Radical-Scavenging Activity of Thiols, Thiobarbituric Acid Derivatives and Phenolic Antioxidants Determined Using the Induction Period Method for Radical Polymerization of Methyl Methacrylate
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
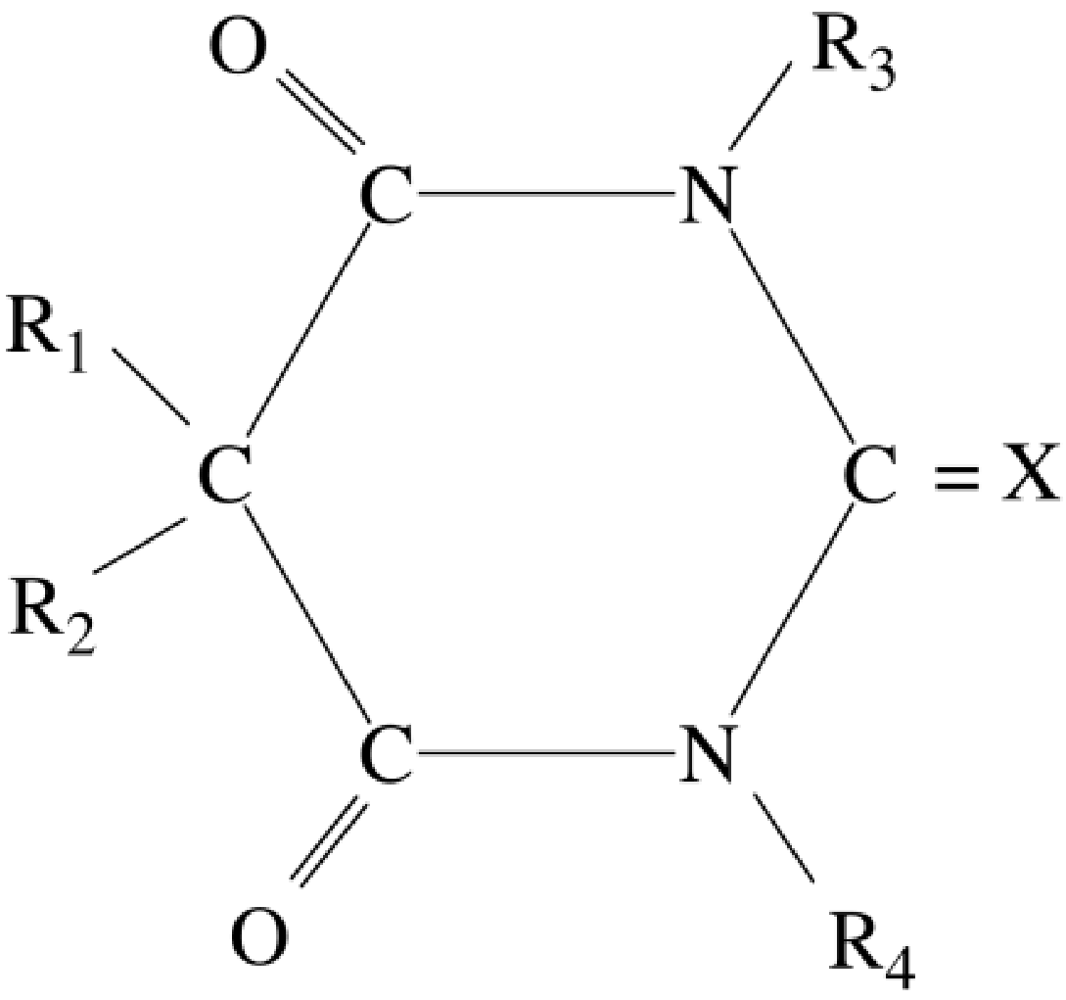
| Compound a | R1 | R2 | R3 | R4 | X |
|---|---|---|---|---|---|
| 1 | CH3 | H | CH3 | CH3 | S |
| 2 | CH3 | CH3 | CH3 | H | S |
| 3 | CH3 | CH3 | H | H | S |
| 4 | CH3CH2CH2CH2 - | H | H | H | S |
| 5 | CH3CH2CH2CH2- | H | H | H | O |
| 6 | H2C=CH-CH2- | H | H | H | S |
| 7 | H2C=CH-CH2- | H | CH3 | CH3 | S |
| 8 | H2C=CH-C6H5-CH2- | H | H | H | S |
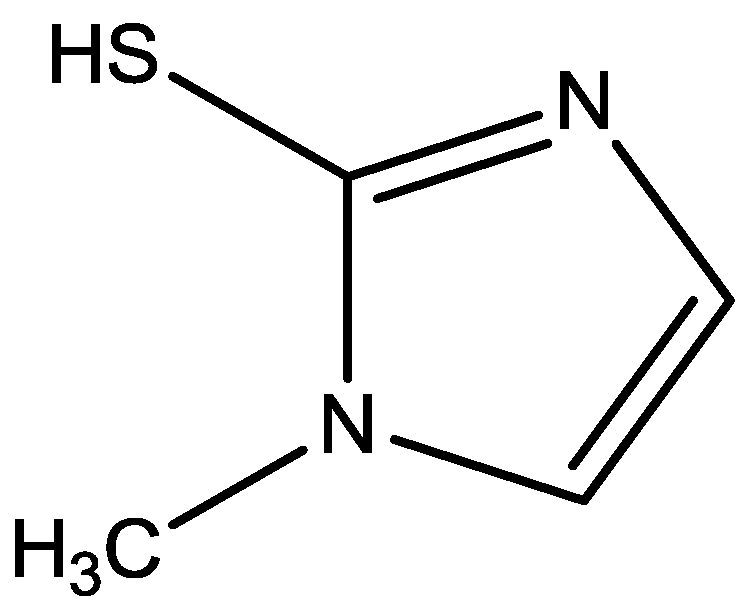
2.2. Induction Period (IP) and Initial Rate of Polymerization (IRP)
2.3. Measurement of Stoichiometric Factor (n)
2.4. Measurement of the Inhibition Rate Constant (kinh)
2.5. Determination of Molecular Weight
3. Results and Discussion
3.1. Thiol
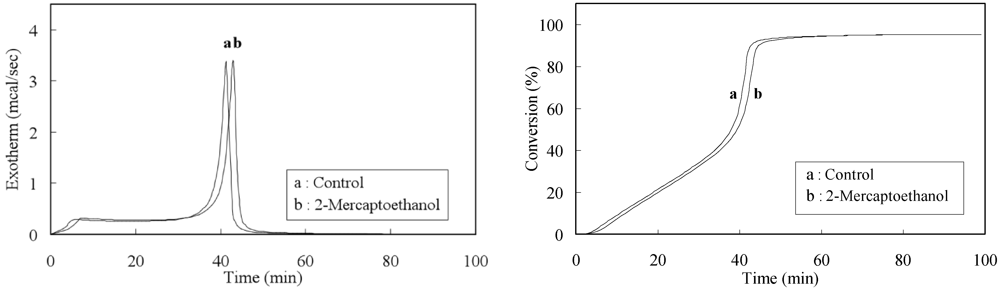
| Additives | Induction period(min) | Initial rate of polymerization (%/min) | Time at maximum exothermic peak (min) | Maximum exothermic rate (mcal/min) | Conversion (%) |
|---|---|---|---|---|---|
| None | 3.38 | 1.321 | 44.18 | 207.54 | 95.9 |
| ME (0.01 mol%) | 4.19 | 1.340 | 41.85 | 200.13 | 93.3 |
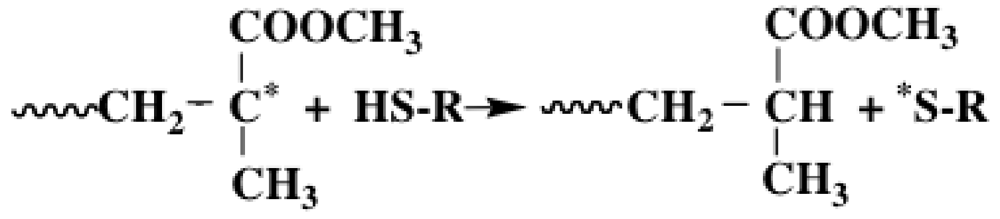
| Additive | Conc. mol% | IP a (min) | n b | IRP c (%/min) | (kinh/kp) d | (CI/CP) e |
|---|---|---|---|---|---|---|
| Experiment 1. Control | – | 0 | – | 1.30 | – | – |
| 2-Mercaptoethanol (ME) f | 0.01 | 1.27 | 0.4 | 1.30 | 60.65 | 0.01 |
| 0.01 | 0.81 g | 0.3 | 1.34 | 94.0 | 0.01 | |
| 0.05 | 4.63 | 0.3 | 1.28 | 16.82 | 0.01 | |
| 0.05 | 4.12 | 0.3 | 1.33 | 18.18 | 0.01 | |
| Experiment 2. Control | – | 0 | – | 1.38 | – | – |
| 2-Mercapto-1-methylimidazole (MMI) | 0.01 | 0.65 | 0.2 | 1.42 | 110.47 | 0.01 |
| 0.02 | 1.36 | 0.3 | 1.35 | 55.52 | 0.01 | |
| 0.05 | 3.54 | 0.3 | 1.33 | 21.57 | 0.01 | |
| Experiment 3. Control | – | 0 | – | 1.25 | – | – |
| 1,3,5-Trimethyl-2-thiobarbituric acid (1) | 1 | −2.06 | – | 1.36 | – | – |
| 1 | 1 | −1.99 | – | 1.42 | – | – |
| 1,5,5-Trimethyl-2-thiobarbituric acid (2) | 1 | 1.03 | 0.004 | 1.24 | 79.66 | 0.85 |
| 5,5-Dimethyl-thiobarbituric acid (3) | 1 | 24.06 | 0.9 | 1.17 | 3.53 | 0.04 |
| 5-Butyl-2-thiobarbituric acid (4) | 1 | 5.7 | 0.02 | 1.29 | 13.57 | 0.14 |
| 5-Butylbarbituric acid (5) | 1 | −0. 3 | – | 1.27 | 44.76 | 0.48 |
| Experiment 4. Control | – | 0 | – | 1.23 | – | – |
| 1 | 1 | −2.07 | – | 1.29 | – | – |
| 5-Allyl-2-thiobarbituric acid (6) | 1 | 5.48 | 0.02 | 1.27 | 14.33 | 0.15 |
| 5-Allyl-1,3-dimethyl-2-thiobarbituric acid (7) | 1 | 0.86 | – | 1.18 | – | – |
| 5-(4-Vinylbenzyl)-2-thiobarbituric acid (8) | 1 | 5.35 | 0.02 | 1.37 | 13.62 | 0.15 |
| Experiment 5. Control | – | 0 | – | 1.30 | – | – |
| p-Cresol (4-Methylphenol) | 1 | 59.0 | 2.2 | 1.29 | 1.48 | 0.02 |
| Eugenol (4-Allyl-2-methoxyphenol) | 1 | 79.0 | 2.0 | 1.08 | 1.17 | 0.01 |
| Guaiacol (2-Methoxyphenol) | 1 | 53.8 | 2.0 | 1.26 | 1.48 | 0.02 |
| Hydroquinone (1,4-Dihydroxybenzene) | 1 | 60.7 | 2.3 | 0.27 | 6.08 | 0.06 |
| Phenol | 1 | 44.0 | 1.7 | 0.64 | 3.5 | 0.04 |
| Thymol (6-Isopropyl-3-methylphenol) | 1 | 63.4 | 2.4 | 1.20 | 1.32 | 0.01 |
| p-Cresol | 0.01 | 3.64 | 1.3 | 1.17 | 23.38 | 0.002 |
| Eugenol | 0.01 | 2.68 | 0.9 | 1.25 | 12.68 | 0.001 |
| Hydroquinone | 0.01 | 4.78 | 1.7 | 1.20 | 17.36 | 0.002 |
| Phenol | 0.01 | 1.61 | 0.7 | 1.22 | 52.22 | 0.006 |
| α-Tocopherol | 0.01 | 4.5 | 1.7 | 1.20 | 18.11 | 0.002 |
| Average molecular weight | Polydispersity index | |||||
|---|---|---|---|---|---|---|
| IP | (× 104) | (× 104) | ||||
| Compound | min | Rinh/Rp | Mn | Mw | Mw/Mn | KCL |
| A. Experiment 3. | ||||||
| Control | 0 | 1 | 14.6 | 49.4 | 3.4 | 315 |
| 1 | −2.02 | 1.11 | 13.0 | 35.1 | 2.7 | 386 |
| 2 | 1.03 | 0.99 | 15.4 | 53.1 | 3.5 | 312 |
| 3 | 24.06 | 1.05 | 14.7 | 46.8 | 3.2 | 294 |
| 4 | 5.7 | 0.94 | 14.5 | 46.6 | 3.2 | 325 |
| 5 | −0.3 | 1.02 | 15.1 | 49.3 | 3.3 | 320 |
| B. Experiment 4. | ||||||
| Control* | 0 | 1 | 14.6 | 49.4 | 3.4 | 315 |
| 6 | 5.48 | 1.03 | 13.0 | 35.1 | 2.7 | 319 |
| 7 | −0.86 | 0.96 | 12.5 | 33.3 | 2.7 | 297 |
| 1 | −2.07 | 1.05 | 10.6 | 23.6 | 2.2 | 325 |
4. Conclusions
References
- Burton, G.W.; Ingold, K.U. Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981, 103, 6472–6477. [Google Scholar] [CrossRef]
- Pryor, W.A.; Strickland, T.; Church, D.F. Comparison of the efficiencies of several natural and synthetic antioxidants in aqueous sodium dodecyl sulfate micelle solution. J. Am. Chem. Soc. 1988, 110, 2224–2229. [Google Scholar]
- Kadoma, Y.; Kumada, W.; Asai, Y.; Sugita, Y.; Yokoe, I.; Fujisawa, S. Radical-scavenging activity of the reaction products of isoeugenol with thiol, thiophenol, mercaptothiazoline or mercaptomethylimodazole using the induction period. Molecules 2007, 12, 1836–1844. [Google Scholar]
- Kadoma, Y.; Imai, Y. Studies on initiator system for cold curing resin using thiobarbituric acid derivatives. J. Japan. Soc. Dent. Mater. Devices 1991, 10, 692–698. [Google Scholar]
- Kadoma, Y.; Imai, Y. Visible-light initiator system using thiobarbituric acid with an allyl group. J. Japan. Soc. Dent. Mater. Devices 1992, 11, 430–435. [Google Scholar]
- Kadoma, Y. Application of 5-(4-vinylbenzyl)-2-thiobarbituric acid to dental materials. J. Japan. Soc. Dent. Mater. Devices 1992, 11, 891–898. [Google Scholar]
- O’Brien, J.L.; Gornick, F. Chain transfer in the polymerization of methyl methacrylates. 1. Transfer with monomer and thiols. The mechanism of termination reaction at 60°. J. Am. Chem. Soc. 1955, 77, 4757–4763. [Google Scholar] [CrossRef]
- Karasu, F.; Arsu, N.; Yagci, Y. 2-Mercapto thioxanthone as a chain transfer agent in free-radical polymerization: A versatile route to incorporate thioxanthone moieties into polymer chain endes. J. Appl. Polym. Sci. 2007, 103, 3766–3770. [Google Scholar]
- Fujisawa, S.; Kadoma, Y.; Yokoe, I. Radical-scavenging activity of butylated hydroxytoluene (BHT) and its metabolites. Chem. Phys. Lipids 2004, 130, 189–195. [Google Scholar]
- Grusibic, Z.; Rempp, P.; Benoit, H. A universal calibration for gel permeation chromatography. J. Polym. Sci. B5 1967, 5, 753–759. [Google Scholar] [CrossRef]
- Okada, Y.; Oono, Y. Polymerization of methyl methacrylates initiated by vitamin B12-formic acid, oxalic acid, or 2-mercaptoethanol system. NIHON KAGAKUKAISHI 1984, 105, 626–630. [Google Scholar]
- Okada, J.; Esaki, T. C-13 NMR spectra of barbituric acid derivatives. Yakugaku Zasshi 1973, 93, 1014–1018. [Google Scholar]
- Kadoma, Y.; Fujisawa, S. Radical-scavenging activity of dietary phytophenols in combination with co-antioxidants using the induction period method. Molecules 2011, 16, 10457–10470. [Google Scholar]
- Fujisawa, S.; Kadoma, Y. Comparative study of the alkyl and peroxy radical scavenging activities of polyphenols. Chemosphere 2006, 62, 71–79. [Google Scholar]
- Amorati, R.; Ferroni, F.; Pedulli, G.F.; Valgimigli, L. Modeling the co-antioxidant behavior of monofunctional phenols. Applications to some relevant compounds. J. Org. Chem. 2003, 68, 9654–9658. [Google Scholar]
- Bain, C.D.; Troughton, E.B.; Tao, Y.T.; Evall, J.; Whitesides, G.M.; Nuzzo, R.G. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 1989, 111, 321–335. [Google Scholar]
- Nuzzo, R.G.; Dubois, L.H.; Allara, D.L. Fundamental studies of microscopic wetting on organic surfaces. 1. Formation and structural characterization of a self-consistent series of polyfunctional organic monolayers. J. Am. Chem. Soc. 1990, 112, 558–569. [Google Scholar] [CrossRef]
- Benali, O.; Larabi, L.; Traisnel, M.; Gengembre, L.; Harek, Y. Electrochemical, theoretical and XPS studies of 2-mercapto-1-methylimidazole adsorption carbon steel in 1 M HClO4. Appl. Surface Sci. 2007, 253, 6130–6139. [Google Scholar]
- Ikemura, K.; Kojima, K.; Endo, T.; Kadoma, Y. Effect of the combination of dithiooctanoate monomers and acidic adhesive monomers on adhesion to precious metals, precious metal alloys and non-precious metal alloys. Dent. Mater. J. 2001, 30, 469–477. [Google Scholar]
- Shimoe, S.; Tanoue, N.; Satoda, T.; Nikawa, H.; Matsumura, H. Evaluation of single liquid primers with organic sulfur compound for bonding between indirect composite material and silver-palladium-copper-gold alloy. Dent. Mater. J. 2010, 29, 25–29. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kadoma, Y.; Fujisawa, S. Radical-Scavenging Activity of Thiols, Thiobarbituric Acid Derivatives and Phenolic Antioxidants Determined Using the Induction Period Method for Radical Polymerization of Methyl Methacrylate. Polymers 2012, 4, 1025-1036. https://doi.org/10.3390/polym4021025
Kadoma Y, Fujisawa S. Radical-Scavenging Activity of Thiols, Thiobarbituric Acid Derivatives and Phenolic Antioxidants Determined Using the Induction Period Method for Radical Polymerization of Methyl Methacrylate. Polymers. 2012; 4(2):1025-1036. https://doi.org/10.3390/polym4021025
Chicago/Turabian StyleKadoma, Yoshinori, and Seiichiro Fujisawa. 2012. "Radical-Scavenging Activity of Thiols, Thiobarbituric Acid Derivatives and Phenolic Antioxidants Determined Using the Induction Period Method for Radical Polymerization of Methyl Methacrylate" Polymers 4, no. 2: 1025-1036. https://doi.org/10.3390/polym4021025




