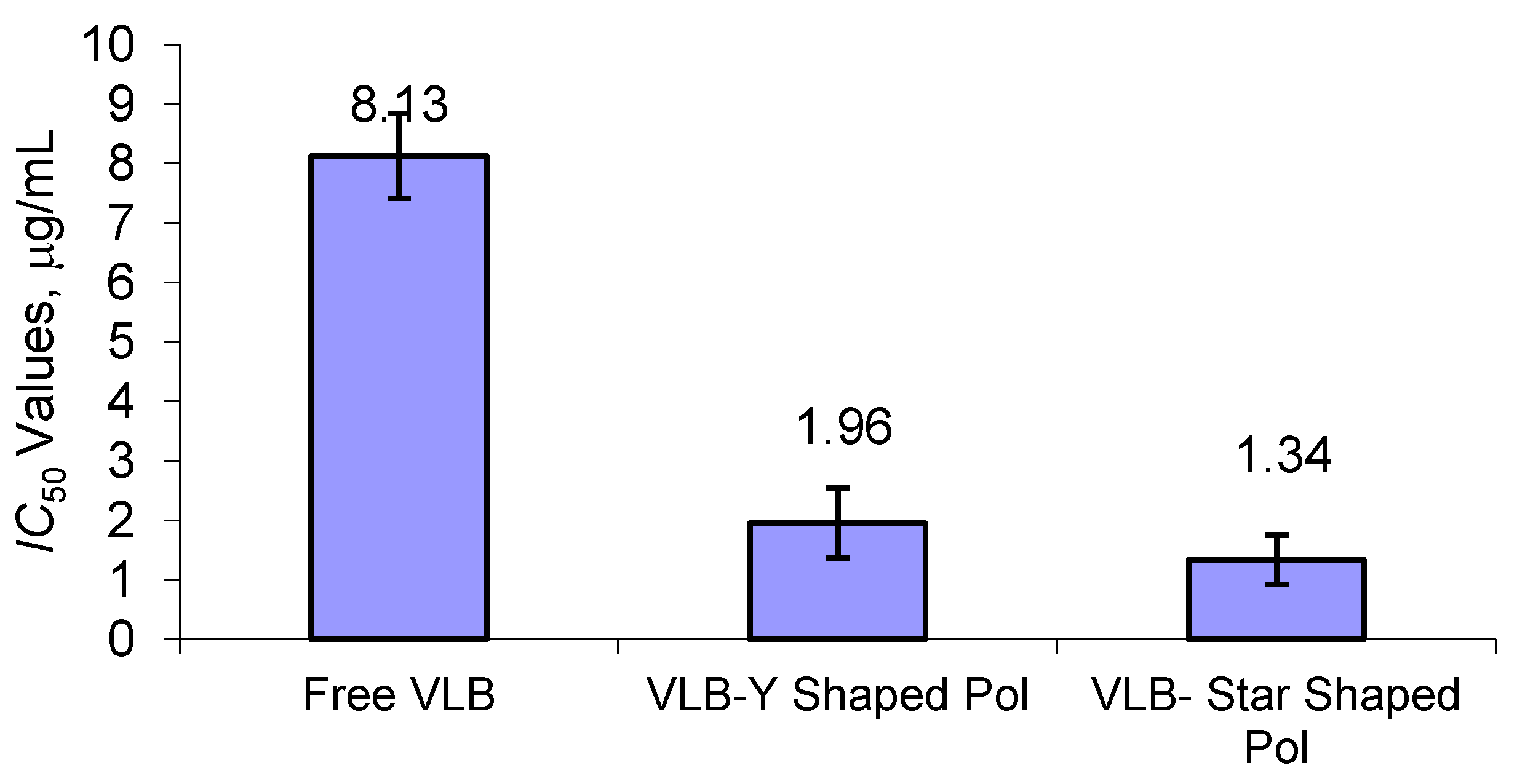
2.8. Investigation of Cytotoxic Activity of Polymeric Nano-VLB Formulations
3. Results and Discussion
3.1. Synthesis of (A-b-B)4 Type Star Polymers
| Polymer | Mn,theo | Mn,NMR | GPC | |
|---|---|---|---|---|
| Mn | Mw/Mn | |||
| PEG-alkyne a | 630 | 685 | 550 | 1.11 |
| (PCL)4b | 6,250 c | 7,200 | 5,400 d | 1.02 |
| (PCL-OTs)4 | 6,900 | 7,850 | 5,250 d | 1.05 |
| (PCL-azide)4 | 6,400 | 7,350 | 5,000 d | 1.05 |
| (PCL)4-(PEG)4 | 10,100 | 9,850 | 8,500 | 1.14 |
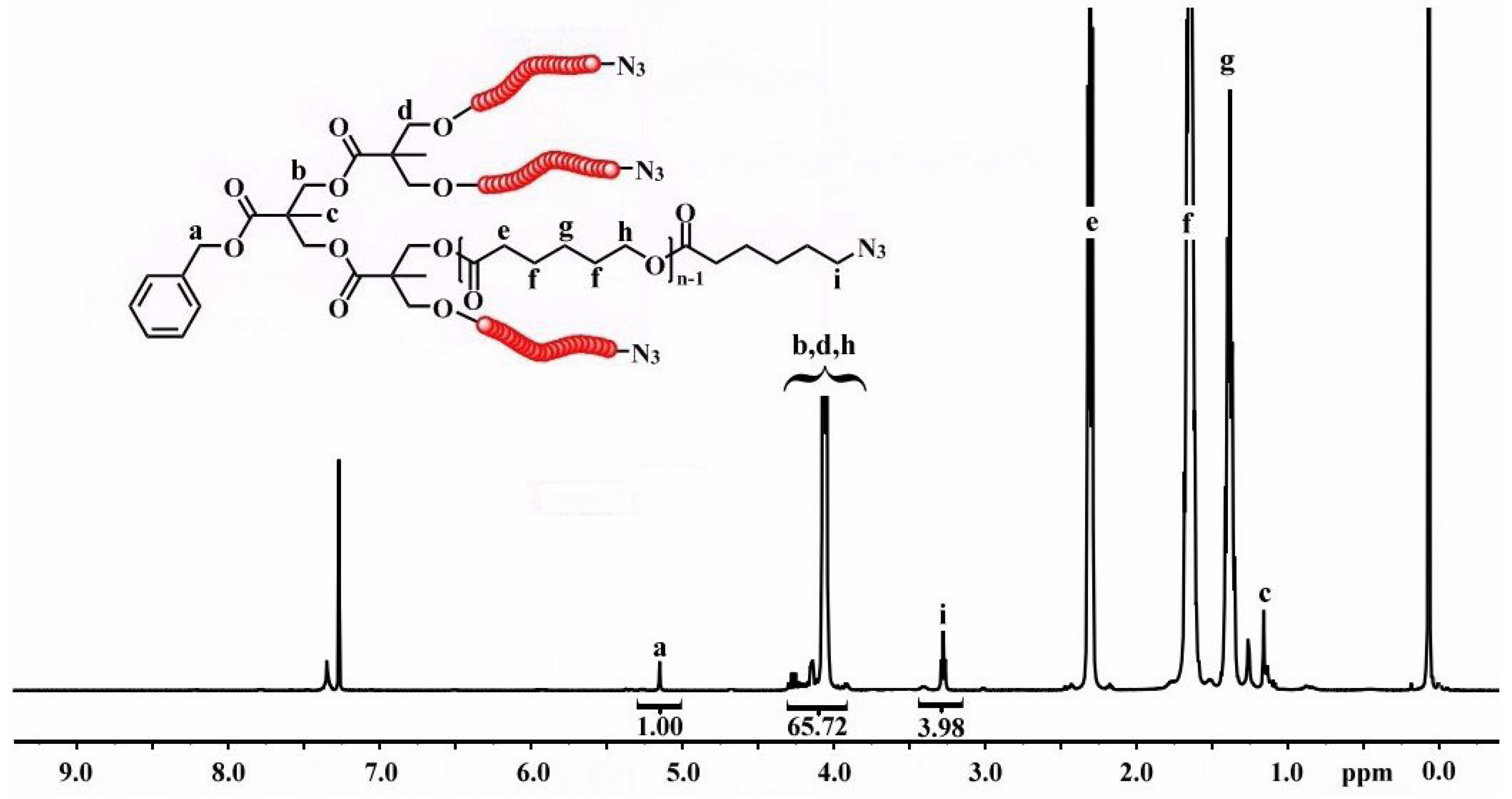

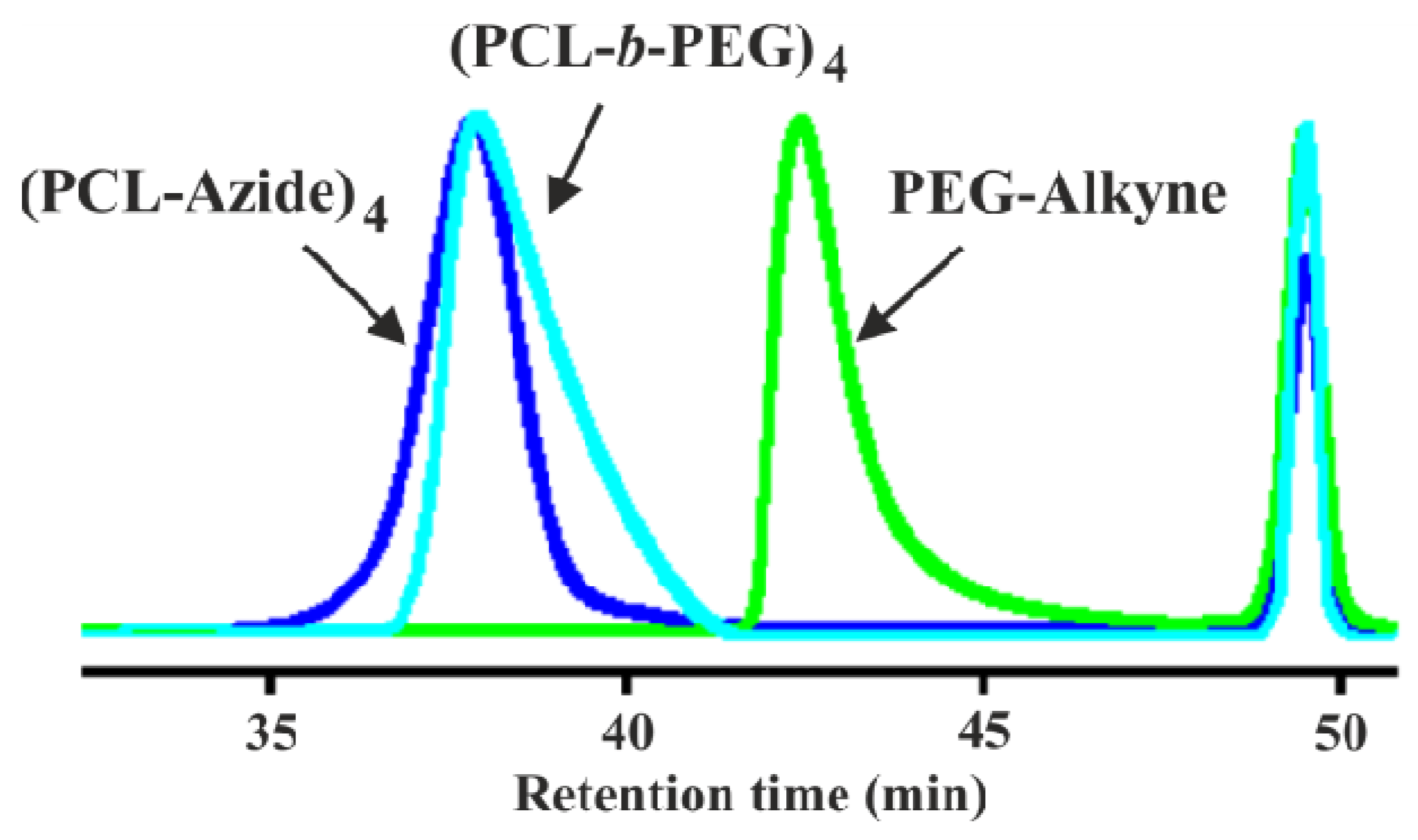
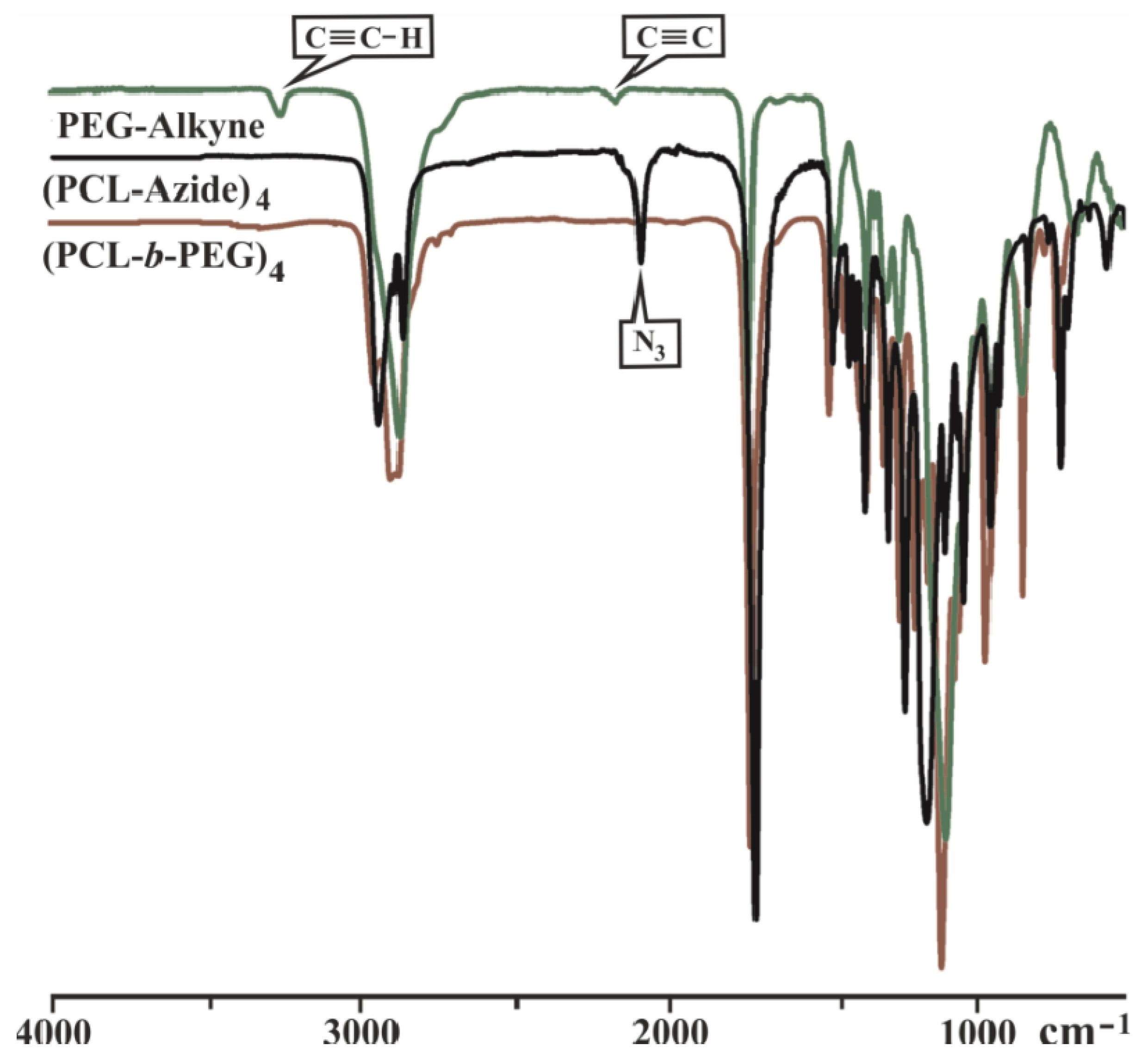
3.2. Synthesis of Y-Shaped Block Copolymer
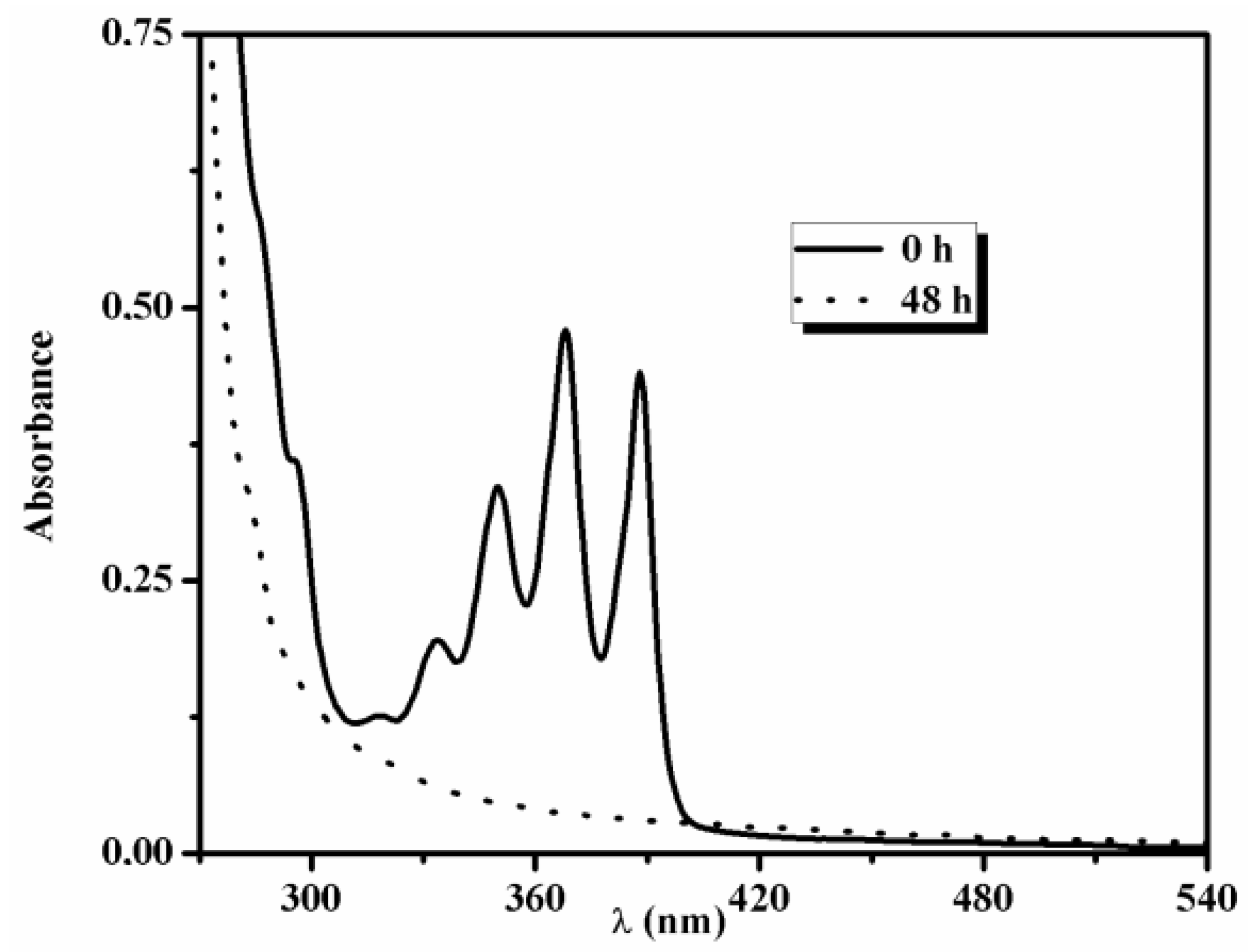
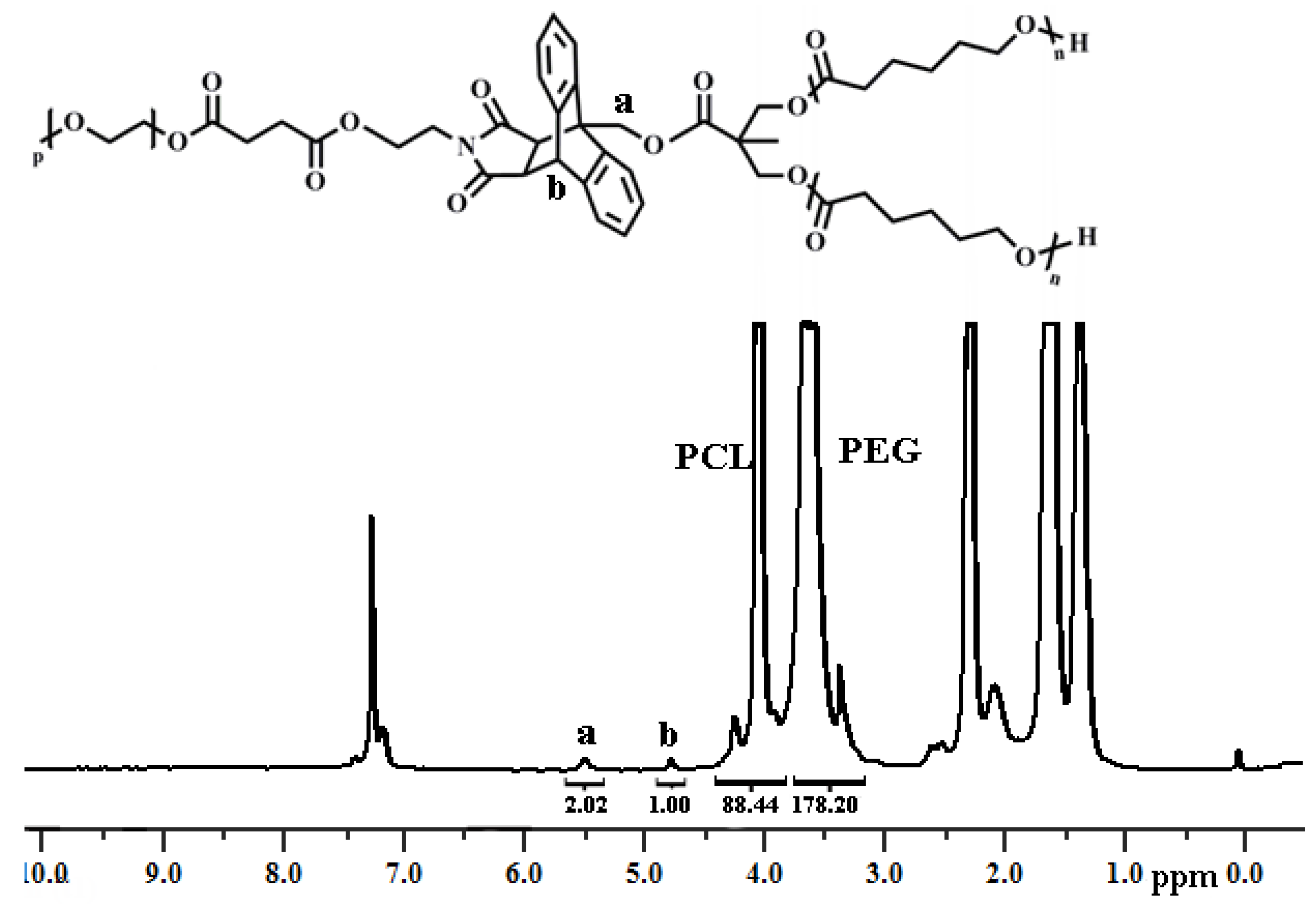
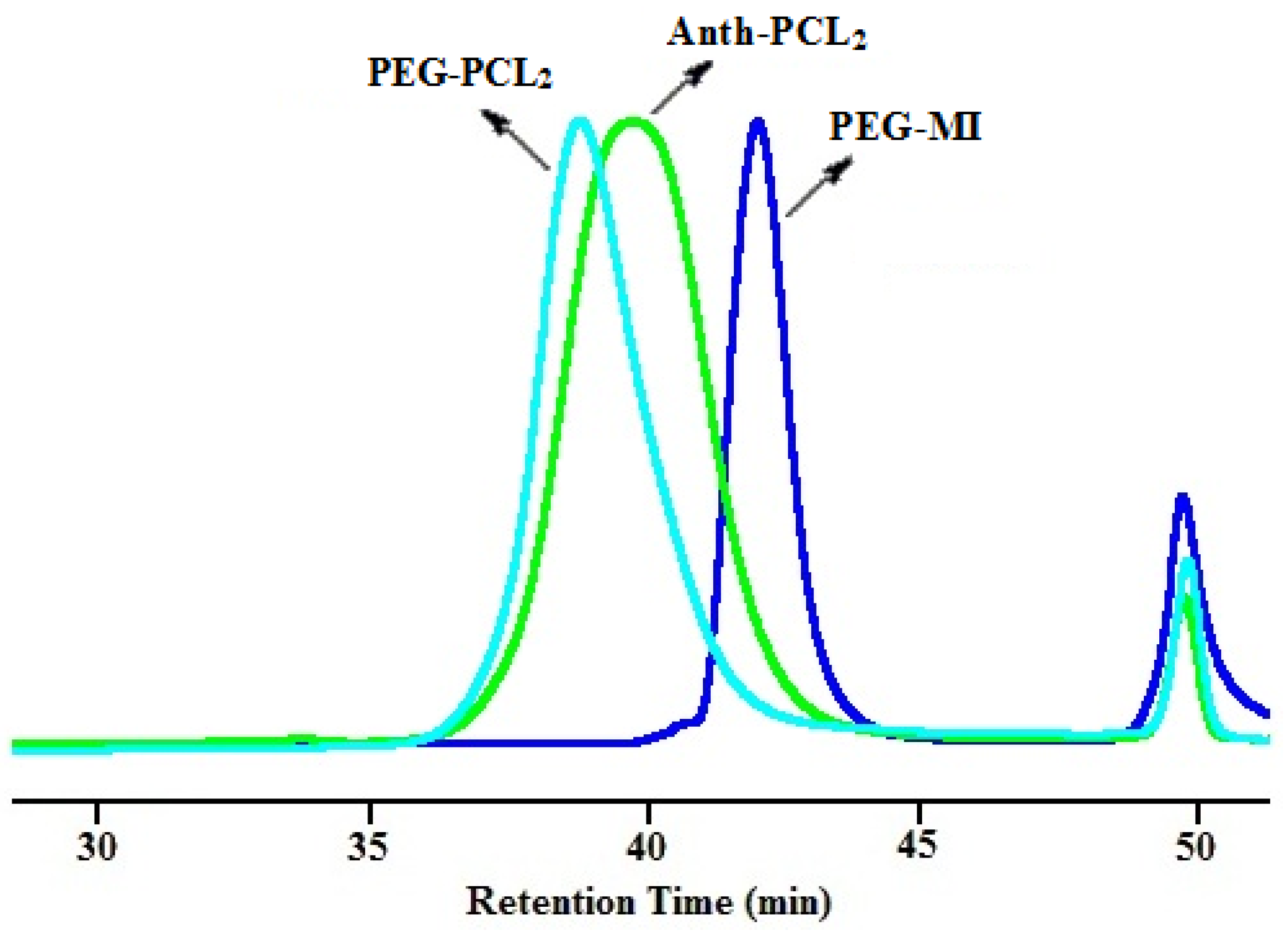
| Polymer | Ini. | Time (h) | Conversionb (%) | Mn,GPC (g/mol) | Mw/Mn | Mn,theo (g/mol) | Mn,NMR (g/mol) | Mn,TD-GPC (g/mol) |
|---|---|---|---|---|---|---|---|---|
| PEG-MI | - | - | - | 3000 | 1.05 | 2350 | 2600 | - |
| Anth-(PCL)2a | 4 | 12 | 85 | 6000 | 1.14 | 4750 c | 5350 | 5100 |
| PEG–(PCL)2 | - | 48 | - | 7700 | 1.10 | 8000 d | 6450 | 5500 |
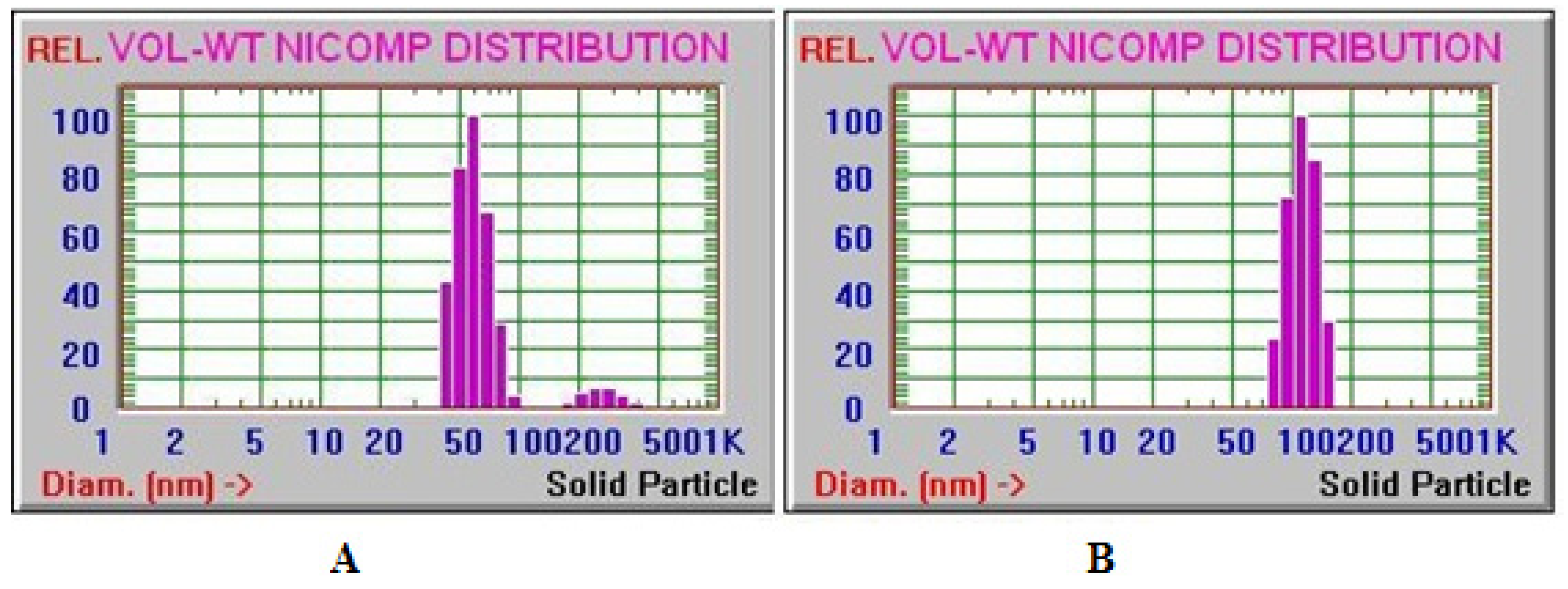
3.3. Particle Size Determination
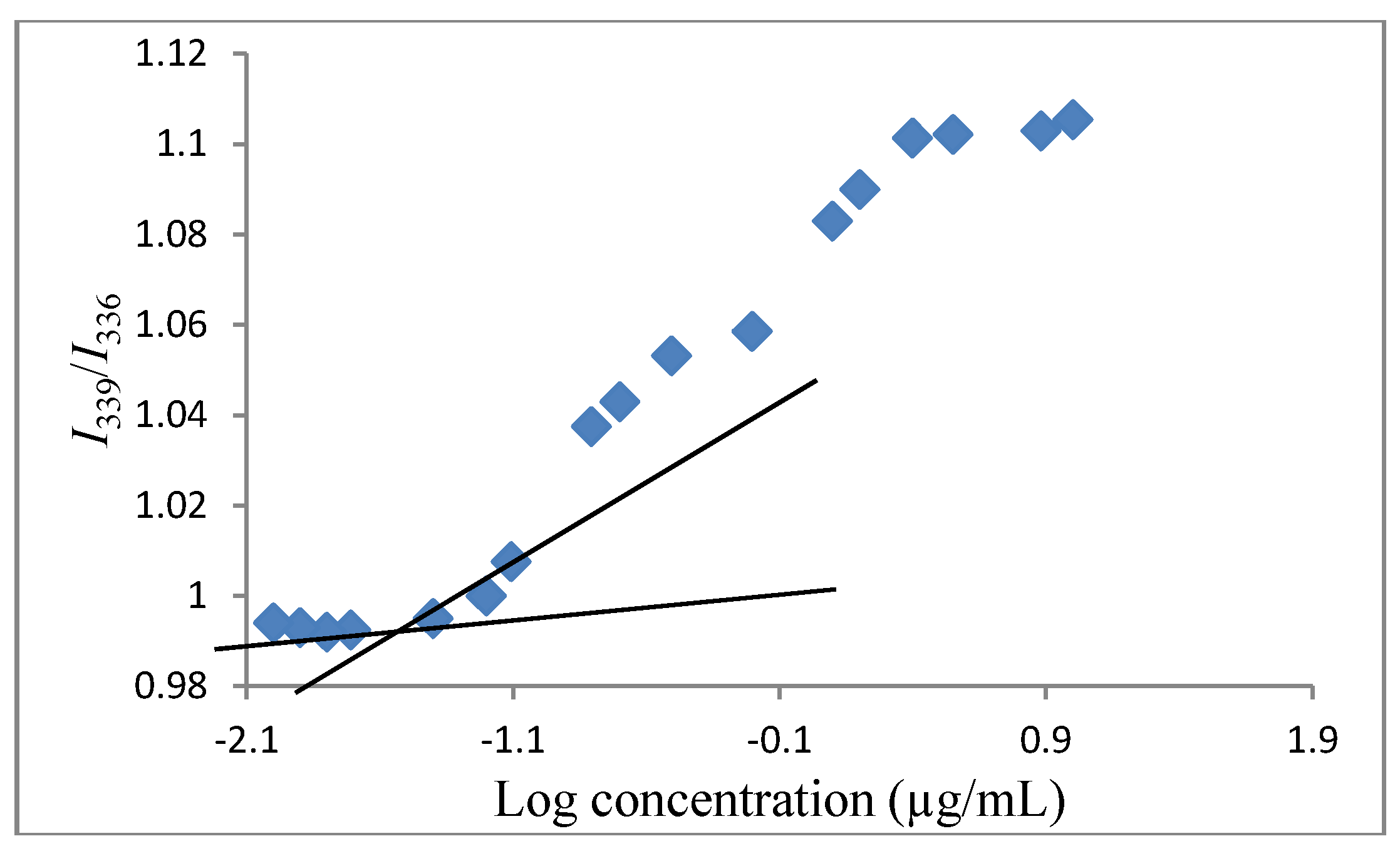
3.4. Determination of the Critical Micelle Concentration (CMC)
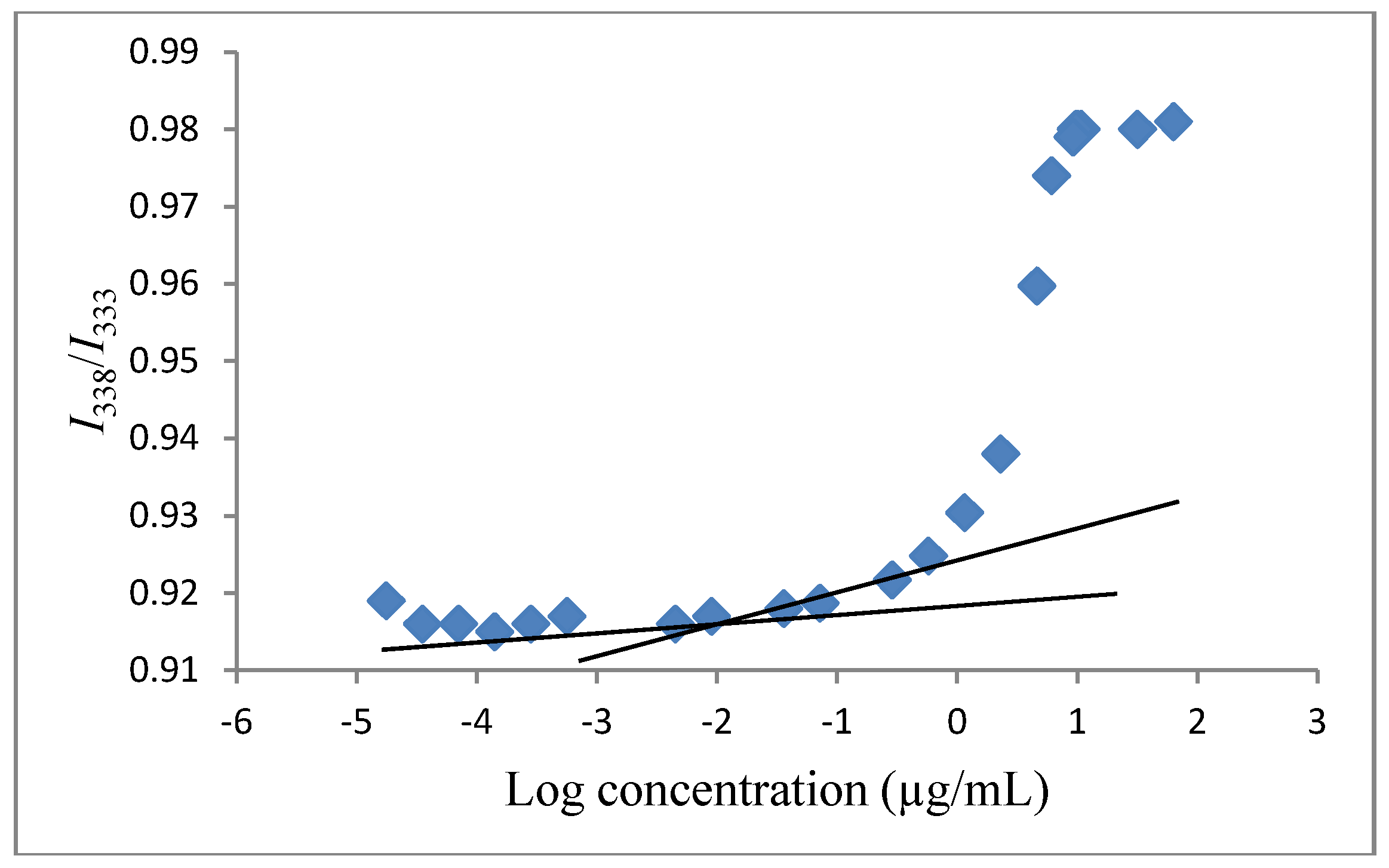
3.5. Determination of Vinorelbine Concentration Associated with Polymeric Micelles by Using ELISA Reader Spectrophotometer
| Total VLB used in the assay (µg/mL) | Drug loading efficacy calculated using regular centrifuge tubes, %/µg·mL−1, (Standard Deviation) | Drug incorporated with micelles calculated using centrifugal filter tubes %/µg·mL−1, (Standard Deviation) | Free VLB dissolved in PBS (µg/mL) |
|---|---|---|---|
| 25 | 100/25 (±0.5) | 20/5 (±0.3) | 20 |
| 50 | 100/50 (±0.5) | 60/30 (±0.7) | 20 |
| 100 | 70/70 (±1.0) | 70/70 ±0.4 | 30 |
| 150 | 63.3/94.5 (±1.5) | 50/75 (±0.4) | 75 |
| 200 | 47.5/95 (±1.5) | 37.5/75 ±0.7 | 125 |
| 250 | 32/80 (±2.5) | 24/60 ±0.9 | 190 |
| 300 | 23.3/69.9 (±1.5) | 16.6/50 ±0.9 | 250 |
| Total VLB used in the assay (µg/mL) | Drug loading efficacy calculated using regular centrifuge tubes %/µg·mL−1, (Standard Deviation) | Drug incorporated with micelles calculated using centrifugal filter tubes %/µg·mL−1, (Standard Deviation) | Free VLB dissolved in PBS (µg/mL) |
|---|---|---|---|
| 150 | 100/150 (±4.6) | 86.6/130 (±0.8) | 20 |
| 200 | 100/200 (±5.8) | 90180 (±0.8) | 20 |
| 250 | 100/250 (±4.6) | 92/230 (±0.4) | 20 |
| 300 | 100/300 (±3.2) | 93.3/280 (±0.9) | 20 |
| 350 | 100/350 (±3.2) | 94.2/330 (±0.1) | 20 |
| 400 | 87.5/350 (±3.6) | 80/320 (±0.5) | 80 |
| 450 | 76.7/345 (±6.4) | 68/305 (±0.6) | 145 |
3.6. Stability of Vinorelbine Associated with Polymeric Micelle Formulations after Lyophilization
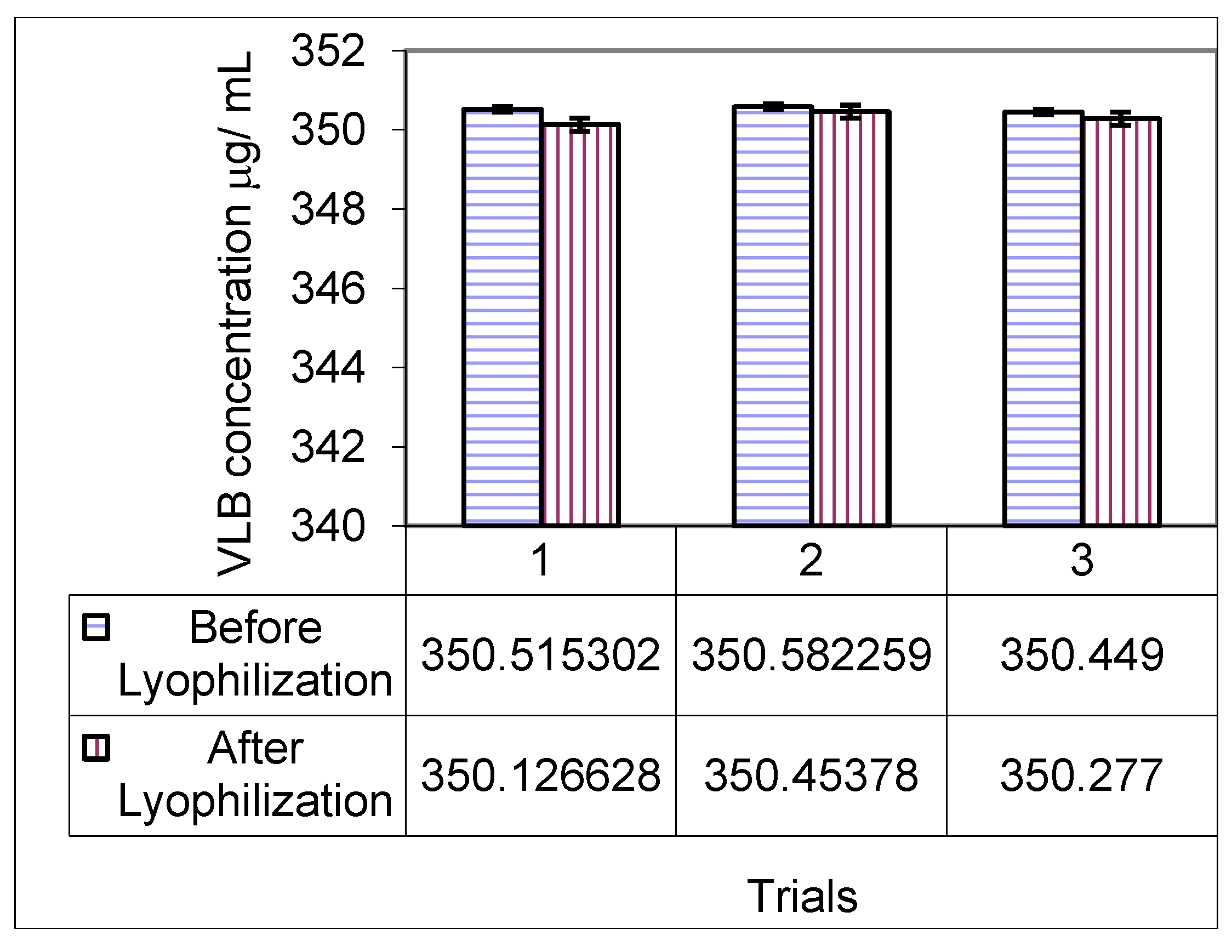
3.7. Drug Release Studies by Dialysis of Polymeric Micelles Containing Vinorelbine (Pol-VLB)

3.8. Cytotoxic Activity of Polymeric Nano-VLB Formulations

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rosler, A.; Vandermeulen, G.W.M.; Klok, H.A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2001, 53, 95–108. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
- Smart, T.; Lomas, H.; Massignani, M.; Flores-Merino, M.V.; Perez, L.R.; Battaglia, G. Block copolymer nanostructures. Nano Today 2008, 3, 38–46. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Wang, F.; Bronich, T.K.; Kabanov, A.V.; Rauh, R.D.; Roovers, J. Synthesis and characterization of star poly(epsilon-caprolactone)-b-poly(ethylene glycol) and poly(l-lactide)-b-poly(ethylene glycol) copolymers: Evaluation as drug delivery carriers. Bioconjug. Chem. 2008, 19, 1423–1429. [Google Scholar] [CrossRef]
- Semple, S.C.; Leone, R.; Wang, J.; Leng, E.C.; Klimuk, S.K.; Eisenhardt, M.L.; Yuan, Z.-N.; Edwards, K.; Maurer, N.; Hope, M.J.; et al. Optimization and characterization of a sphingomyelin/cholesterol liposome formulation of vinorelbine with promising antitumor activity. J. Pharm. Sci. 2005, 94, 1024–1038. [Google Scholar] [CrossRef]
- Zheng, C.; Qiu, L.; Yao, X.; Zhu, K. Novel micelles from graft polyphosphazenes as potential anti-cancer drug delivery systems: Drug encapsulation and in vitro evaluation. Int. J. Pharm. 2009, 373, 133–140. [Google Scholar] [CrossRef]
- Kim, J.H.; Emoto, K.; Iijima, M.; Nagasaki, Y.; Aoyagi, T.; Okano, T.; Sakurai, Y.; Kataoka, K. Core-stabilized polymeric micelle as potential drug carrier: Increased solubilization of taxol. Polym. Adv. Technol. 1999, 10, 647–654. [Google Scholar] [CrossRef]
- Liu, H.B.; Jiang, A.; Guo, J.A.; Uhrich, K.E. Unimolecular micelles: Synthesis and characterization of amphiphilic polymer systems. J. Polym. Sci. A Polym. Chem. 1999, 37, 703–711. [Google Scholar] [CrossRef]
- Kim, K.H.; Cui, G.H.; Lim, H.J.; Huh, J.; Ahn, C.H.; Jo, W.H. Synthesis and micellization of star-shaped poly(ethylene glycol)-block-poly(epsilon-caprolactone). Macromol. Chem. Phys. 2004, 205, 1684–1692. [Google Scholar] [CrossRef]
- Deng, M.X.; Chen, X.S.; Piao, L.H.; Zhang, X.F.; Dai, Z.L.; Jing, X.B. Synthesis of four-armed poly(epsilon-caprolactone)-block-poly(ethylene oxide) by diethylzinc catalyst. J. Polym. Sci. A Polym. Chem. 2004, 42, 950–959. [Google Scholar] [CrossRef]
- Zeng, F.Q.; Lee, H.; Chidiac, M.; Allen, C. Synthesis and characterization of six-arm star poly(δ-valerolactone)-block-methoxy poly(ethylene glycol) copolymers. Biomacromolecules 2005, 6, 2140–2149. [Google Scholar]
- Roovers, J.; Toporowski, P.M.; Douglas, J. Thermodynamic properties of dilute and semidilute solutions of regular star polymers. Macromolecules 1995, 28, 7064–7070. [Google Scholar] [CrossRef]
- Iijima, M.; Nagasaki, Y.; Okada, T.; Kato, M.; Kataoka, K. Core-polymerized reactive micelles from heterotelechelic amphiphilic block copolymers. Macromolecules 1999, 32, 1140–1146. [Google Scholar] [CrossRef]
- Wang, F.; Bronich, T.K.; Kabanov, A.V.; Rauh, R.D.; Roovers, J. Synthesis and evaluation of a star amphiphilic block copolymer from poly(ε-caprolactone) and poly(ethylene glycol) as a potential drug delivery carrier. Bioconjug. Chem. 2005, 16, 397–405. [Google Scholar] [CrossRef]
- Nystrom, A.M.; Fadeel, B. Safety assessment of nanomaterials: Implications for nanomedicine. J. Control. Release 2012, 161, 403–408. [Google Scholar] [CrossRef]
- Jia, L.; Yan, L.; Li, Y. Synthesis of biodegradable amphiphilic Y-shaped block co-polymers via Ring-opening polymerization for drug delivery. J. Biomater. Sci. Polym. Ed. 2011, 22, 1197–1213. [Google Scholar]
- Zhang, H.-H.; Huang, Z.-Q.; Sun, B.-W.; Guo, J.-X.; Wang, J.-L.; Chen, Y.-Q. Y-shaped poly(ethylene glycol) and poly(trimethylene carbonate) amphiphilic copolymer: Synthesis and for drug delivery. J. Polym. Sci. A Polym. Chem. 2008, 46, 8131–8140. [Google Scholar] [CrossRef]
- Rao, J.; Zhang, Y.; Zhang, J.; Liu, S. Facile preparation of well-defined AB2Y-shaped miktoarm star polypeptide copolymer via the combination of ring-opening polymerization and click chemistry. Biomacromolecules 2008, 9, 2586–2593. [Google Scholar] [CrossRef]
- Sun, J.; Chen, X.; Guo, J.; Shi, Q.; Xie, Z.; Jing, X. Synthesis and self-assembly of a novel Y-shaped copolymer with a helical polypeptide arm. Polymer 2009, 50, 455–461. [Google Scholar] [CrossRef]
- Kohno, E.; Murase, S.; Nishikata, M.; Okamura, N.; Matzno, S.; Kuwahara, T.; Matsuyama, K. Methods of preventing vinorelbine-induced phlebitis: An experimental study in rabbits. Int. J. Med. Sci. 2008, 5, 218–223. [Google Scholar]
- Taylor, R.L.; Williams, D.M.; Craven, P.C.; Graybill, J.R.; Drutz, D.J.; Magee, W.E. Amphotericin-B in liposomes—A novel therapy for histoplasmosis. Am. Rev. Respir. Dis. 1982, 125, 610–611. [Google Scholar]
- Koukoulitsa, C.; Kyrikou, I.; Demetzos, C.; Mavromoustakos, T. The role of the anticancer drug vinorelbine in lipid bilayers using differential scanning calorimetry and molecular modeling. Chem. Phys. Lipids 2006, 144, 85–95. [Google Scholar] [CrossRef]
- Tunca, U.; Ozyurek, Z.; Erdogan, T.; Hizal, G. Novel miktofunctional initiator for the preparation of an ABC-type miktoarm star polymer via a combination of controlled polymerization techniques. J. Polym. Sci. A Polym. Chem. 2004, 42, 4228–4236. [Google Scholar] [CrossRef]
- Durmaz, H.; Dag, A.; Altintas, O.; Erdogan, T.; Hizal, G.; Tunca, U. One-pot synthesis of ABC type triblock copolymers via in situ click [3 + 2] and Diels-Alder [4 + 2] reactions. Macromolecules 2006, 40, 191–198. [Google Scholar]
- Altintas, O.; Hizal, G.; Tunca, U. Synthesis of an ABCD 4-miktoarm star quaterpolymer through a Diels-Alder click reaction. Des. Monomers Polym. 2009, 12, 83–98. [Google Scholar] [CrossRef]
- Dag, A.; Durmaz, H.; Hizal, G.; Tunca, U. Preparation of 3-arm star polymers (A3) via Diels-Alder click reaction. J. Polym. Sci. A Polym. Chem. 2008, 46, 302–313. [Google Scholar] [CrossRef]
- Ihre, H.; Hult, A.; Fréchet, J.M.J.; Gitsov, I. Double-stage convergent approach for the synthesis of functionalized dendritic aliphatic polyesters based on 2,2-bis(hydroxymethyl)propionic acid. Macromolecules 1998, 31, 4061–4068. [Google Scholar] [CrossRef]
- Ward, G.A. Measurements of binding thermodynamics in drug discovery. Drug Discov. Today 2005, 10, 1543–1551. [Google Scholar] [CrossRef]
- Lv, Q.; Yu, A.; Xi, Y.; Li, H.; Song, Z.; Cui, J.; Cao, F.; Zhai, G. Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int. J. Pharm. 2009, 372, 191–198. [Google Scholar] [CrossRef]
- Markovsky, E.; Koroukhov, N.; Golomb, G. Additive-free albumin nanoparticles of alendronate for attenuating inflammation through monocyte inhibition. Nanomedicine 2007, 2, 545–553. [Google Scholar] [CrossRef]
- Zhou, X.J.; Placidi, M.; Rahmani, R. Uptake and metabolism of vinca alkaloids by freshly isolated human hepatocytes in suspension. Anticancer Res. 1994, 14, 1017–1022. [Google Scholar]
- Alkan Onyuksel, H.; Ramakrishnan, S.; Chai, H.B.; Pezzuto, J.M. A mixed micellar formulation suitable for the parenteral administration of taxol. Pharm. Res. 1994, 11, 206–212. [Google Scholar] [CrossRef]
- Onyuksel, H.; Mohanty, P.S.; Rubinstein, I. VIP-grafted sterically stabilized phospholipid nanomicellar 17-allylamino-17-demethoxy geldanamycin: A novel targeted nanomedicine for breast cancer. Int. J. Pharm. 2009, 365, 157–161. [Google Scholar] [CrossRef]
- Dagar, S.; Krishnadas, A.; Rubinstein, I.; Blend, M.J.; Onyuksel, H. VIP grafted sterically stabilized liposomes for targeted imaging of breast cancer: In vivo studies. J. Control. Release 2003, 91, 123–133. [Google Scholar] [CrossRef]
- Krishnadas, A.; Rubinstein, I.; Onyuksel, H. Sterically stabilized phospholipid mixed micelles: In vitro evaluation as a novel carrier for water-insoluble drugs. Pharm. Res. 2003, 20, 297–302. [Google Scholar] [CrossRef]
- Ashok, B.; Arleth, L.; Hjelm, R.P.; Rubinstein, I.; Onyuksel, H. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: Effects of PEG chain length and PC incorporation. J. Pharm. Sci. 2004, 93, 2476–2487. [Google Scholar] [CrossRef]
- Onyuksel, H.; Koo, O.M.; Sethi, V.; Rubinstein, I. Sterically stabilized phospholipid micellar human vasoactive intestinal peptide: A novel disease-modifying drug for rheumatoid arthritis. Arthritis Rheum. 2005, 52, S262–S262. [Google Scholar] [CrossRef]
- Cho, Y.W.; Lee, J.; Lee, S.C.; Huh, K.M.; Park, K. Hydrotropic agents for study of in vitro paclitaxel release from polymeric micelles. J. Control. Release 2004, 97, 249–257. [Google Scholar] [CrossRef]
- Morris, P.G.; Fornier, M.N. Microtubule active agents: Beyond the taxane frontier. Clin. Cancer Res. 2008, 14, 7167–7172. [Google Scholar] [CrossRef]
- Michalowski, C.B.; Guterres, S.S.; Dalla Costa, T. Microdialysis for evaluating the entrapment and release of a lipophilic drug from nanoparticles. J. Pharm. Biomed. Anal. 2004, 35, 1093–1100. [Google Scholar] [CrossRef]
- Johnston, M.J.W.; Semple, S.C.; Klimuk, S.K.; Edwards, K.; Eisenhardt, M.L.; Leng, E.C.; Karlsson, G.; Yanko, D.; Cullis, P.R. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim. Biophys. Acta Biomembr. 2006, 1758, 55–64. [Google Scholar]
- Onyuksel, H.; Jeon, E.; Rubinstein, I. Nanomicellar paclitaxel increases cytotoxicity of multidrug resistant breast cancer cells. Cancer Lett. 2009, 274, 327–330. [Google Scholar] [CrossRef]
- Allahverdiyev, A.; Duran, N.; Ozguven, M.; Koltas, S. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedicine 2004, 11, 657–661. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B Biointerfaces 1999, 16, 3–27. [Google Scholar] [CrossRef]
- Vagberg, L.J.M.; Cogan, K.A.; Gast, A.P. Light-scattering study of starlike polymeric micelles. Macromolecules 1991, 24, 1670–1677. [Google Scholar] [CrossRef]
- Cogan, K.A.; Gast, A.P. Effect of water on diblock copolymers in oil–Large aggregates, micelles, and microemulsions. Macromolecules 1990, 23, 745–753. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bahadori, F.; Dag, A.; Durmaz, H.; Cakir, N.; Onyuksel, H.; Tunca, U.; Topcu, G.; Hizal, G. Synthesis and Characterization of Biodegradable Amphiphilic Star and Y-Shaped Block Copolymers as Potential Carriers for Vinorelbine. Polymers 2014, 6, 214-242. https://doi.org/10.3390/polym6010214
Bahadori F, Dag A, Durmaz H, Cakir N, Onyuksel H, Tunca U, Topcu G, Hizal G. Synthesis and Characterization of Biodegradable Amphiphilic Star and Y-Shaped Block Copolymers as Potential Carriers for Vinorelbine. Polymers. 2014; 6(1):214-242. https://doi.org/10.3390/polym6010214
Chicago/Turabian StyleBahadori, Fatemeh, Aydan Dag, Hakan Durmaz, Nese Cakir, Hayat Onyuksel, Umit Tunca, Gulacti Topcu, and Gurkan Hizal. 2014. "Synthesis and Characterization of Biodegradable Amphiphilic Star and Y-Shaped Block Copolymers as Potential Carriers for Vinorelbine" Polymers 6, no. 1: 214-242. https://doi.org/10.3390/polym6010214
APA StyleBahadori, F., Dag, A., Durmaz, H., Cakir, N., Onyuksel, H., Tunca, U., Topcu, G., & Hizal, G. (2014). Synthesis and Characterization of Biodegradable Amphiphilic Star and Y-Shaped Block Copolymers as Potential Carriers for Vinorelbine. Polymers, 6(1), 214-242. https://doi.org/10.3390/polym6010214