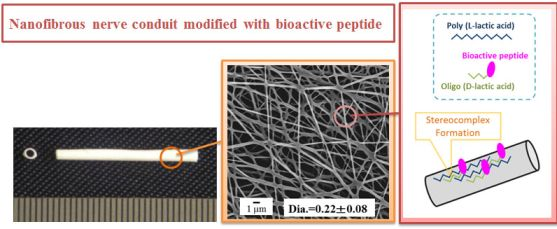
Surface Modification of Poly(L-lactic acid) Nanofiber with Oligo(D-lactic acid) Bioactive-Peptide Conjugates for Peripheral Nerve Regeneration
Abstract
: In some traumatic nerve injuries, autologous nerve grafting is the first choice for bridging the gap between the severed nerve ends. However, this therapeutic strategy has some disadvantages, including permanent loss of donor function and requirement of multiple surgeries. An attractive alternative to this therapeutic technique is the use of artificial nerve conduit. Poly (L-lactic acid) (PLLA) is widely used as a substrate for artificial nerve conduit because it is readily biodegradable, but it is not inherently biologically active. In this study, we developed a PLLA nanofibrous nerve conduit, modified with a conjugate of oligo (D-lactic acid) (ODLA) and the neurite outgrowth, thereby promoting peptide AG73 (RKRLQVQLSIRT) to improve nerve regeneration. PLA/ODLA-AG73 nanofibrous conduit was fabricated by electrospinning and then transplanted at the 10 mm gap of rat sciatic nerve. After six months, electrophysiological evaluation revealed that it achieved better functional reinnervation than silicone tube (used as a reference) or unmodified PLLA nanofibrous conduit.1. Introduction
Nerve injuries—especially the most serious type, neurotmesis—typically result in significant nerve gaps and loss of motor and sensory functions. If the gap is short, the severed nerve stumps can often be reconnected surgically owing to non-tension; if it is long, an autologous nerve graft from the patient's own body is used to bridge the injury site. However, autologous nerve grafts have several disadvantages, including permanent loss of donor function, requirement of multiple surgeries, and size mismatch between the injured nerve and the graft nerves. Therefore, artificial nerve conduit is of great interest for bridging the gap between severed nerve stumps [1,2].
Various biodegradable polymers are used as substrates for nerve conduit. Poly(lactic acid) (PLA) [3-5] and its copolymers are preferred because they are non-enzymatically hydrolyzed to low toxic lactic acid and are metabolized via the tricarboxylic acid cycle in vivo [6]. In addition, PLA has attractive mechanical properties and excellent shaping and molding properties, and can be fabricated into porous, nanofibrous, and many other types of structures. However, PLA exhibits poor hydrophilicity and lacks congenital biological activity, so it is usually modified with biologically active molecules. For nerve conduit, the proteins collagen and laminin are used as modifiers, because their biologically active sequences promote nerve regeneration [7]. However, these proteins are animal-derived, and can display high antigenicity because of unnecessary biologically active sequences and enzymatically digested fragments [8].
Many biologically active peptides are reported to be useful for tissue regeneration. Focusing on nerve regeneration, the peptides RGD, IKVAV, and YIGSR, isolated from fibronectin or laminin, are preferred to improve the biological activities of nerve conduit [9]. Another advantage is that, although they are biologically active, they are prepared by chemical procedures.
There are many reports of nerve conduit being fabricated with combinations of biodegradable polymer substrates (at this point, typically chitosan) and biologically active peptides. For example, a nanofibrous nerve conduit combined with YIGSR peptide has been shown to promote nerve repair in rats [10]. However, although it is easy to introduce bioactive molecules into chitosan via amino groups, it is difficult to control their biodegradability. In contrast, PLA biocompatibility can be controlled by molecular weight regulation or by copolymerization with other hydroxyl acids such as glycolic acid. However, PLA is not easily modified with bioactive molecules because it lacks functional groups.
Many investigators have attempted to add functional groups to PLA in order to enhance its biological activity via copolymerization or chemical grafting with other polymers [11], plasma treatment [12], chemical modification [13], and physical adsorption [14].
In our previous study, we developed amphiphilic oligo(D-lactic acid)-RGD (ODLA-RGD) conjugates to impart cell adhesion properties to poly(L-lactic acid) (PLLA) films [15]. The two enantiomeric PLAs, PLLA and poly(D-lactic acid) (PDLA), are known to form a stereocomplex by strong interaction between their L-lactyl and D-lactyl units [16-18]. The bioactive RGD sequence was stably immobilized onto PLLA scaffolds via stereocomplex formation of ODLA segment and PLLA, resulting in improved 3T3 cell adhesion.
PLLA nanofibrous nerve conduit was modified with a novel conjugate composed of ODLA and neurite outgrowth, thereby promoting peptide AG73 (RKRLQVQLSIRT) has been fabricated by electrospinning PLLA and ODLA-AG73 conjugate solutions [19]. AG73 found in the 1 chain C domain of laminin-I is known to strongly promote cell adhesion, wound healing, and neurite outgrowth [20,21].
With the goal of developing novel PLLA nanofibrous nerve conduit modified with the neurite outgrowth-promoting peptide for nerve regeneration, we analyzed the surface of PLLA/ODLA-AG73 nanofibrous conduit to determine whether or not the AG73 peptides are stably immobilized on the PLLA nanofiber. We then, implanted conduit in a 10 mm gap in rat sciatic nerve to bridge the nerve stumps, and performed histological and electrophysiological evaluations of the repaired nerve after six months.
2. Experimental Section
2.1. Materials
The amino acid derivatives dimethylformamide (DMF), piperidine, and trifluoroacetatic acid (TFA) were purchased from Watanabe Chemical Industries (Hiroshima, Japan). PAL-PEG resin was purchased from Applied Biosystems (Foster City, CA, USA). The condensation agents 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride n-hydrate (DMT-MM) and N-methylmorpholine (NMM) were obtained from Kokusan Chemical Company (Tokyo, Japan) and Nacalai Tesque (Kyoto, Japan), respectively. Acetic anhydrate and L-lactic acid were purchased from Wako Pure Chemical Industries (Osaka, Japan). PLLA (mw = 130,000) was obtained from Mitsui Chemicals (Tokyo, Japan).
2.2. Methods
2.2.1. Synthesis of ODLA-AG73 Conjugates
ODLA-AG73 conjugates were synthesized on resin from 9-fluorenylmethoxycarbonyl (Fmoc) by a solid-phase procedure (Scheme 1) [22].
All Fmoc-protected amino acids (Fmoc-Arg(Pbf)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Leu-OH, Fmoc-Gln(tBu)-OH, Fmoc-Val-OH, Fmoc-Ser(tBu)-OH, Fmoc-Ile-OH, and Fmoc-Thr-OH) were sequentially elongated on PAL–PEG resin by DMT-MM and NMM in DMF. After the coupling reaction, the absence of free amino groups was qualitatively checked by the Kaiser test. Fmoc deprotection was performed with 20% piperidine in DMF. After deprotection of the final Fmoc group, acetylated ODLA, synthesized by direct condensation polymerization, was introduced using DMT-MM and NMM in DCM. Deprotection of the side chains and ODLA-AG73 cleavage was performed with 95 v% TFA aqueous solution. Filtration followed by precipitation from diethyl ether yielded the ODLA-AG73 conjugate, as characterized by MALDI-TOF/MS.
2.2.2. Fabrication of PLLA/ODLA-AG73 Nanofibrous Nerve Conduit
PLLA/ODLA-AG73 nanofibrous nerve conduit was fabricated by electrospinning (Figure 1). For the inner layer of the conduit, PLLA/ODLA-AG73 solution was prepared at 10 w% in hexafluoroisopropanol (HFIP) with 3 w% ODLA-AG73 conjugate for PLLA. For the outer layer of the conduit, 10 w% PLLA solution in HFIP containing 1 w% PEG for PLLA was prepared. Electrospinning of PLLA/ODLA-AG73 solution was performed using a plastic syringe equipped with a stainless steel needle (length = 15.0 mm, diameter = 20 G) at a constant feed rate of 50 μL/min. A rotating stainless steel tube (outer diameter = 1.0 mm, speed = 1,500 rpm) was used as a target; the distance between the needle tip and the target was 100 mm. Positive-charged PLLA/ODLA-AG73 (inner layer) and PLLA/PEG (outer layer) solutions were injected using a needle to the target at high voltage (13 kV) for 1 and 9 min, respectively. PLLA/ODLA-AG73 nanofibrous nerve conduit was removed from the target, cut to lengths of 12 mm, washed with Milli-Q water and ethanol, and dried in vacuo to remove the remaining organic solvents. Surface morphology was observed by scanning electron microscopy (SEM; JCM-5700, JEOL, Tokyo, Japan).
2.2.3. Determination of Conduit Surface Composition
The surface composition of PLLA/ODLA-AG73 nanofibrous nerve conduit was determined by X-ray photoelectron spectroscopy (XPS; ESCA-3400, Shimadzu Co., Kyoto, Japan). The X-ray source was a monochromatic Mg Kα X-ray from a rotating anode. Survey scans were performed from 0 to 1,200 eV. Peak positions and areas were analyzed, and C1s, N1s, and O1s ratios were calculated with the software provided by the manufacturer.
2.2.4. Conduit Implantation
A total of six SD rats (seven weeks old, male) (Japan SLC, Shizuoka, Japan) were used to compare PLLA nanofibrous nerve conduit with and without ODLA-AG73 and silicone tubes (N = 2). Silicone tubes (length = 12 mm, inner diameter = 1.0 mm, outer diameter = 2.0 mm) (Sogo Laboratory Glass Works Company, Kyoto, Japan), sterilized with ethylene oxide, were used as negative control because they do not collapse and conglutinate with surrounding tissues after implantation [23].
The rats were anesthetized with Escain isoflurane (Mylan Inc., Canonsburg, PA, USA), the right sciatic nerve was exposed, and a 10 mm segment was removed from the distal portion of the nerve. A conduit was implanted so as to bridge the stumps of the removed nerve. The conduit was filled with physiological saline solution and sutured in place with 10–0 vicryl (Ethicon, Somerville, NJ, USA). The muscle incision and skin were closed with 3–0 silk suture (Ethicon).
2.2.5. Histological Evaluation
The rats were sacrificed six months after implantation and the nerve conduits, including regenerative nerve, were carefully dissected.
Specimens were fixed with 10% formalin neural buffer solution, dehydrated in ethanol series, embedded in paraffin, cut into thin cross sections ∼2–4 mm from a distal connection of 3.0 μm thickness with an ultramicrotome, and stained with the following: (1) hematoxylin and eosin (HE), (2) anti-glial fibrillary acidic protein (GFAP) rabbit polyclonal antibody (Dako, Glostrup, Denmark), and (3) anti-neurofilament 200 kD (NF200) rabbit polyclonal antibody (Sigma-Aldrich). Immunohistochemical analysis was performed by standard immunoperoxidase techniques.
After de-paraffinization, the sections were treated as follows. Sections for NF200 staining were incubated in TBS buffer solution [50 mM tris-HCl (pH 7.6) and 0.15 M NaCl] containing 0.65 mg/mL of proteinase K (Dako) for 5 min at room temperature and then washed with TBST buffer solution [50 mM tris-HCl (pH 7.6), 0.05% Tween-20, and 0.15 M NaCl]; endogenous peroxidase activity was blocked with 3.0% hydrogen peroxide in methanol for 10 min. Sections for GFAP and NF200 staining were incubated with an appropriate dilution of a primary antibody for 30 min at room temperature and overnight at 4 °C, respectively. After rinsing the primary antibody using a TBST buffer, the sections were incubated for 30 min at room temperature with a secondary antibody (N-Histofine Simple Stain Rat MAX PO, Nichirei Biosciences, Tokyo, Japan). Then, the slides were incubated with DAB+ solution (Dako) for 30 min and rinsed gently with distilled water. Counterstaining was carried out with hematoxylin for 1 min. The slides were rinsed in water until clear, dehydrated with ethanol, and washed with xylene. Finally, a coverslip was applied with Malinol (Muto Pure Chemical Company, Tokyo, Japan) and the slides were observed by light microscopy (COOLSCOPE II, Nicon, Tokyo, JAPAN).
2.2.6. Electrophysiological Analysis
Rats (N = 2) were anesthetized with Escain isoflurane (Mylan Inc.) and the implanted nerve conduits were exposed from the proximal to the distal portions of the sciatic nerve and the tibialis muscle. An electromyogram of the tibialis muscle, a measure of myogenic potential, was obtained by stimulating the nerve at a point proximal to the implanted nerve conduit. Stimulation was achieved by dissecting this portion of the nerve free from surrounding tissue and positioning on it a pair of platinum wire stimulating electrodes. A pair of recording electrodes was inserted into the tibialis muscle. Both stimulating and recording electrodes were connected to an electric stimulator (SEN-3401, Nihon Kohden, Tokyo, Japan) and a data acquisition system (PowerLab 8/30, ADInstruments, Colorado Springs, CO, USA). The stimulation parameters were as follows: strength = 1 V, duration = 10 s, pulse = 1 Hz. The myogenic potential of the tibial muscle was amplified with PowerLab system (ADInstruments, Burlingame, CA, USA), and the average of 50 traces was recorded.
3. Results and Discussion
3.1. Preparation and Characterization of PLLA/ODLA-AG73 Nanofibrous Conduit
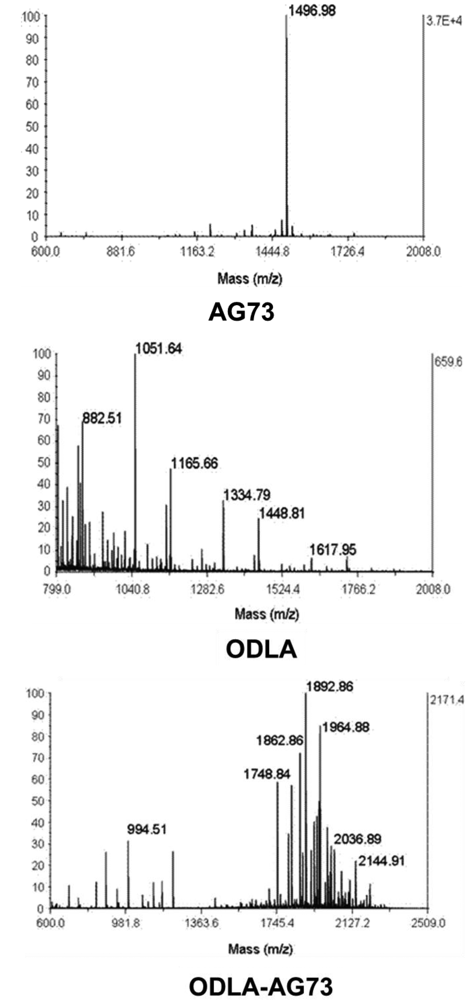
ODLA-AG73 conjugates were synthesized on resin from Fmoc in a solid-phase procedure. The conjugates consisted of 3- to 10-mer ODLA and AG73 sequences, with molecular weights of ∼2 kDa (Figure S1).
PLLA/ODLA-AG73 nanofibrous nerve conduit was fabricated by electrospinning solutions of PLLA or PLLA/ODLA-AG73 (97:3) (Figure 2). The diameters of the PLLA and PLLA/ODLA-AG73 nanofibers were approximately 280 and 220 μm, respectively, and the conduits were tightly reticulate.
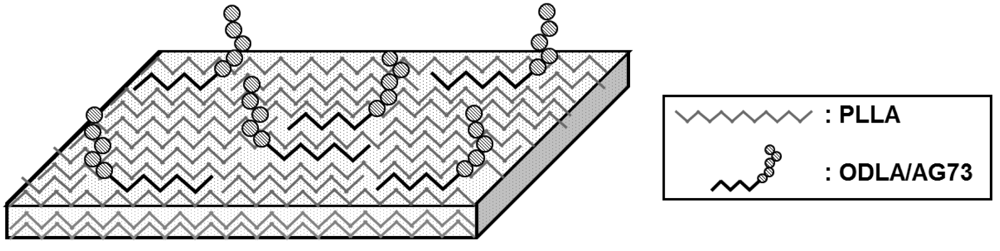
A nanofibrous conduit is thought to be the most suitable structure for suppressing connective tissue ingression and allowing permeation of liquid elements into the conduit. In our previous study, we incorporated ODLA-RGD conjugates into the PLLA crystal structure via stereocomplex formation by means of electrospinning, resulting in stable immobilization of RGD peptide on a PLLA nanofiber [15]. Similarly, we thought that we could immobilize AG73 peptides on a PLLA nanofiber using ODLA-AG73 (Figure 1). In fact, neurite outgrowth in PC12 cells (the rat pheochromocytoma cell line) is slightly better on PLLA/ODLA-AG73 nanofiber than on PLLA nanofiber in vitro (Figure S2).
To confirm that AG73 peptide was introduced onto the PLLA nanofiber using ODLA-AG73 conjugates, we analyzed the surface by XPS (Figure 3). The spectrum of the PLLA nanofibrous conduit lacks an N1s peak because the PLLA molecule does not containnitrogen. However, after thorough washing, the spectrum of PLLA/ODLA-AG73 has a slight N1s peak (N1s/C1s ratio = 0.03 ± 0.01) corresponding to amino acid, indicating that AG73 was stably immobilized on the PLLA nanofiber (Table 1). The ODLA region of the ODLA-AG73 conjugate might have incorporated or formed stereocomplex crystallites with PLLA, as reported in our previous study [15]. Because AG73 is hydrophilic, AG73 at the nanofiber surface must be exposed to liquid in order to express its biological activity in a physiological environment.
3.2. Evaluation of Nerve Regeneration
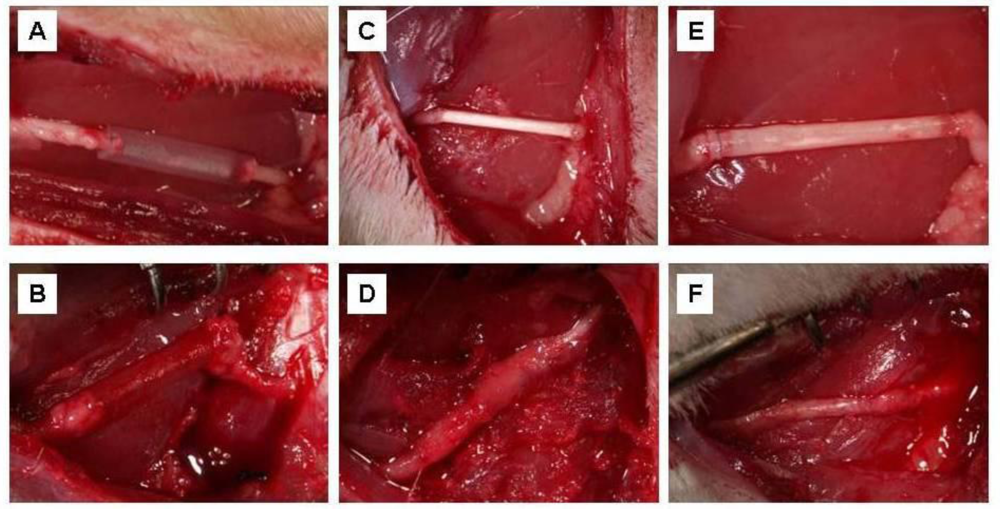
Silicon tubes (used as a control), PLLA fibrous conduit, and PLLA/ODLA-AG73 fibrous conduit were implanted at the 10 mm gap of rat peripheral nerve (Figure 4(A,C,E)). Both nerve stumps were pulled inside the tube and sutured. After six months, regenerated nerve-like tissue was visually observed inside the semitransparent silicone tube. In contrast, the PLLA and PLLA/ODLA-AG73 conduits were almost degraded and the boundaries between nerve and conduit were barely distinguishable. The silicone tube allowed the peripheral nerve to be exteriorized easily (Figure 4(B)), but the PLLA and PLLA/ODLA-AG73 conduits adhered severely to the surrounding tissue and muscle (Figure 4(D,F)). Woo et al. reported that the nanofibrous architecture of PLA scaffolds enhances the adsorption of cytoadherence proteins such as fibronectin and vitronectin, and allows cell attachment [24]. Accordingly, the PLLA and PLLA/ODLA-AG73 conduits might have promoted protein adsorption, and thus, adhesion of surrounding tissue.
We performed histological analyses of cross sections near the distal connection. Figure 5(A–C) shows the case for a healthy nerve. Figure 5(D–F) shows the case for a silicone tube (used as a control). GFAP-positive cells and NF200-positive axons appear at the center, resulting in overlap. The regenerating nerve is round in shape and lacks ingression of connective tissue because the tube is not biodegradable and does not allow permeation of liquid elements and cells from outside and neural-cell attachment inside the tube. Figures 5(G–I) and 5(J–L) show the cases for PLLA conduit and PLLA/ODLA-AG73 conduit, respectively. In both cases, the regenerating nerve is also visible, the appearance of GFAP-positive cells and NF200-positive axons is similar, and fragments of degraded conduit are evident around the regenerating nerve. Ingression of connective tissue between the nerve stumps inhibits nerve regeneration, so it is necessary to control the degradation ratio of the conduit.
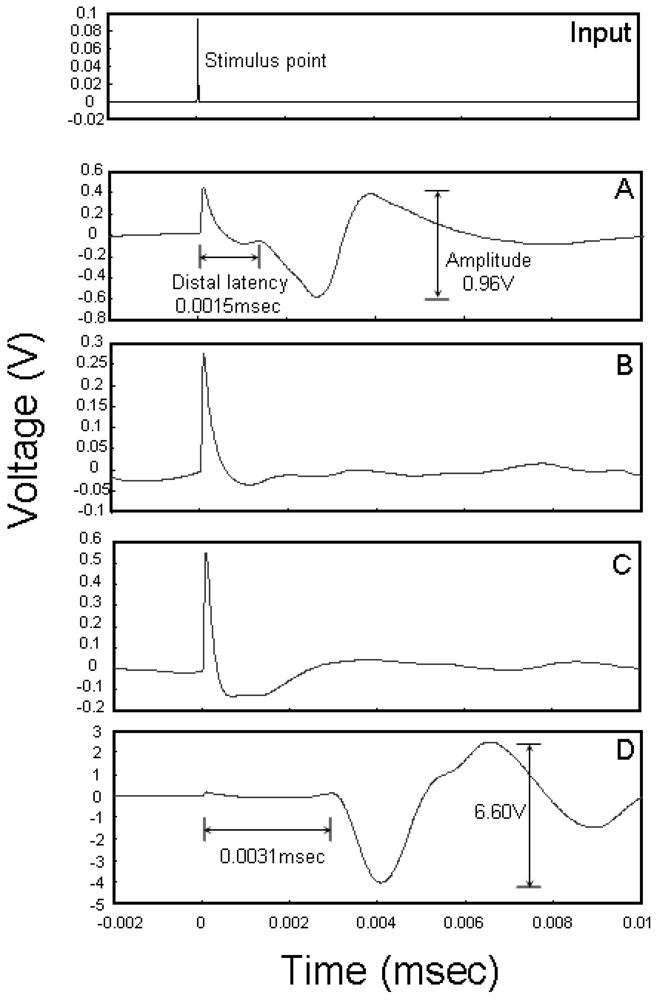
We performed electrophysiological analyses to evaluate functional reinnervation through the conduits. Figure 6(A) shows the case for a healthy nerve; the distal latency is 0.0015 ms and amplitude is 0.96 V. Figure 6(B,C) shows the cases for the silicone tube and PLLA conduit, respectively. The myogenic potential of the tibialis muscle is not detectable, suggesting that the functional reinnervation was not established within the measured time. Figure 6(D) shows the case for the PLLA/ODLA-AG73 conduit. The myogenic potential pattern is similar to that for healthy nerve, indicating that the PLLA/ODLA-AG73 conduit achieves enhanced functional reinnervation. However, its distal latency (0.0031 ms) is longer than that of a healthy nerve because of delayed axonal remyelination [25,26]. As in Figure 5(K), more GFAP-positive non-myelinating Schwann cells [27] are observed in the PLLA/ODLA-AG73 conduit than in a healthy nerve. In addition, the myogenic potential (6.60 V) is much larger than that for a healthy nerve, perhaps because unwanted reinnervation between a sciatic nerve and surrounding muscle occurs when the conduit degrades and loses its barrier function. Although two rats were used for the electrophysiological analysis of the PLLA/ODLA-AG73 conduit, electromyograms of both were similar. Thus, our results suggest that the PLLA/ODLA-AG73 conduit enhances nerve regeneration and its degradation rate should be controlled to achieve functional reinnervation.
4. Conclusions
We fabricated PLA nanofibrous nerve conduit modified with ODLA-AG73 conjugate by electrospinning, implanted them into rat sciatic nerves, and observed the subsequent regeneration. XPS analysis shows that neurite outgrowth-promoting peptide AG73 was stably immobilized on the PLLA nanofiber. Electrophysiological analysis in vivo shows that the PLLA/ODLA-AG73 conduit achieved enhanced functional reinnervation. The degradation ratio of the PLLA/ODLA-AG73 conduit should be optimized to suppress unwanted reinnervation with the surrounding muscles and to develop further suitable conduit for clinical applications.

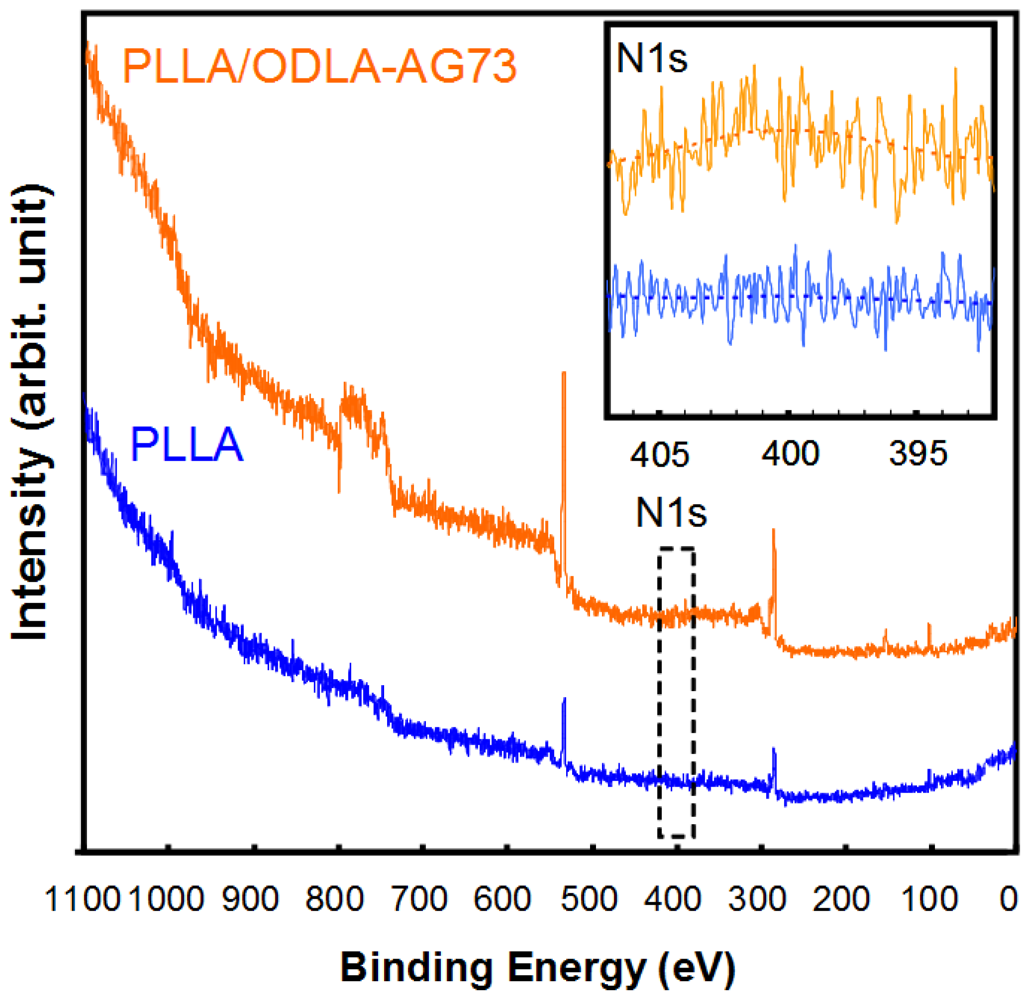


| N1s/C1s | O1s/C1s | |
|---|---|---|
| PLLA | N.D. | 1.23 ± 0.08 |
| PLLA-ODLA/AG73 | 0.03 ± 0.01 | 1.57 ± 0.27 |
Acknowledgments
A part of this work was supported by Grant-in-Aid for Scientific Research on Innovation Areas (20106014).
Appendix
References
- Hadlock, T.; Elisseeff, J.; Langer, R.; Vacanti, J.; Cheney, M. A tissue-engineered conduit for peripheral nerve repair. Arch. Otolaryngol. Head Neck. Surg. 1998, 124, 1081–1086. [Google Scholar]
- Meek, M.F.; Coert, J.H. Clinical use of nerve conduits in peripheral-nerve repair: Review of the literature. J. Reconstr. Microsurg. 2002, 18, 97–110. [Google Scholar]
- Mligiliche, N.L.; Tabata, Y.; Kitada, M.; Endoh, K.; Okamoto, K.; Fujimoto, E.; Ide, C. Poly lactic acid-caprolactone copolymer tube with a denatured skeletal muscle segment inside as a guide for peripheral nerve regeneration: A morphological and electrophysiological evaluation of the regenerated nerves. Anat. Sci. Int. 2003, 78, 156–161. [Google Scholar]
- Sundback, C.; Hadlock, T.; Cheney, M.; Vacanti, J. Manufacture of porous polymer nerve conduits next term by a novel low-pressure injection molding process. Biomaterials 2003, 24, 819–830. [Google Scholar]
- Oh, S.H.; Kim, J.H.; Song, K.S.; Jeon, B.H.; Yoon, J.H.; Seo, T.B.; Namgung, U.; Lee, I.W.; Lee, J.H. Peripheral nerve regeneration within an asymmetrically porous PLAG/Pluronic F127 nerve guide conduit. Biomaterials 2008, 29, 1601–1609. [Google Scholar]
- Sakai, Y.; Matsuyama, Y.; Takahashi, K.; Sato, T.; Hattori, T.; Nakashima, S.; Ishiguro, N. New artificial nerve conduits made with photocrosslinked hyaluronic acid for peripheral nerve regeneration. Bio-Med. Mater. Eng. 2007, 17, 191–197. [Google Scholar]
- Inada, Y.; Morimoto, S.; Moroi, K.; Endo, K.; Nakamura, T. Surgical relief of causaligia with an artificial nerve guide tube: Successful surgical treatment of causalgia (Complex regional pain syndrome type II) by in situ tissue engineering with a polyglicolic acid-collagen tube. Pain 2005, 117, 251–258. [Google Scholar]
- Andair-Kirk, T.L.; Senior, R.M. Fragments of extracellular matrix as amediators of inflammation. Int. J. Biochem. Cell Biol. 2008, 40, 1101–1110. [Google Scholar]
- Itoh, S.; Suzuki, M.; Yamaguchi, I.; Takakuda, K.; Kobayashi, H.; Shinomiya, K.; Tanaka, J. Development of a nerve scaffold using a tendon chitosan tube. Artif. Organs 2003, 27, 1079–1088. [Google Scholar]
- Wang, W.; Itoh, S.; Matuda, A.; Aizawa, T.; Demura, M.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Enhanced nerve regeneration through a bilayered chitosan tube: The effect of introduction of glycine spacer into the CYIGSR sequence. J. Biomed. Mater. Res. 2008, 85A, 919–928. [Google Scholar]
- Jiao, Y.P.; Cui, F.Z. Surface modification of polyester biomaterials for tissue engineering. Biomed. Mater. 2007, 2, R24–R37. [Google Scholar]
- Khorasani, M.T.; Mirzadeh, H.; Irani, S. Plasma surface modification of poly(L-lactic acid) and poly(lactic-co-glycolic acid) films for improvement of nerve cells adhesion. Radiat. Phys. Chem. 2008, 77, 280–287. [Google Scholar]
- Ma, Z.; Gao, C.; Ji, J.; Ji, J.; Shen, J. Protein immobilization on the surface of poly-L-lactic acid films for improvement of cellular interactions. Eur. Polym. J. 2002, 38, 2279–2284. [Google Scholar]
- Kakinoki, S.; Yamaoka, T. Stable modification of poly(lactic acid) surface with neurite outgrowth-promoting peptides via hydrophobic collagen-like sequence. Acta Biomater. 2010, 6, 1925–1930. [Google Scholar]
- Yamaoka, T.; Uchida, S.; Higami, T.; Murakami, A. Immobilization of Bioactive Molecules onto PLLA Porous Matrices for Tissue Regeneration. Proceedings of International Chemical Congress of Pacific Basin Societies, Hawaii, HI, USA, 15–20 December 2005. Program No. 482.
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar]
- Fujiwara, T.; Mukose, T.; Yamaoka, T.; Yamane, H.; Sakurai, S.; Kimura, Y. Novel thermo-responsive formation of a hydrogel by stereo-complexation between PLLA-PEG-PLLA and PDLA-PEG-PDLA block copolymers. Macromol. Biosci. 2001, 1, 204–208. [Google Scholar]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar]
- Weeks, B.S.; Nomizu, M.; Ramachandran, R.S.; Yamada, Y.; Kleinman, H.K. Laminin-1 and the RKRLQVQLSIRT Laminin-1 α1 globular domain peptide stimulate matrix metalloproteinase secretion by PC12 cells. Exp. Cell Res. 1998, 243, 375–382. [Google Scholar]
- Mochizuki, M.; Kadoya, Y.; Wakabayashi, Y.; Kato, K.; Okazaki, I.; Yamada, M.; Sato, T.; Sakairi, N.; Nishi, N.; Nomizu, M. Laminin-1 peptide-conjugated chitosan membranes as a novel approach for cell engineering. FASEB J. 2003, 17, 875–877. [Google Scholar]
- Weng, C.C.; Peter, D.W. Fmoc Solid Phase Peptide Synthesis; Oxford University Press: New York, NY, USA, 2000; pp. 61–62. [Google Scholar]
- Francel, P.C.; Francel, T.J.; Mackinnon, S.E.; Hertl, C. Enhancing nerve regeneration across a silicone tube conduit by using interposed short-segment nerve grafts. J. Neurosurg 1997, 87, 887–892. [Google Scholar]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. 2003, 67A, 531–537. [Google Scholar]
- Navarro, X.; Rodriguez, F.J.; Labrador, R.O.; Buti, M.; Ceballos, D.; Gomez, N.; Guadras, J.; Perego, G. Peripheral nerve regeneration through bioresorbable and durable nerve guides. J. Peripher. Nerv. Syst. 1996, 1, 53–64. [Google Scholar]
- Ahmed, M.R.; Vairamuthu, S.; Shafiuzama, M.D.; Basha, S.H.; Jayakumar, R. Microwave irradiated collagen tubes as a better matrix for peripheral nerve regeneration. Brain Res. 2005, 1046, 55–67. [Google Scholar]
- Jessen, K.R.; Morgan, L.; Stewart, H.J.S.; Mirsky, R. Tree markers of adult non-myelin-forming Schwann cells, 217c(Ran-1), A5E3 and GFAP: development and regulation by neuron-Schwann cell interactions. Development 1990, 109, 91–103. [Google Scholar]
©2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kakinoki, S.; Uchida, S.; Ehashi, T.; Murakami, A.; Yamaoka, T. Surface Modification of Poly(L-lactic acid) Nanofiber with Oligo(D-lactic acid) Bioactive-Peptide Conjugates for Peripheral Nerve Regeneration. Polymers 2011, 3, 820-832. https://doi.org/10.3390/polym3020820
Kakinoki S, Uchida S, Ehashi T, Murakami A, Yamaoka T. Surface Modification of Poly(L-lactic acid) Nanofiber with Oligo(D-lactic acid) Bioactive-Peptide Conjugates for Peripheral Nerve Regeneration. Polymers. 2011; 3(2):820-832. https://doi.org/10.3390/polym3020820
Chicago/Turabian StyleKakinoki, Sachiro, Sho Uchida, Tomo Ehashi, Akira Murakami, and Tetsuji Yamaoka. 2011. "Surface Modification of Poly(L-lactic acid) Nanofiber with Oligo(D-lactic acid) Bioactive-Peptide Conjugates for Peripheral Nerve Regeneration" Polymers 3, no. 2: 820-832. https://doi.org/10.3390/polym3020820