Preparation and Characterization of Organic-Inorganic Hybrid Hydrogel Electrolyte Using Alkaline Solution
Abstract
: Organic-inorganic hybrid hydrogel electrolytes were prepared by mixing hydrotalcite, cross-linked potassium poly(acrylate) and 6 M KOH solution. The organic-inorganic hybrid hydrogel electrolytes had high ionic conductivity (0.456–0.540 S cm−1) at 30 °C. Moreover, the mechanical strength of the hydrogel electrolytes was high enough to form a 2–3 mm thick freestanding membrane because of the reinforcement with hydrotalcite.1. Introduction
Solid polymer electrolytes permitted safer, thinner and more flexible battery designs [1], but their ionic conductivity was insufficient to use in practice. Polymer gel electrolytes, which can stably hold considerable amount of electrolyte solution within polymer matrices, significantly improved ionic conductivity [2,3]. Thus lithium polymer secondary batteries with polymer gel electrolytes have actively been researched and developed. Meanwhile, alkaline secondary batteries such as nickel-metal hydride (Ni-MH) batteries are well-known as safe and environmentally friendly systems compared to lithium ion batteries and lithium polymer secondary batteries. Moreover, nickel-zinc and silver-zinc batteries are promising systems for their high energy density.
Several researchers have tried applying polymer electrolyte or gel electrolyte to alkaline secondary batteries [4-7]. Iwakura et al. firstly prepared a polymer hydrogel electrolyte (PHE) in which a KOH aqueous solution was held in a network structure of the cross-linked potassium poly(acrylate) (PAAK). They found that the PHE had liquidlike ionic conductivity, and applied it to all solid state Ni-MH batteries [8-10]. Inoue et al. developed a clay-based inorganic hydrogel electrolyte using hydrotalcite (HT) and KOH aqueous solution, and clarified that it had ionic conductivity comparable to the PHE [11]. However, these hydrogel electrolytes showed too low mechanical strength to form a freestanding membrane. In this work, we tried preparing organic-inorganic hybrid hydrogel electrolytes using PAAK, HT and KOH aqueous solution to realize not only high ionic conductivity but also high mechanical strength. Dispersing carbon black or silica as nanofiller is a popular strategy to reinforce rubber or organic polymer materials [12,13]. In addition, Croce et al. found that the nanocomposite polymer electrolytes using polyethylene oxide and LiClO4 containing TiO2 and Al2O3 nanoparticle had high ionic conductivity [14]. Therefore, it is reasonable to apply HT as nanofiller to reinforce the PHE.
2. Experimental Section
2.1. Preparation of Organic-Inorganic Hybrid Hydrogel Electrolytes
KOH and HT were purchased from Wako Pure Chemical Industries, Ltd. PAAK whose potassium content was 11 wt% was purchased from Sigma-Aldrich. Milli-Q (Millipore) water with resistivity of 18.2 M Ω cm was used in this study. Three kinds of organic-inorganic hybrid hydrogel electrolytes were prepared according to the following procedure: 0.8, 1.6 or 2.4 g of HT was added to 10 mL of 6 M KOH aqueous solution. After being stirred for 5 min, 1 g of PAAK was added to the resultant suspension, followed by stirring for a few minutes and standing for 3 days, resulting in homogeneous milky hydrogel. After that, air bubbles in the gel were removed under a vacuum. All procedures were performed at room temperature. Each hydrogel electrolyte is called hybrid hydrogel(0.8), hybrid hydrogel(1.6) and hybrid hydrogel(2.4), respectively.
2.2. Electrochemical Measurements
Electrochemical measurements were carried out using a potentiostat (SI1287 and SI1260; Solartron Analytical) with a three-electrode configuration cell. An Hg/HgO electrode was used as the reference electrode. Two Pt plates with apparent surface area of 1 cm2 were used as the counter and working electrodes, respectively. Ionic conductivity of 6 M KOH solution and three hydrogels was evaluated by ac impedance measurement at a frequency of 100 kHz with the perturbation of 5 mV. A handmade two-electrode conductivity cell consisted of two Pt black-coated Pt electrodes with apparent surface area of 1 cm2 was used.
2.3. X-Ray Diffraction Analysis
X-ray diffraction (XRD) spectra of HT and hybrid hydrogel electrolytes were measured by using an X-ray diffractometer (XRD-6100; Shimadzu) equipped with a Cu Kα source (λ = 0.1541 nm, 50 kV, 30 mA).
3. Results and Discussion
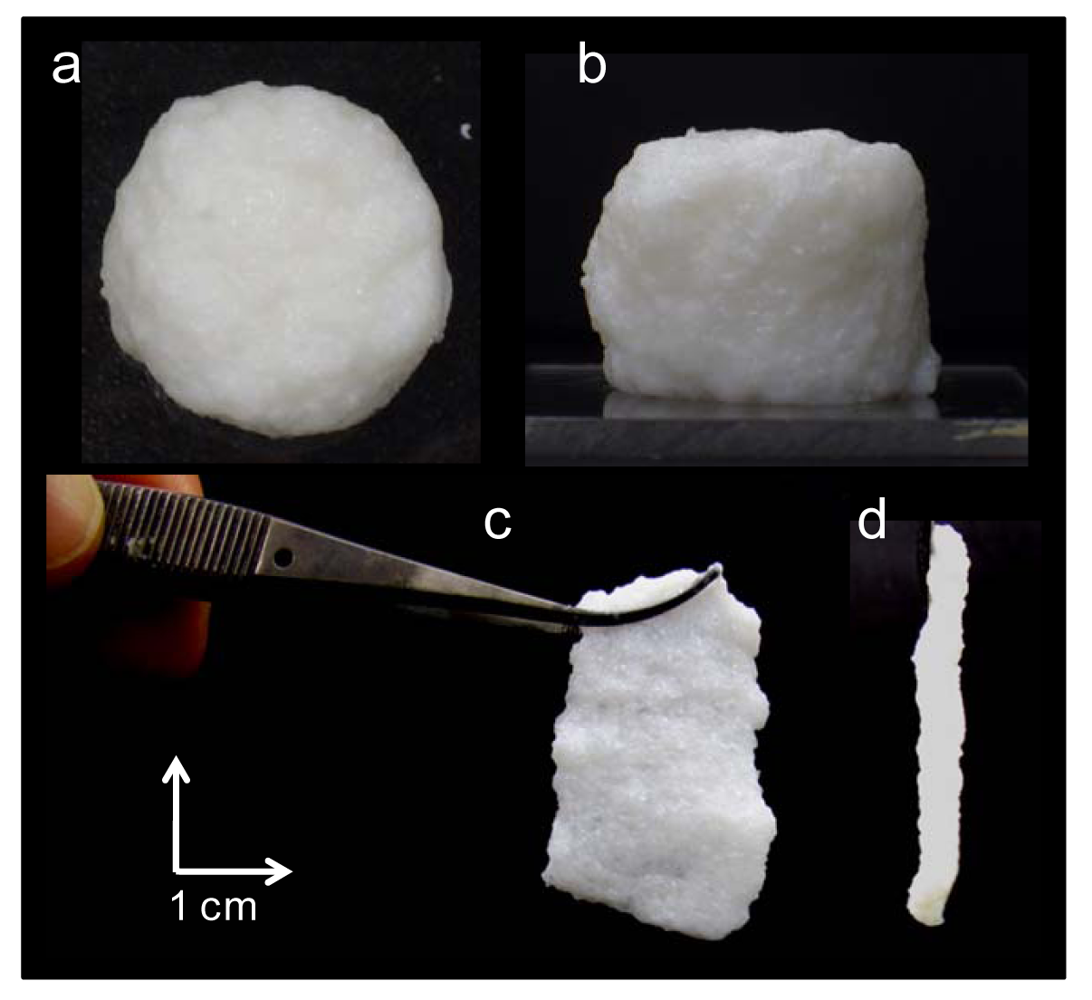
Figure 1 shows pictures of the hybrid hydrogel(1.6) and its freestanding membrane. The hybrid hydrogel(1.6) showed white color and tolerance to alkali. The hybrid hydrogel(1.6) kept its shape for hours when it was put on a flat surface (Figure 1(a,b)). in addition, it was possible to form a freestanding membrane with a thickness of 2–3 mm (Figure 1(c,d)). These strongly suggest the hydrogel electrolyte has high mechanical strength. The mechanical strength of the hydrogel electrolyte using only PAAK or HT was too low to form a self-standing membrane. Conventional polymer-clay composites containing aggregated nanolayer tactoids ordinarily improve rigidity, but they often sacrifice elongation and toughness [15]. However, the present hybrid hydrogel electrolytes keep both viscosity and elasticity. Thus, HT is homogeneously dispersed in PAAK matrix and HT works well as nanofiller to reinforce the PHE. Moreover, it should be noted that the hybrid hydrogel electrolytes are prepared just by mixing organic polymer, clay and KOH aqueous solution for only a few minutes. In this way, the hybrid hydrogel electrolytes are readily and quickly prepared.
Figure 2 shows XRD spectra of the hybrid hydrogel(1.6) and pristine HT. The hybrid hydrogel(1.6) had diffraction peaks similar to the pristine HT. The lattice distance of the pristine HT was evaluated as 0.78 nm from the (003) peak and that of HT in the hybrid hydrogel(1.6) was 0.77 nm. In the case of the inorganic hydrogel electrolyte with only HT, lattice distance was increased to 0.81 nm because carbonate was replaced with OH− upon ion exchange or the degree of hydration increased [11]. In the present case, it is deduced that the hybrid hydrogel(1.6) has lower degree of hydration than the inorganic hydrogel electrolyte. Another is less replacement of carbonate in the interlayer of HT lamellae with OH− ion. According to the Scherrer's equation, the crystallite size of HT was evaluated as 26.7 nm for the pristine HT and 24.6 nm for the hybrid hydrogel(1.6). Therefore, the number of HT lamellae is estimated to be 35 for the pristine HT and 33 for the hybrid hydrogel(1.6), indicating that most of the HT lamellae were not delaminated in the hybrid hydrogel.
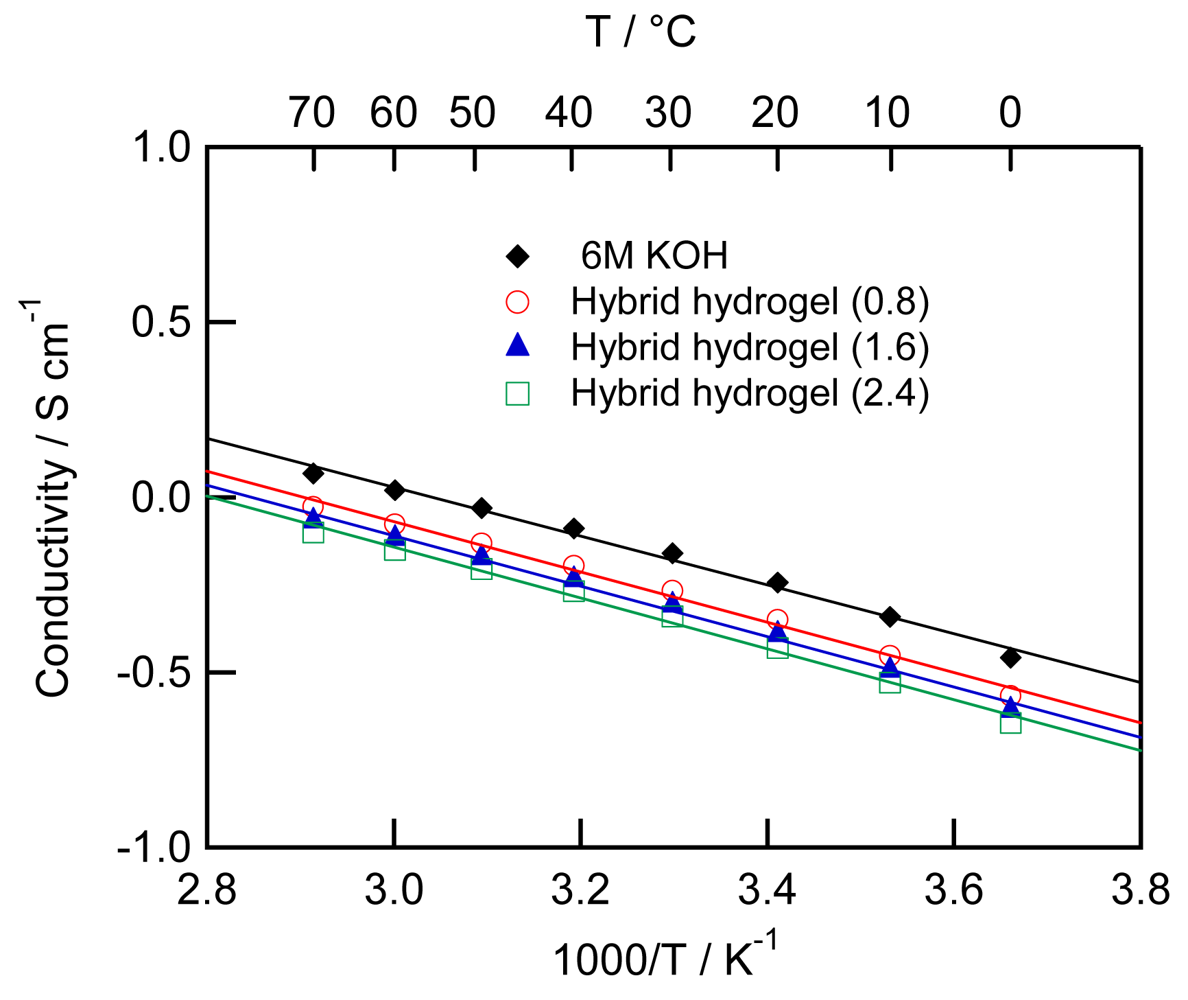
Figure 3 shows temperature dependence of ionic conductivity for 6 M KOH aqueous solution and three hybrid hydrogel electrolytes. Arrhenius equation is given as follows:
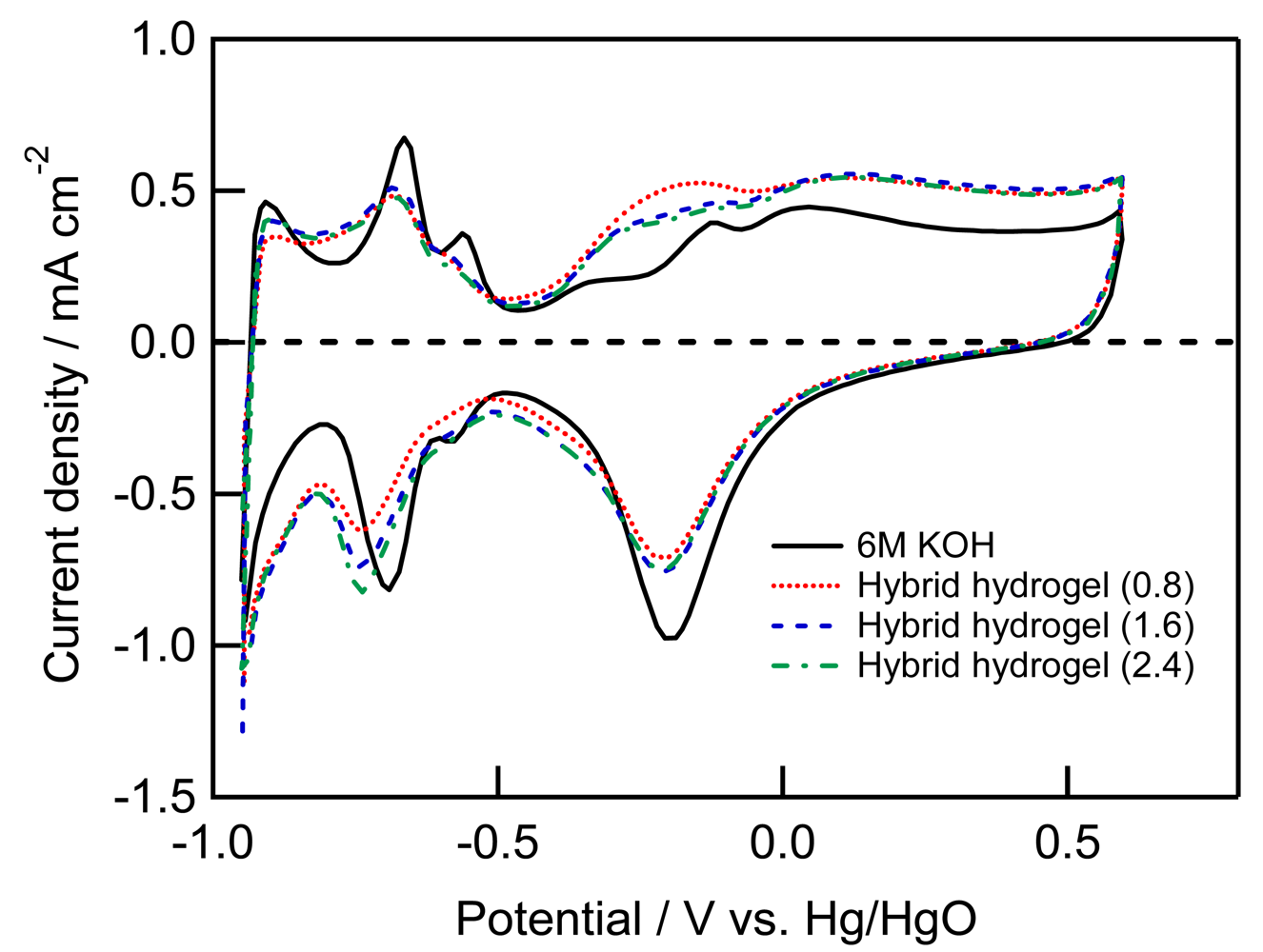
Figure 4 shows cyclic voltammograms (CVs) of a Pt working electrode in 6 M KOH aqueous solution and three hybrid hydrogel electrolytes. Typical oxidation and reduction peaks due to the formation of Pt oxide and its reduction and hydrogen adsorption and desorption were observed in the CV in each hybrid hydrogel electrolyte. However, there was no significant current for any other reactions in each voltammogram. Therefore we can say the electrochemical window of Pt in the hybrid hydrogel electrolytes is comparable to that in 6 M KOH aqueous solution, which clearly indicates the hybrid hydrogel electrolytes are promising solid electrolytes for alkaline batteries, capacitors and fuel cells compatible with 6 M KOH aqueous solution.
4. Conclusions
The mechanical strength of the hybrid hydrogel electrolytes containing both PAAK and HT is high enough to form freestanding membranes. Their ionic conductivity is 0.456–0.540 S cm−1 at 30 °C and their potential window is comparable to that in KOH solution. Moreover, the hybrid hydrogel electrolytes can be prepared just by mixing the HT, PAAK and KOH solution without polymerization and thermal treatment. The hybrid hydrogel electrolytes have potential for safe, economical and environmentally friendly energy applications like batteries, capacitors and fuel cells.

| Hybrid hydrogel(0.8) | Hybrid hydrogel(1.6) | Hybrid hydrogel(2.4) | |
|---|---|---|---|
| κ at 30 °C/S cm−1 | 0.540 | 0.491 | 0.456 |
| ΔE0/kJ mol−1 | 13.8 | 13.8 | 13.9 |
| ln (σ0/S cm−1) | 4.80 | 4.72 | 4.69 |
References
- Meyer, W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar]
- Abraham, K.M.; Alamgir, M. Li+-Conductive solid polymer electrolytes with liquid-like conductivity. J. Electrochem. Soc. 1990, 137, 1657–1658. [Google Scholar]
- Sotomura, T.; Tatsuma, T.; Oyama, N. An organosulfur polymer cathode with a high current capability for rechargeable batteries. J. Electrochem. Soc. 1996, 143, 3152–3157. [Google Scholar]
- Fauvarque, J.F.; Guinot, S.; Bouzir, N.; Salmon, E.; Penneau, J.F. Alkaline Poly(ethylene oxide) solid polymer electrolytes. Application to nickel secondary batteries. Electrochim. Acta 1995, 40, 2449–2453. [Google Scholar]
- Vassal, N.; Salmon, E.; Fauvarque, J.F. Nickel/metal hydride secondary batteries using an alkaline solid polymer electrolyte. J. Electrochem. Soc. 1999, 146, 20–26. [Google Scholar]
- Lewandowski, A.; Skorupska, K.; Malinska, J. Novel poly(vinyl alcohol)-KOH-H2O alkaline polymer electrolyte. Solid State Ion. 2000, 133, 265–271. [Google Scholar]
- Kumar, G.G.; Sampath, S. Electrochemical and spectroscopic investigations of a gel polymer electrolyte of Poly(Methylmethacrylate) and zinc triflate. Solid State Ion. 2005, 176, 773–780. [Google Scholar]
- Iwakura, C.; Furukawa, N.; Ohnishi, T.; Sakamoto, K.; Nohara, S.; Inoue, H. Nickel/metal hydride cells using an alkaline polymer gel electrolyte based on potassium salt of crosslinked of Poly (Acrylic Acid). Electrochemistry (Tokyo, Jpn.) 2001, 69, 659–663. [Google Scholar]
- Iwakura, C.; Ikoma, K.; Nohara, S.; Furukawa, N.; Inoue, H. Charge-discharge and capacity retention characteristics of new type Ni/MH batteries using polymer hydrogel electrolyte. J. Electrochem. Soc. 2003, 150, A1623–A1627. [Google Scholar]
- Iwakura, C.; Nohara, S.; Furukawa, N.; Inoue, H. The possible use of polymer gel electrolytes in nickel/metal hydride battery. Solid State Ion. 2002, 148, 487–492. [Google Scholar]
- Inoue, H.; Inaba, Y.; Okuda, S.; Higuchi, E.; Nohara, S. Inorganic hydrogel electrolyte with liquidlike ionic conductivity. Electrochem. Solid-State Lett. 2009, 12, A58–A60. [Google Scholar]
- Ueno, K.; Hata, K.; Katakabe, T.; Kondoh, M.; Watanabe, M. Nanocomposite ion gels based on silica nanoparticles and an ionic liquid: Ionic transport, viscoelastic properties, and microstructure. J. Phys. Chem. B 2008, 112, 9013–9019. [Google Scholar]
- Haraguchi, K.; Takehisa, T. Nanocomposite hydrogels: A unique organic-inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar]
- Croce, F.; Appetechi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–459. [Google Scholar]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chiku, M.; Tomita, S.; Higuchi, E.; Inoue, H. Preparation and Characterization of Organic-Inorganic Hybrid Hydrogel Electrolyte Using Alkaline Solution. Polymers 2011, 3, 1600-1606. https://doi.org/10.3390/polym3041600
Chiku M, Tomita S, Higuchi E, Inoue H. Preparation and Characterization of Organic-Inorganic Hybrid Hydrogel Electrolyte Using Alkaline Solution. Polymers. 2011; 3(4):1600-1606. https://doi.org/10.3390/polym3041600
Chicago/Turabian StyleChiku, Masanobu, Shoji Tomita, Eiji Higuchi, and Hiroshi Inoue. 2011. "Preparation and Characterization of Organic-Inorganic Hybrid Hydrogel Electrolyte Using Alkaline Solution" Polymers 3, no. 4: 1600-1606. https://doi.org/10.3390/polym3041600




