Abstract
A new esterase from Thermobifida halotolerans (Thh_Est) was cloned and expressed in E. coli and investigated for surface hydrolysis of polylactic acid (PLA) and polyethylene terephthalate (PET). Thh_Est is a member of the serine hydrolases superfamily containing the -GxSxG- motif with 85–87% homology to an esterase from T. alba, to an acetylxylan esterase from T. fusca and to various Thermobifida cutinases. Thh_Est hydrolyzed the PET model substrate bis(benzoyloxyethyl)terephthalate and PET releasing terephthalic acid and mono-(2-hydroxyethyl) terephthalate in comparable amounts (19.8 and 21.5 mmol/mol of enzyme) while no higher oligomers like bis-(2-hydroxyethyl) terephthalate were detected. Similarly, PLA was hydrolyzed as indicated by the release of lactic acid. Enzymatic surface hydrolysis of PET and PLA led to a strong hydrophilicity increase, as quantified with a WCA decrease from 90.8° and 75.5° to 50.4° and to a complete spread of the water drop on the surface, respectively.
1. Introduction
Enzymes have been used for a long time in many industrial processes [,,,] and consumer applications [,,]. They allow simplification of complex chemical processes [], synthesis of new molecules [], or as additives they can increase the performance of the product in which they are formulated [,]. One of the fields in which enzymes are increasingly capturing the interest of researchers across the globe is material science. There are several interesting examples describing the functionalization of natural fibers using enzymes in order to increase antibacterial properties [], antioxidant capacity [,,], or to increase compatibility with synthetic materials []. Nevertheless, it is still a challenge to find suitable enzymes for the biotransformation of non-natural and polymeric substrates.
Recently, enzymes have been demonstrated to be suitable alternative tools for hydrophilization of polymer surfaces. Hydrophilization of polyesters is essential for numerous applications of PLA and PET, ranging from textiles to composites and medical devices [,]. Typically, chemical methods [,] or plasma technology are used to increase the hydrophilicity of polymers [,] or to create reactive groups on the surface of inert materials []. Enzyme-based approaches would avoid the use of harsh chemicals [] and/or require considerable less energy [].
Poly(alkyleneterephthalates) (PAT), and particularly polyethylene terephthalate (PET), are one of the most widely used synthetic polymers worldwide [] which can be functionalized via limited enzymatic hydrolysis of the ester bond of the polymer backbone [,,,,]. Cutinases [,,], lipases [,], esterases [,,] including paranitrobenzyl esterase [] have been assessed for this purpose.
Among cutinases, representatives from Aspergilllus oryzae, Humicola insolens [], Penicillium citrinum [], Fusarium solani [,], Thermobifida fusca [] and Thermobifida cellulolysitica [] have been described to hydrolyze PET. Considerably fewer lipases have been described to hydrolyze PET, including enzymes from Candida antarctica [], Thermomyces lanuginosus [], Burkholderia spp. [] and Triticum aestivum []. Esterases were believed to have less potential for surface hydrolysis of polyesters than cutinases or lipases [,].
Here we demonstrate for the first time that the esterase Thh_Est from Thermobifida halotolerans can efficiently hydrolyze PET and PLA thereby considerably increasing surface hydrophilicity of these materials.
2. Materials and Methods
2.1. Chemicals
Methanol and acetonitrile were purchased from Roth (Carl Roth, Karlsruhe, Germany). All other chemicals were of analytical grade from SIGMA (Germany) except the model substrate bis(benzoyloxyethyl)terephthalate (3PET) which was synthesized in two steps as previously described [].
2.2. Bacterial Strains, Plasmids and Culture Conditions
Thermobifida halotolerans DSM44931 was obtained from the German Resource Centre for Biological Material (DSMZ, Germany). The strain was maintained on LB/agar plates and cultivated in 500 mL shaking flasks (200 mL LB medium, 37 °C, 160 rpm, 24 h). Cells were harvested by centrifugation (3,200× g, 4 °C, 20 min). Vector pET26b(+) (Novagen, Germany) was used for expression of Thh_Est in E. coli BL21-Gold (DE3) (Stratagene, Germany).
2.3. General Recombinant DNA Techniques
All DNA manipulations described in this work were performed by standard methods []. The PCR was performed in a Gene Amp® PCR 2200 thermocycler (Applied Biosystems, USA). Digestion of DNA with restriction endonucleases (New England Biolabs, USA), dephosphorylation with alkaline phosphatase (Roche, Germany) and ligation with T4 DNA-ligase (Fermentas, Germany) were performed in accordance to the manufacturer’s instructions. Plasmid Mini Kit from Qiagen (Germany) was used to prepare plasmid DNA. Plasmids and DNA fragments were purified by Qiagen DNA purification kits (Qiagen, Germany).
2.4. Cloning of Thh_est
The gene Thh_est was amplified from the respective genomic DNA of T. halotolerans DSM44931 by standard polymerase chain reaction (PCR). On the basis of the known sequences of genes coding for cutinases from T. fusca YX (Genbank accession numbers YP_288944 and YP_288943) two primers were designed, 5'-CCCCCGCTCATATGGCCAACCCCT ACGAGCG-3' (forward primer) and 5'-GTGTTCTAAGCTTCAGTGGTGGTGGTGGTGGTGCTCGAGTGCCAGGCACGAGA GTAGT-3' (reverse primer), facilitating amplification of the respective gene without signal peptide and introduction of the 6xHis-Tag at the C-terminus of the esterase. The designed primers included the restriction sites NdeI and HindIII for cloning the gene into the vector pET26b(+). The PCR was done in a volume of 50 µL with genomic DNA as template, 0.4 µM of each primer, 0.2 mM dNTP’s, 5 units PhusionTM DNA polymerase (Finnzymes) and 1× reaction buffer provided by the supplier. The DNA amplification was performed in 30 cycles according to the instructions for the PhusionTM DNA polymerase. The amplified PCR-products were purified, digested with NdeI and HindIII, ligated to pET26b(+) and transformed into E. coli BL21-Gold(DE3). The nucleotide sequence was determined by DNA sequencing.
2.5. DNA Sequencing, Alignments and Deposition of Sequence Data
DNA was sequenced as custom service by Agowa (Germany). DNA analysis was performed with Vector NTI Suite 10 (Invitrogen, USA). BLAST search was performed using the ExPASy (Expert Protein Analysis System) proteomics server of the Swiss Institute of Bioinformatics, and sequences of related proteins were aligned using the Clustal W program (Swiss EMBnet node server). The nucleotide sequence of Thh_est has been deposited in the GenBank database under submission JQ339742.
2.6. Expression and Purification
Thh_Est was expressed in shake flasks. Therefore the plasmid was freshly transformed in E. coli BL21-Gold(DE3). Transformants were used to inoculate 20 mL Luria-Bertani (LB) broth supplemented with 40 µg/mL kanamycin. The culture was grown overnight on a rotary shaker at 30 °C and 160 rpm. For inoculation of the main culture, 1 mL of the overnight culture was transferred to 200 mL of the same media and incubated at 30 °C. At an optical density (600 nm) between 0.6 and 0.8 the culture was cooled down to 20 °C and induced by addition of 0.05 mM IPTG. After induction for 24 h the cells were harvested by centrifugation (3,200 g, 4 °C, 20 min). Cell pellet was disrupted by sonification using three-times 30-s pulses under ice cooling (cell disruptor from BRANSON Ultrasonics). Purification of the enzyme was performed by affinity chromatography according to the manufacturer’s instructions (IBA BioTAGnology, Germany). Afterwards the HisTag elution buffer was exchanged with 100 mM Tris HCl pH 7.0 by the use of PD-10 desalting columns (Amersham Biosciences).
2.7. Esterase Activity Assay
Esterase activity using p-nitrophenyl acetate (PNPA) and p-nitrophenyl butyrate (PNPB) as substrates was measured at 25 °C. For the final assay mixture, 200 µL of the substrate solution was diluted in 50 mM Tris HCl buffer pH = 7 and mixed with 20 µL enzyme solution. Activity was determined by measuring the increase of the absorbance at 405 nm, which indicated an increase of p-nitrophenol (ε405nm = 11.86 mmol−1cm−1) due to hydrolysis of PNPA or PNPB. A Spectromax Plus 384 plate reader (Molecular Devices) was used to follow the reaction. The activity was calculated in units, where 1 unit had been defined as being the amount of enzyme required to hydrolyze 1 µmol of substrate per minute under the given assay condition. A blank was measured using 20 µL buffer instead of sample.
2.8. PET and PLA Hydrolysis
Prior to the enzyme treatment, PET and PLA films were cut into pieces of 10 × 20 mm and washed in a three consecutive steps. In a first step, each PET piece was washed with a solution of Triton-X100, in a second step 100 mM Na2CO3 and finally deionized water was used. Each washing step lasted 30 min and was performed at 50 °C for PET and 37 °C for PLA. The final enzyme concentration was 100 mg/mL diluted in tris buffer (100 mM) pH = 7. Incubations were made at 130 rpm and 50 °C for PET and 37 °C for PLA. Hydrolysis products were measured by using high performance liquid chromatography - (HPLC) as described below. Controls were performed with heat-inactivated enzyme.
2.9. Hydrolysis of PET Model Substrate bis(benzoyloxyethyl)terephthalate
Hydrolysis of the PET model substrate bis(benzoyloxyethyl)terephthalate (3PET) was performed in 2 mL Eppendorf tubes as previously described []. 10 mg 3PET were incubated in 1000 µL of 100 mM K2HPO4/KH2PO4 containing 100 µg/mL enzyme at 50 °C and pH 7.0 for 2 hours and 350 rpm. The released products were analyzed by using HPLC as described below.
2.10. Analysis of PET, 3PET and PLA Hydrolysis Released Products
PET and 3PET soluble released products were analyzed as previously described by Eberl et al. []. After enzymatic treatment proteins were precipitated using 1:1 (v/v) methanol abs. on ice. Samples were centrifuged (Hettich MIKRO 200 R, Tuttlingen, Germany) at 16,000× g at 0 °C for 15 min. The supernatant for measurement was brought to an HPLC vial and acidified by adding 1 μL of HCl concentrated. The HPLC used was a DIONEX P-580 PUMP (Dionex Cooperation, Sunnyvale, USA) equipped with an ASI-100 automated sample injector and a PDA-100 photodiode array detector. Analysis of TA, benzoic acid (BA), 2-hydroxyethyl benzoate (HEB), mono-(2-hydroxyethyl) terephthalate (MHET) and bis-(2-hydroxyethyl) terephthalate (BHET) a reversed phase column RP-C18 (Discovery® HS-C18, 5 μm, 150 × 4.6 mm with precolumn, Supelco, Bellefonte, USA) was used.
Lactic Acid (LA) was also determined using the same conditions. Analysis was carried out with 20% acetonitrile, 20% 10 mM sulfuric acid and 60% (v/v) water as eluent. The flow rate was set to 1 mL min-1 and the column was maintained at a temperature of 25 °C. The injection volume was 10 μL. Detection of the analytes was performed with a photodiode array detector at the wavelength of 241 nm.
2.11. PET and PLA Hydrophilicity Measurements
Water contact angles of the PET and PLA films after enzymatic treatment were measured using a Contact Angle and Surface Tension Analyzer (Kruss GmbH, Hamburg, Germany). Deionized water was used as test liquid with a drop size of 3 µL and the speed of 300 µL/s. Contact angles were measured after 3 s and data are obtained from the averages of the measurements taken from at least 5 different points of the sample surface.
3. Results and Discussion
3.1. Cloning, Sequence Analysis and Heterologous Expression in E. coli of an Esterase from Thermobifida Halotolerans
Cutinases from Thermobifida fusca, T. cellulosilytica and T. alba have shown to hydrolyze PET-films [,]. However, for industrial processes, more efficient enzymes are still needed while their potential to surface hydrophylize PLA has been largely neglected. In 2008, Thermobifida halotolerans has been isolated from a salt mine sample collected from Yunnan province (Southwest China, []) and classified as a novel species of Thermobifida. In order to identify homologous cutinases from this novel species, primers were designed, based on the genome sequences of cutinases from T. fusca YX. By the use of the designed primers, the gene coding for Thh_Est was amplified from the genomic DNA of T. halotolerans. The open reading frame of the gene (789 bp) encodes for a protein comprising 262 amino acids and a calculated mass of 28.7 kDa. The gene comprised a GC content of 68% which is expected for the genus Thermobifida. Sequence analysis of Thh_Est (Supporting Information File 1) revealed that the enzyme is a member of the serine hydrolases superfamily containing the –GxSxG- motif in which the catalytic Ser131 is embedded.
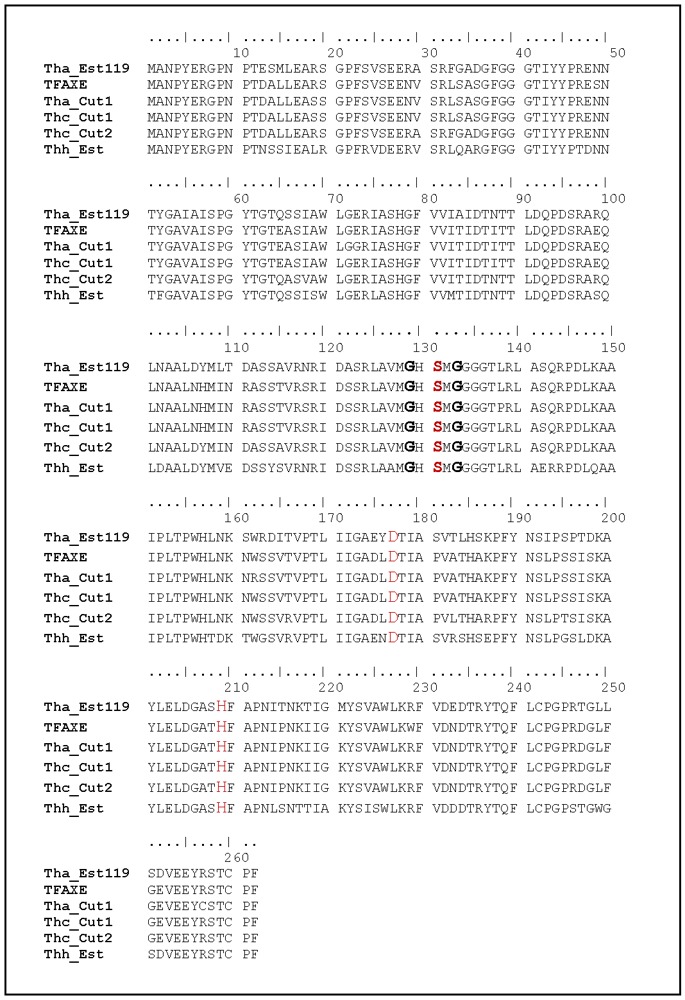
A BLAST search for homologous proteins listed an esterase from T.alba (Est119, Genbank BAK48590.1; []), two cutinases from T.cellulosilytica (Thc_Cut1 and Thc_Cut2, Genbank ADV92526 and ADV92527), the cutinase from T.alba (Tha_Cut1, Genbank ADV92526) as well as an acetylxylan esterase from T.fusca (TFAXE, Genbank ADM47605.1; []) as hits showing 85–87% similarity to Thh_Est (Table 1, Figure 1 and Appendix).

Table 1.
Comparison of hydrolases from Thermobifida based on % homology of primary sequences.
| Thh_Est | Tha_Est119 | Thc_Cut2 | TFAXE | Tha_Cut1 | Thc_Cut1 | |
|---|---|---|---|---|---|---|
| Thh_Est | 0 | 87% | 87% | 86% | 85% | 87% |
| Tha_Est119 | 0 | 93% | 89% | 88% | 89% | |
| Thc_Cut2 | 0 | 95% | 94% | 95% | ||
| TFAXE | 0 | 98% | 99% | |||
| Tha_Cut1 | 0 | 98% | ||||
| Thc_Cut1 | 0 |
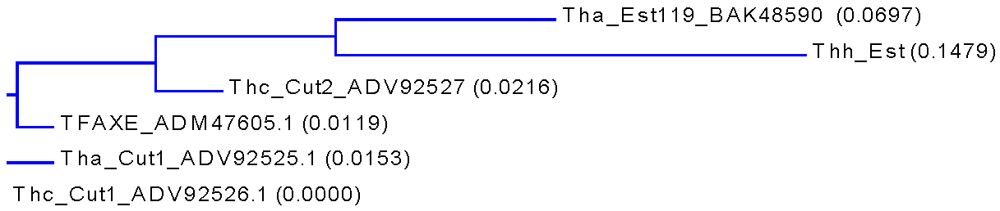
Figure 1.
Phylogenetic tree based on amino sequences of the Thh_Est and other highly homologous hydrolases from Thermobifida. The calculation is based on a sequence distance method and utilizes the Neighbor Joining (NJ) algorithm of Saitou and Nei (1987). The tree displays the calculated distance values in parenthesis following the molecule name and the GenBank number on the tree.
The gene coding for Thh_Est was cloned through the NdeI/HindIII restriction sites into pET26b(+) for expression without the pelB leader sequence. The recombinant protein harboring a C-terminal hexahistidine tag (29.8 kDa) was expressedisTag in E. coli BL21-Gold(DE3) at 20 °C by addition of 0.05 mM IPTG. The recombinant esterase was purified in one step by metal chelate affinity chromatography from the cell free E. coli extract to highest purity (Figure 2). Only low amounts of inclusion bodies were observed after 10h of induction (Figure 2, lane 11). At pH 7.0 and 25 °C, recombinant Thh_Est revealed specific activities of 500 U/mg with pNP butyrate and 550 U/mg with pNP acetate.
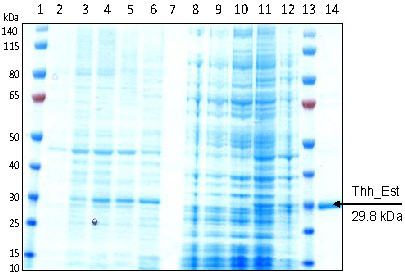
Figure 2.
SDS-PAGE analysis of Thh_Est expressed in E. coli BL21-Gold (DE3). Samples were withdrawn at different times of induction, centrifuged, disrupted and centrifuged again. Lane 1 and 13: PageRuler Prestained Protein Ladder (Fermentas); lane 2-6: cleared lysates from induced cells after 0, 3, 6 and 10 h of induction with 0.05 mM IPTG; lane 7: empty; lane 8-12: insoluble cell fractions from induced cells after 0, 3, 6 and 10 h of induction; lane 14: purified Thh_Est.
3.2. Hydrolysis of PLA Films
The ability of the Thh_Est to hydrolyze PLA films was assessed by analyzing the released products via HPLC-UV after hydrolysis and measurements of the hydrophilicity increase as WCA. The only released product found in the incubation solution was lactic acid in a concentration of 14.5 μM while no higher oligomers were detected. The degree of hydrophilization achieved was significant since it was not possible to measure a final value of the WCA due to the fact that the water drop was a complete spread on the polymer surface.
3.3. Hydrolysis of the PET Model Substrate bis(benzoyloxyethyl)terephthalate
Hydrolysis of the model substrate bis(benzoyloxyethyl)terephthalate (3PET) has been proven first to be valuable for the screening of enzymes capable of PET hydrolysis [] and to provide important mechanistic information [,,,] about the type of hydrolysis mechanism. In the case of the new esterase Thh_Est, the hydrolysis product found in highest concentration was BA, followed by HEB, both in a comparable concentration higher than 50 mmol per mol of enzyme. In contrast, MHET and TA were detected in a lower amount, whereas no BHET was observed (Figure 3).
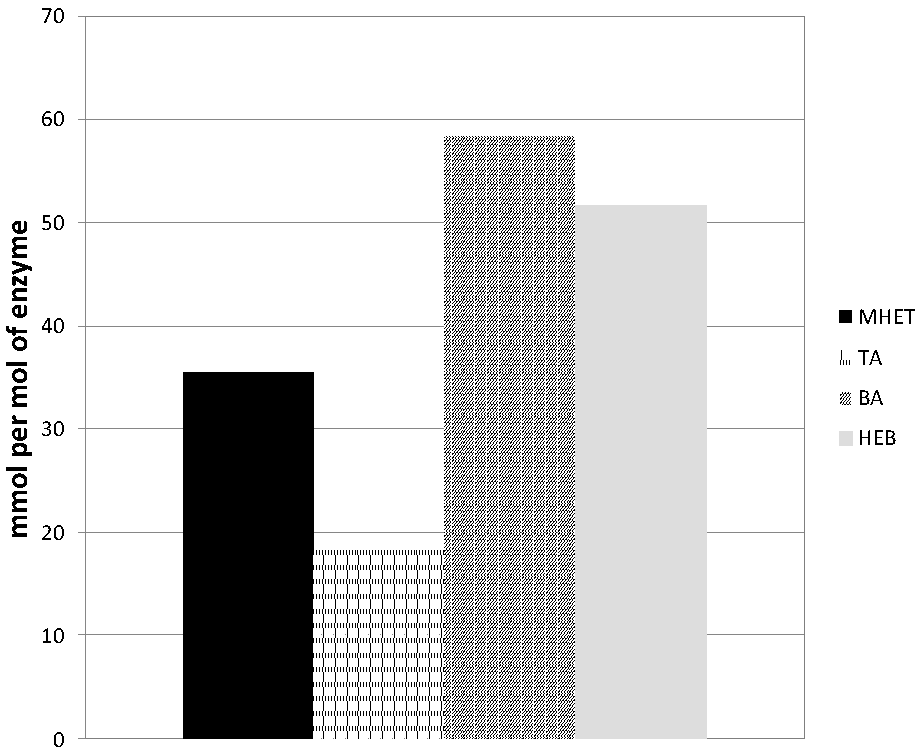
Figure 3.
Hydrolysis of the PET model substrate 3PET with the esterase Thh_Est.
The 3PET hydrolysis product pattern of Thh_Est was similar to the profile previously reported for the cutinase 2 from Thermobifida cellulosilytica (Thc_Cut2). Other Thermobifida fusca cutinases [,] likewise released comparable amounts of BA and HEB whereas the ratio between MHET and TA was higher for Thh_Est. This might indicate a more exo-type hydrolysis mechanism of the Thh_Est esterase when compared to Thermobifida cutinases. In contrast, a Humicola insolens (Hi_Cut) cutinase released the terminal BA and MHET as major hydrolysis products []. A very similar hydrolysis profile was found for the para-nitrobenzylesterase from Bacillus subtilis (BsEstB) [], although in this case the amount of release products was much lower than for Thh_Est or Hi_Cut. Interestingly, the overall amount of released products per mol of Thh_Est was significantly higher than the values reported for cutinases from Thermobifida species Thc_Cu1, Thc_Cut2 and Thf_42 [].
3.4. Hydrolysis of PET Films
Upon incubation with PET, the new esterase from T. halotolerans only released MHET and TA while no BHET was detected. The amount of MHET and TA released were comparable with around 19.8 and 21.5 mmol/mol of enzyme, respectively. Previously, for a lipase from T. lanuginosus [] a ten times higher release of MHET than TA from amorphous fibers and 3 times more from semi-crystalline PET fibers was reported. Interestingly, only in the case of amorphous fibers was BHET detected. Similarly, Eberl et al. reported higher amounts of MHET than of TA when the same lipase was used []. In the case of cutinase from F. solani again higher MHET to TA ratios were found and BHET was detected in non-significant amounts []. For cutinases from Fusca sp. comparable amounts of MHET and TA released from PET were reported, with no detection of BHET, when PET fabrics of around 40% crystallinity were used as substrate []. On the other hand, cutinases from Thermobifida fusca DSM 44342 and cutinase 1 from Thermobifida cellulolysitca showed an opposite trend with higher amounts of TA than MHET. However, prolonged incubation together with the ability of the both enzymes to endo-wise hydrolyze the polyester may lead to this view [].
3.5. Hydrophilicity Measurement of Enzymatically Treated PLA and PET
Thh_Est was able to hydrolyze films of both PLA and PET (Table 2). Provided a complete removal of the enzyme adhered in the polymer surface it is possible to compare the degree of hydrophilicity achieved by Water Contact Angle (WCA) measurement []. In the case of PLA films the hydrophilicity increase achieved was significantly higher than obtained for PET. The WCA in the case of PLA decreased from 75.5° to a complete spread of the water drop on the polymer surface after deposition. For PET it was possible to decrease the WCA from 90.8° to 50.4°.

Table 2.
Hydrophilicity increase (quantified as Water contact angle WCA decrease) of PET and PLA upon surface hydrolysis with the esterase Thh_Est.
| Initial | Control | Enzyme treated | |
|---|---|---|---|
| PET | 90.8° ± 3.3° | 74.8° ± 2.3° | 50.4° ± 9.9° |
| PLA | 75.5° ± 2.7° | 68.4° ± 2.3° | <20° |
Enzymatic hydrophilization of polyester surfaces has applications [] ranging from improved coating with PVC [] to more efficient textile finishing processes []. The advantage of enzymes derived from Thermobifida species is high thermal stability which allows working at temperatures below but close to the glass transition temperature of the polymer. These working conditions facilitate a high mobility of the polymer chains leading to higher accessibility of the polymer for enzymatic attack.
Concluding the results of this paper, the new esterase Thermobifida Thh_Est has a potential for both PET and PLA surface hydrolysis, similar to cutinases from the same genus. This is rather unexpected since cutinases were previously believed to be superior to esterases in terms of polyester hydrolysis [,].
Acknowledgements
This study was performed within the Austrian Centre of Industrial Biotechnology ACIB, the MacroFun project and COST Action 868. This work has been supported by the Federal Ministry of Economy, Family and Youth (BMWFJ), the Federal Ministry of Traffic, Innovation and Technology (bmvit), the Styrian Business Promotion Agency SFG, the Standortagentur Tirol and ZIT–Technology Agency of the City of Vienna through the COMET-Funding Program managed by the Austrian Research Promotion Agency FFG.
References
- Guebitz, G.M.; Cavaco-Paulo, A. Enzymes go big: Surface hydrolysis and fictionalization of synthetic polymers. Trends Biotechnol. 2008, 26, 32–38. [Google Scholar]
- Choct, M. Enzymes for the feed industry: Past, present and future. World’s Poult. Sci. J. 2006, 62, 5–16. [Google Scholar] [CrossRef]
- Kirk, O.; Borchert, T.V.; Fuglsang, C.C. Industrial enzyme applications. Curr. Opin. Biotechnol. 2002, 13, 345–351. [Google Scholar]
- Bajpai, P. Application of enzymes in the pulp and paper industry applicaton. Biotechnol. Progr. 1999, 15, 147–157. [Google Scholar]
- Hassan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzyme Microb. Technol. 2006, 39, 235–251. [Google Scholar]
- Maurer, K.H. Detergent proteases. Curr. Opin. Biotechnol. 2004, 15, 330–334. [Google Scholar]
- Hakamada, Y.; Koike, K.; Yoshimatsu, T.; Moir, H.; Kobayashi, T.; Ito, S. Thermostable alkaline cellulase from an alkaliphilic isolate, bacillus Sp. KSM-S237. Extremophiles 1997, 1, 151–156. [Google Scholar] [CrossRef]
- Huisman, G.W.; Gray, D. Towards novel processes for the fine-chemical and pharmaceutical industries. Curr. Opin. Biotechnol. 2002, 13, 352–358. [Google Scholar]
- Koeller, K.M.; Wong, C.H. Enzymes for chemical synthesis. Nature 2001, 409, 232–240. [Google Scholar]
- Souter, P.F.; Perez-Prat, E.M.; Herrero Acero, E.; Pricelius, S.; Guebitz, G.M. Cleaning and/or Treatment Compositions. WO/2009/090576, 23 July 2009. [Google Scholar]
- Svendsen, A.; Schroeder Gald, S.O.; Fukuyama, S.; Matsui, T. Cutinase Variants. US6960459 B2, 2005. [Google Scholar]
- Schroeder, M.; Aichernig, N.; Guebitz, G.M.; Kokol, V. Enzymatic coating of lignocellulosic surfaces polyphenols. Biotechnol. J. 2007, 2, 334–341. [Google Scholar]
- Božič, M.; Gorgieva, S.; Kokol, V. Laccase-mediated functionalization of chitosan by caffeic and galic acids for modulating antioxidant and antimicrobial properties. Carbohydr. Polym. 2011, in press.. [Google Scholar]
- Silva, C.; Matamá, T.; Kim, S.; Padrão, J.; Nugroho Prasetyo, E.; Kudanga, T.; Nyanhongo, G.S.; Guebitz, G.M.; Casal, M.; Cavaco-Paulo, A. Antimicrobial and antioxidant linen via laccase-assisted grafting. React. Funct. Polym. 2011, 71, 713–720. [Google Scholar]
- Mai, C.; Milstein, O.; Hüttermann, A. Fungal laccase grafts acrylamide onto lignin in presence of peroxides. Appl. Environ. Microbiol. 1999, 51, 527–531. [Google Scholar]
- Buchheit, O.; Eddoumy, F.; Sorrenti, E.; Di Martino, J.; Ruch, D. Modifications of the polylactic acid surface properties by DBD plasma treatment at atmospheric pressure. J. Eng. Mater. Technol. 2011, 133, 030903–1. [Google Scholar]
- Kim, H.A.; Choi, J.H.; Takizawa, S. Comparison of initial filtration resistance by pretreatment processes in the nanofiltration for drinking water treatment. Separ. Purif. Technol. 2007, 56, 352–362. [Google Scholar]
- Asatekin, A.; Kang, S.; Elimelech, M.; Mayes, A.M. Anti-fouling ultrafiltration membranes containing polyacrylonitrile-graft poly(ethyleneoxide) comb copolymer additives. J. Membr. Sci. 2007, 298, 136–146. [Google Scholar]
- Zhu, L.; Wang, C.; Qiu, Y. Influence of the amount of absorbed moisture in nylon fibers on atmospheric pressure plasma processing. Surf. Coat. Technol. 2007, 201, 7453–7461. [Google Scholar]
- Zhang, W.; Chu, P.K.; Ji, J.; Zhang, Y.; Liu, X.; Fu, R.K.Y.; Ha, P.C.T.; Yan, Q. Plasma surface modification of poly vinyl chloride for improvement of antibacterial properties. Biomaterials 2006, 27, 44–51. [Google Scholar]
- Schroeder, M.; Fatarella, E.; Kovac, J.; Guebitz, G.M.; Kokol, V. Laccase induced grafting on plasma-pretreated polyprene. Biomacromolecules 2008, 9, 2735–2741. [Google Scholar]
- Tressaud, A.; Durand, E.; Labrugère, C.; Kharitonov, A.P.; Kharitonova, L.N. Modification of surface properties of carbon-based and polymeric materials through fluorination routes: From fundamental research to industrial applications. J. Fluor. Chem. 2007, 128, 378–391. [Google Scholar]
- Guebitz, G.M.; Cavaco-Paulo, A. New substrates for reliable enzymes: Enzymatic modification of polymers. Curr. Opin. Biotechnol. 2003, 14, 577–582. [Google Scholar]
- Nylon Chain Report 2010. YNFX Yarns and Fiber Exchange. Dated-23 Feb 2011.
- Vertommen, M.A.M.E.; Nierstrasz, V.A.; Veer, M.; Warmoeskerken, M.M.C.G. Enzymatic surface modification of poly(ethylene terephthalate). J. Biotechnol. 2005, 120, 376–386. [Google Scholar]
- Oeser, T.; Wei, R.; Baumgarten, T.; Billig, S.; Foellner, C.; Zimmermann, W. High level expression of a hydrophobic poly(ethylene terephthalate) hydrolyzing carboxylesterase from thermobifida fusca KW3 in escherichia coli BL21(DE3). J. Biotechnol. 2010, 146, 100–104. [Google Scholar]
- Herrero Acero, E.; Ribitsch, D.; Steinkellner, G.; Gruber, K.; Greimel, K.; Eiteljoerg, I.; Trotscha, E.; Wei, R.; Zimmermann, W.; Zinn, M.; et al. Enzymatic surface hydrolysis of PET: Effect of structural diversity on kinetic properties of cutinases from Thremobifida. Macromolecules 2011, 44, 4632–4640. [Google Scholar]
- Donelli, I.; Freddi, G.; Nierstrasz, V.A.; Taddei, P. Surface structure and properties of poly-(ethylene terephthalate) hydrolyzed by alkali and cutinase. Polym. Degrad. Stab. 2010, 95, 1542–1550. [Google Scholar]
- Brueckner, T.; Eberl, A.; Heumann, S.; Rabe, M.; Guebitz, G.M. Enzymatic and chemical hydrolysis of poly(ethylene terephthalate) fabrics. J. Polym. Sci. Part A: Polym. Chem. 2008, 46, 6435–6443. [Google Scholar] [CrossRef]
- Alisch-Mark, M.; Herrmann, A.; Zimmermann, W. Increase of the hydrophilicity of polyethylene terephthalate fibers by hydrolases from thermomonospora fusca and fusarium solani f. sp pisi. Biotechnol. Lett. 2006, 28, 681–685. [Google Scholar] [CrossRef]
- Araújo, R.; Silva, C.; O’Neill, A.; Micaelo, N.; Guebitz, G.; Soares, C.M.; Casal, M.; Cavaco-Paulo, A. Tailoring cutinase activity towards polyethylene terephthalate and polyamide 6,6 fibers. J. Biotechnol. 2007, 128, 849–857. [Google Scholar]
- Eberl, A.; Heumann, S.; Brueckner, T.; Araújo, R.; Cavaco-Paulo, A.; Kaufmann, F. Enzymatic surface hydrolysis of poly(ethylene terephthalate) and bis(benzoyloxyethyl) terephthalate by lipase and cutinase in the presence of surface active molecules. J. Biotechnol. 2009, 143, 207–212. [Google Scholar]
- Yoon, M.; Kellis, J.; Poulouse, A.J. Enzymatic modification of polyester. AATCC Rev. 2002, 2, 33–36. [Google Scholar]
- Ribitsch, D.; Heumann, S.; Trotscha, E.; Herrero Acero, E.; Greimel, K.; Leber, R.; Birner-Gruenberger, R.; Deller, S.; Eiteljoerg, I.; Remler, P.; et al. Hydrolysis of polyethyleneterephthalate by para-nitrobenzylesterase from bacillus subtilis. Biotechnol. Prog. 2011, 27, 951–960. [Google Scholar]
- Ronkvist, Å.M.; Xie, W.; Lu, W.; Gross, R.A. Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar]
- Liebminger, S.; Eberl, A.; Sousa, F.; Heumann, S.; Fisher-Colbrie, G.; Cavaco-Paulo, A.; Guebitz, G.M. Hydrolysis of PET and bis-(benzoyloxyethyl) terephthalate with a new polyesterase from penicillium citrinum. Biocatal. Biotransform. 2007, 25, 171–177. [Google Scholar]
- O’Neill, A.; Araújo, R.; Casal, M.; Guebitz, G.M.; Cavaco-Paulo, A. Effect of the agitation on the adsorption and hydrolytic efficiency of cutinases on polyethylene terephthalate fibres. Enzyme Microb. Technol. 2007, 7, 1801–1805. [Google Scholar]
- Lee, C.W.; Do Chung, J. Synthesis and biodegradation behavior of poly(ethylene terephthalate) oligomers. Polymer-Korea 2009, 33, 198–202. [Google Scholar]
- Nechwatal, A.; Blokesch, A.; Nicolai, M.; Krieg, A.; Kolbe, A.; Wolf, M.; Gerhardt, M. A contribution to the investigation of enzyme-catalysed hydrolysis of poly(ethylene terephthalate) oligomers. Macromol.Mater. Eng. 2006, 291, 1486–1494. [Google Scholar]
- Heumann, S.; Eberl, A.; Pobeheim, H.; Liebminger, S.; Fischer-Colbrie, G.; Almansa, E.; Cavaco-Paulo, A.; Guebitz, G.M. New model substrates for enzymes hydrolysing polyethyleneterephthalate and polyamide fibers. J. Biochem. Bioph. Methods 2006, 69, 89–99. [Google Scholar]
- Sambrook, J.E.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor: New York, NY, USA, 1989. [Google Scholar]
- Ribitsch, D.; Herrero Acero, E.; Greimel, K.; Eiteljoerg, I.; Trotscha, E.; Freddi, G.; Schwab, H.; Guebitz, G.M. Characterization of a new cutinase from Thermobifida Alba regarding PET-surface hydrolysis. Biocatal. Biotransform. 2012, in press.. [Google Scholar]
- Yang, L.-L.; Tang, S.-K.; Zhang, Y.-Q.; Zhi, X.-Y.; Wang, D.; Xu, L.-H.; Li, W.-J. Thermobifida Halotolerans sp. nov., isolated from a salt mine sample, and emended description of the genus Thermobifida. Int. J. Syst. Evol. Microbiol. 2008, 58, 1821–1825. [Google Scholar] [CrossRef]
- Hu, H.; Thumarat, U.; Zhang, X.; Tang, M.; Kawai, F. Diversity of polyester-degrading bacteria in compost and molecular analysis of a thermoactive esterase from Thermobifida Alba AHK119. Appl. Environ. Microbiol. 2010, 87, 771–779. [Google Scholar]
- Huang, Y.C.; Chen, G.H.; Chen, Y.F.; Chen, W.L.; Yang, C.H. Heterologous expression of thermostable acetylxylan esterase gene from thermobifida fusca and its synergistic action with xylanase for the production of xylooligosaccharide. Biochem. Biophys. Res. Commun. 2010, 400, 718–723. [Google Scholar]
- Almansa, E.; Heumann, S.; Eberl, A.; Fischer-Colbrie, G.; Martinkova, L.; Marek, J.; Cavaco-Paulo, A.; Guebitz, G.M. Enzymatic surface hydrolysis of PET enhances bonding in PVC coating. Biocatal. Biotrans. 2008, 26, 365–370. [Google Scholar]
Appendix
Alignment of the primary sequence of Thh_Est, esterase from T. alba (Tha_Est119, Genbank Genbank BAK48590.1), cutinases from T. cellulosilytica (Thc_Cut1 and Thc_Cut2, Genbank ADV92526 and ADV92527), cutinase 1 from T. alba (Tha_Cut1, Genbank ADV92526) and acetylxylan esterase from T. fusca (TFAXE, Genbank ADM47605.1). Red: amino acids of the catalytic triade. Bold: conserved GxSxG motif.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).