1. Introduction
Stimuli responsive hydrogels are three dimensional systems composed of a solid crosslinked matrix network and interstitial fluid with or without ionic species [
1]. Several responsive hydrogels such as pH [
2,
3], temperature [
4,
5] and enzyme [
6,
7] stimulated systems are currently under research. However, substance release from these responsive hydrogels mostly relies on internal stimulus for activation and cannot be controlled. Electro-conductive hydrogels (ECHs), with electrically tunable properties, primarily consist of an inherently conducting polymer within a tridimensional crosslinked network of a polymer-based hydrogel. The combination of conductive properties of electro-active polymers (EAPs) and the swelling/de-swelling capabilities of the hydrogel, make them dynamic and versatile for various biomedical purposes [
8], such as implantable drug delivery systems, thus allowing for patient-controlled release of drugs at the times of need directly into the target area.
There are various EAPs which may be used as conducting polymers, such as polypyrrole [
9,
10], polythiophene [
11,
12], poly(3,4-ethylenedioxythiophene) (PEDOT) [
13] and polyaniline (PANi) [
14]. Among these, the application of PANi as an EAP has attracted much attention in a wide variety of fields such as artificial muscles [
15,
16], technological applications such as rechargeable batteries, electromagnetic interference shielding, microwave and radar absorbing materials, anti-static and anti-corrosive coatings [
17,
18], biosensors [
19] and conducting polymers [
20]. This is attributed to the simplicity of its synthesis, either chemically or electrochemically, high degree of chemical stability and easy doping process [
21]. PANi, the inherently conductive polymer, has also been shown to be biocompatible and is therefore the EAP of choice for the synthesis of Electro-Conductive Hydrogel (ECH) [
22-
25]. When EAPs such as PANi or polypyrrole (PPy) are used alone, they display a lack of solubility and fragility, thus requiring the blending of the EAP into a co-matrix system making it an essential step for the synthesis of the ECH [
26]. The preparation of a hydrogel can be performed by either using a natural or a synthetic polymer [
27]. Synthetic polymers are usually preferred since natural polymers, although more biocompatible, may not have sufficient mechanical strength and may cause inflammation due to contamination from pathogens [
28]. Synthetic polymers, on the other hand, can be tailored to suit individual needs in terms of functionality. The use of a synthetic polymer as a drug delivery system generally causes concern regarding its biocompatibility. With the myriads of synthetic polymers being discovered, several with the potential to be used in a drug delivery system have been investigated regarding their biocompatibility. Although not all, some EAPs with favorable characteristics such as the PANi-derivatives and the PPy-derivatives have also shown to be biocompatible. Those non-biocompatible are required to be re-modified or otherwise be utilized as an
ex vivo drug delivery system where there is no risk of causing any untoward reactions in the patients. Polymers such as polyacrylic acid [
29], polyacrylamide [
30], polysaccharides [
31], poly(
N-isopropyl acrylamide) [
32] and poly(vinyl alcohol) (PVA) [
30,
31] all have potential for the synthesis of a hydrogel. PVA was selected as the hydrogel component in this study based on its favorable water-soluble, desirable physicochemical properties and its biocompatibility [
33]. Furthermore, chemically crosslinked PVA hydrogel has been gaining increasing attention in the field of biomedics [
34].
This study focused on developing an ECH that is capable of electro-actuable release of the model drug indomethacin in the presence of an electric field by co-blending PANi with PVA and subsequently crosslinking with diethyl acetamidomalonate (DAA), as a polymeric hydrogel system. The diethyl group of DAA, a biocompatible crosslinking agent [
35], renders it chemically reactive and was responsible for the crosslinking action [
36]. A hydrogel may be synthesized as a blend, a copolymer, a graft copolymer, an interpenetrating polymer network (IPN) or a composite and each is structured differently. A blend hydrogel is one which is synthesized by interaction between two or more reagents. One example would be a hydrogel formed via the esterification of PVA and gelatine [
37]. The esterification occurs between the hydroxyl group of the PVA and the carboxyl group of the gelatine, thus resulting in the hydrogel. Sometimes, the hydrogel may be as a result of two monomers reacting together to form a polymer, or by incorporation of one polymer into another. These hydrogels are termed co-polymer hydrogels. A study done by Henderson and co-workers has managed to form a hydrogel consisting of a crosslinked tri-block copolymer network [
38]. A graft copolymeric hydrogel is similar to a copolymer hydrogel, as the polymer which is used to form the hydrogel is a graft co-polymer [
39]. A graft copolymer is a type of branched copolymer with the side chain being different and separate from the main chain. As in the case of the graft copolymer, the copolymer formed usually combines the properties of both polymers which forms the copolymer [
40].
The next hydrogel structure which may be formed is the IPN, which is essentially a network of a component within the hydrogel network itself. It may be classified as a class of polymer blends in network form, in which at least one component is polymerized in the presence of the other [
41]. EAPs are commonly incorporated into the hydrogel in the form of IPN. This may increase the conductivity of the electrical stimulation into the hydrogel and thus improve the electro-response of the hydrogel. The IPN hydrogel may be formed by initially creating a solution containing both components. The initiator and crosslinker is then added into the solution simultaneously. The crosslinking and polymerization is then allowed to continue for several hours until the hydrogel is formed [
42]. IPN hydrogel is commonly synthesized with an EAP incorporated.
The last class of hydrogel is the composite hydrogel. A composite hydrogel is formed with a particle being embedded within the hydrogel network. These particles do not have any direct interactions with the hydrogel. Composite hydrogels can be synthesized as a responsive hydrogel, where the drug was entrapped within the hydrogel. The responsive hydrogel then swells/deswells when exposed to various stimuli and thus results in drug release [
43]. One more important reason for using DAA as a crosslinker is that it offers adequate structural integrity of the ECH without compromising its swelling capability. Indomethacin was incorporated into the ECH as the model drug for controlled release via electro-actuation. Indomethacin is used for the treatment of chronic pain and it is well known that repeated oral dosing of indomethacin causes undesirable side-effects such as gastric irritation [
44-
46]. Therefore, the incorporation of indomethacin into the ECH may allow for the controlled release of indomethacin in the targeted area, thus reducing side-effects. This work investigated the effect of electric-actuation of drug release from the ECH in addition to characterization studies.
3. Experimental Section
3.1. Materials
Diethyl acetamidomalonate (DAA) was purchased from Fluka Chemie AG, Buchs, Switzerland. The commercially available polymers, polyvinyl alcohol (PVA) (Sigma-Aldrich, Steinhelm, Germany; MW = 89,000–98,000 g/mol) and polyaniline (PANi) (Sigma-Aldrich, Steinhelm, Germany; MW = 20,000 g/mol) were used. Indomethacin was procured from Sigma-Aldrich (St Louis, MO, U.S.). Double deionized water was obtained from a Milli-Q water purification system (Milli-Q, Millipore, Billerica, MA, U.S.). All other reagents used were of analytical grade and were employed as purchased.
3.2. Preparation of the Electro-Conductive Hydrogel
The ECH formulations were synthesized by crosslinking PVA with DAA, thus entrapping indomethacin and the EAP within the hydrogel matrix. Initially, 0.8 g of PVA was dissolved in 10 mL of double deionized water while 0.0689 g of DAA and 100mg indomethacin were dissolved in acetone in separately. After the DAA and the indomethacin were completely dissolved, 1.3418 %w/w PANi was added to the mixture. The two solutions were then blended and agitated with a glass rod in order to facilitate the crosslinking process. Crosslinking occurred spontaneously and was complete within one minute. The crosslinked polymer composite was then rinsed with distilled water and air-dried for 24 hours at room temperature.
3.3. Optimization Studies Employing a Box-Behnken Experimental Design Template
In order to produce an optimized ECH with high drug entrapment and minimum erosion, 15 ECH formulations with various combinations of the independent variables were synthesized and tested for their drug releasing ability, drug entrapment efficiency and rate of erosion (
Table 1). The results were guided through a 3-factor, 3 centre points Box-Behnken quadratic design employing Minitab Statistical Software, V15 (Minitab Inc., State College, PA, U.S.). The three independent variables employed for the synthesis of the ECH included the hydrogel component formed by PVA, the crosslinker DAA used to increase the structural integrity of the hydrogel and the EAP, namely PANi, to enhance the conductivity of the ECH.
Table 1 represents the upper and lower limits for each of the variables. The limits for these variables were based on their ability to form a stable ECH with optimal drug release kinetics (maximum quantity of drug released before depletion) with a minimal erosion rate and maximum drug entrapment efficiency.
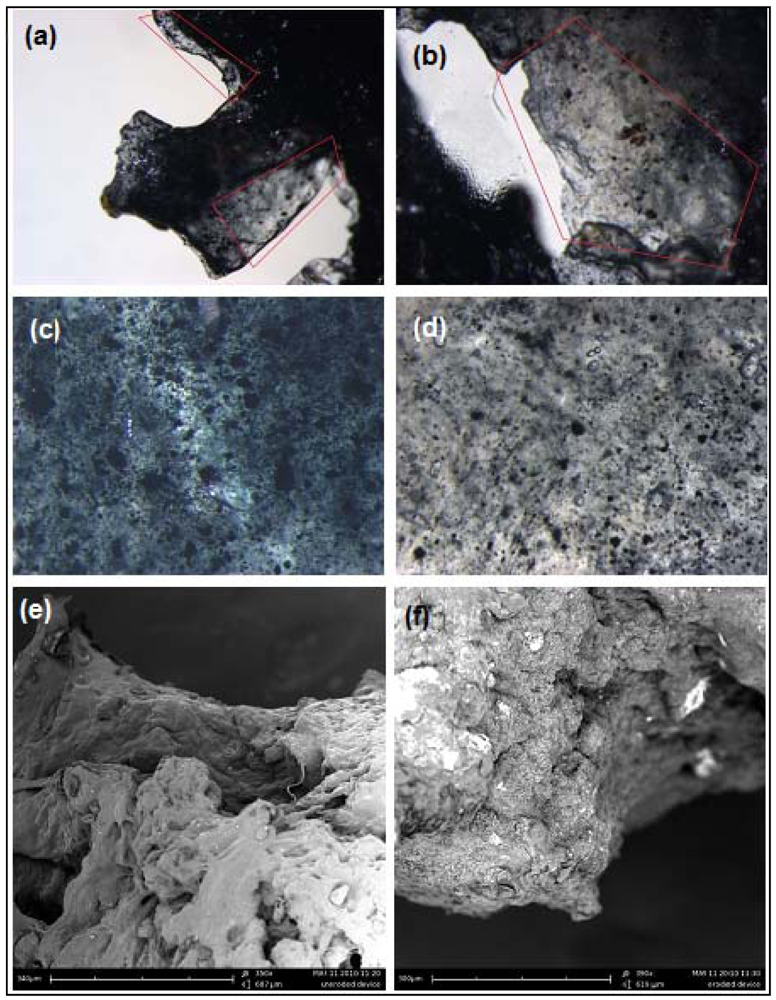
3.4. Determination of the Surface Morphology of the Electro-Conductive Hydrogel
An assessment of the surface morphology facilitated the determination of the extent of erosion on the ECH. Surface morphology was assessed using light microscopy (Olympus SZX7 ILLD2-200, Olympus, Tokyo, Japan) and scanning electron microscopy (SEM) (Phenom™, FEI Company, Hillsboro, Oregon, U.S.).
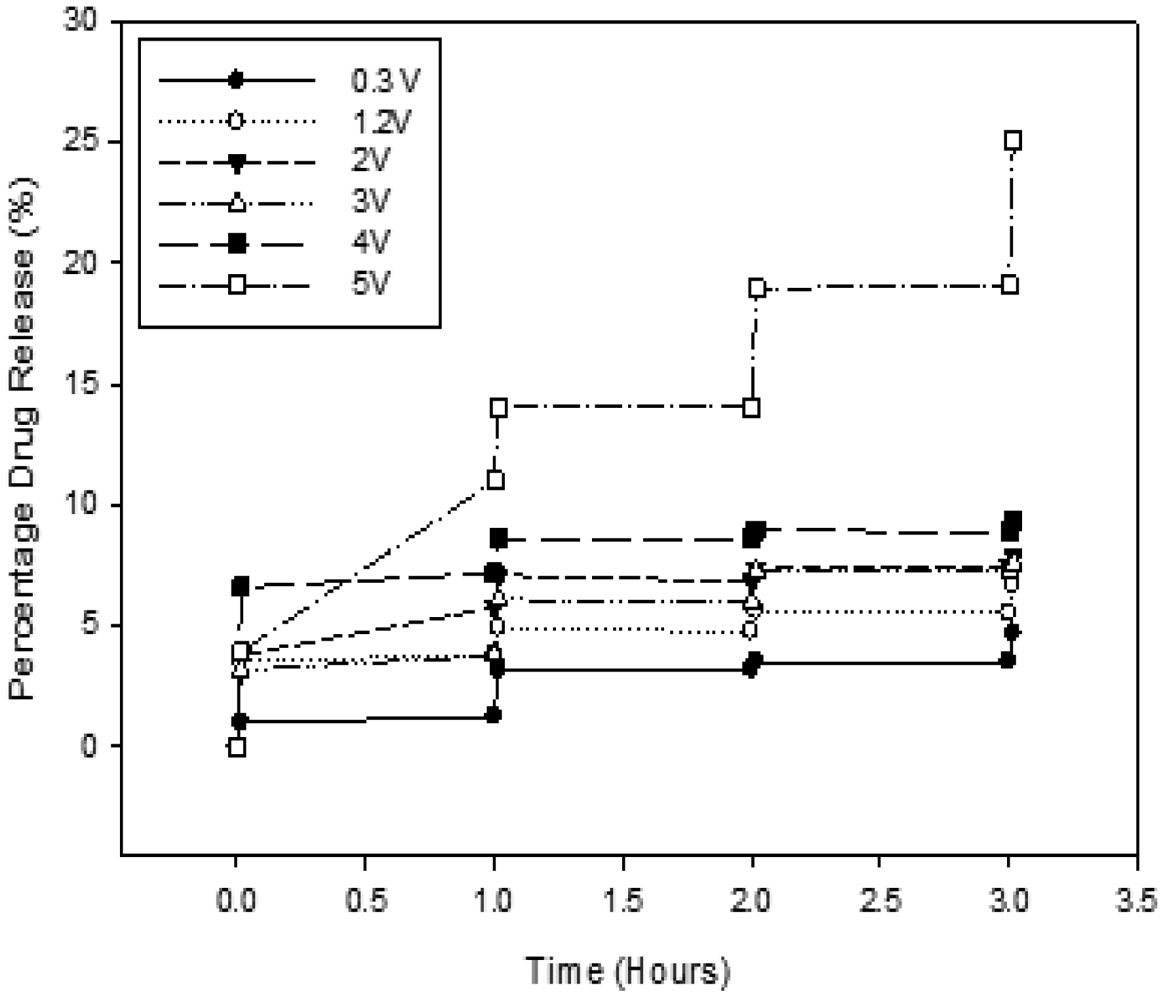
3.5. Assessment of the Drug Release from the Electro-Conductive Hydrogel
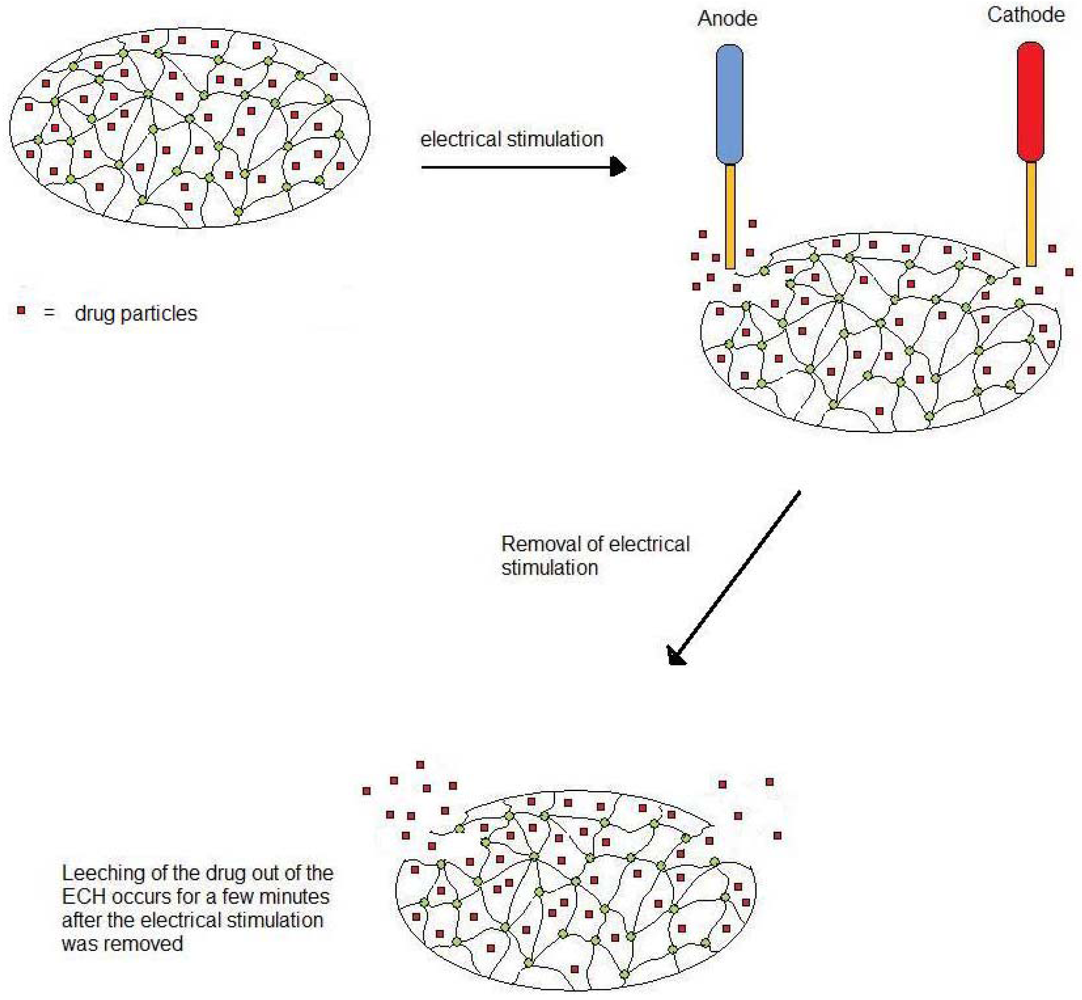
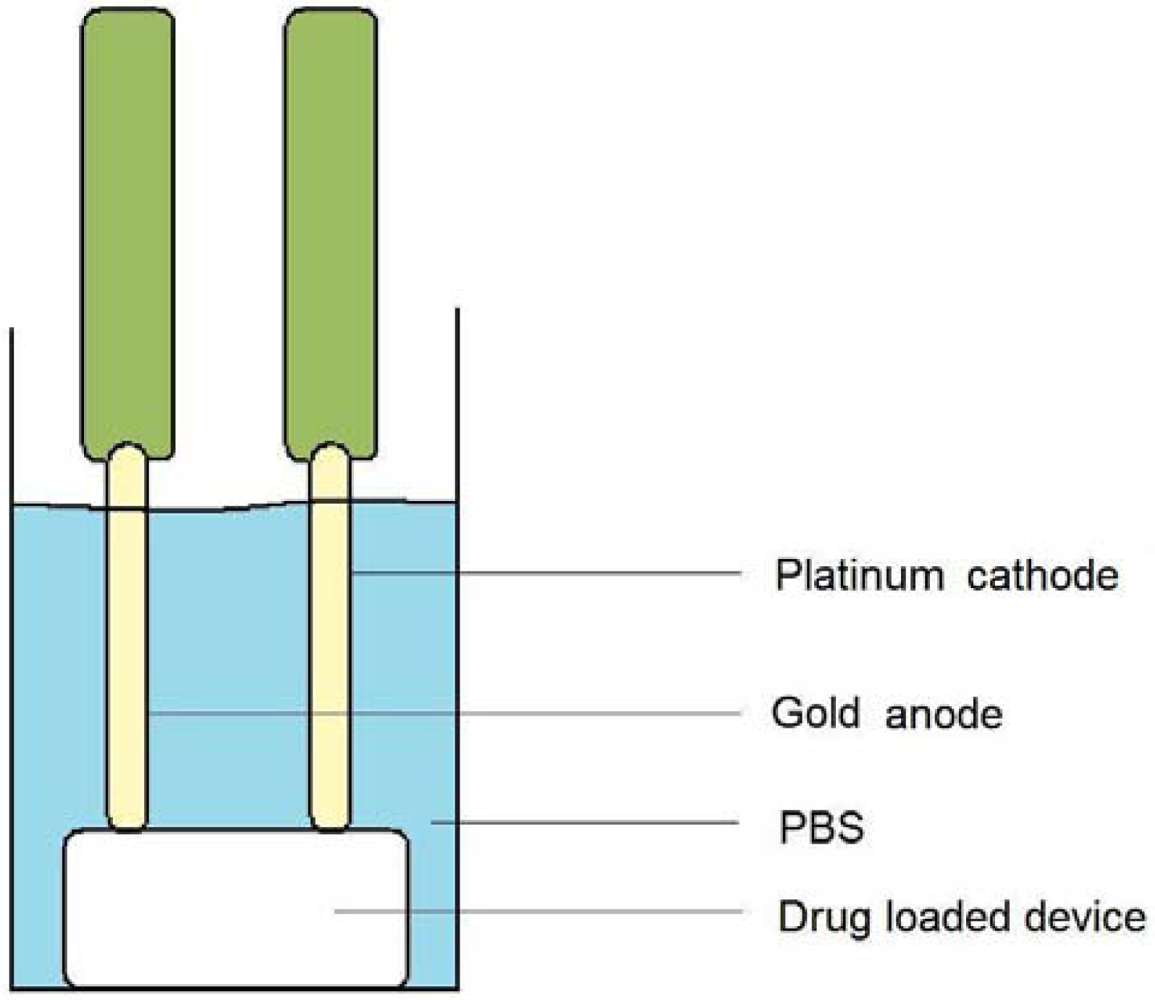
In order to assess the drug release from the ECH, the system was subjected to electrical stimulation while immersed in phosphate-buffered saline (PBS; pH 7.4; 37 °C). This was performed by immersing the ECH in 20 mL of PBS and allowing a potential difference of 1.2 V to be maintained between the two electrodes. Platinum was used as the cathode and gold as the anode. The setup of the experiment is depicted in
Figure 10.
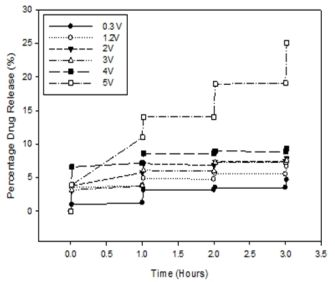
Electrical stimulation was maintained for a duration of 60 seconds before 2 mL samples were withdrawn for analysis of drug concentration. This was repeated four times, after which the samples were then analyzed by UV/visible spectroscopy (Lambda 25 UV/vis Spectroscopy, Perkin-Elmer, Shelton, CT, U.S.) for any presence in drug via computation from a standard linear curve of indomethacin in PBS (pH 7.4; 37 °C) (R2 = 0.99).
3.6. Determination of the Drug Entrapment Efficiency
Drug Entrapment Efficiency (DEE) studies were performed on the ECH by initially removing a section of the ECH. The removed section was accurately weighed and completely dissolved in 100 mL of PBS. The PBS solution was then analyzed at λ
318 nm for indomethacin content using a standard linear curve [
53,
54].
Equation 1 was used to compute the DEE.
Where, DEE% is the drug entrapment efficiency, Ia is the actual quantity (mg) of indomethacin as determined by the UV/visible spectroscopy and It is the theoretical quantity (mg) of indomethacin which was added into the ECH.
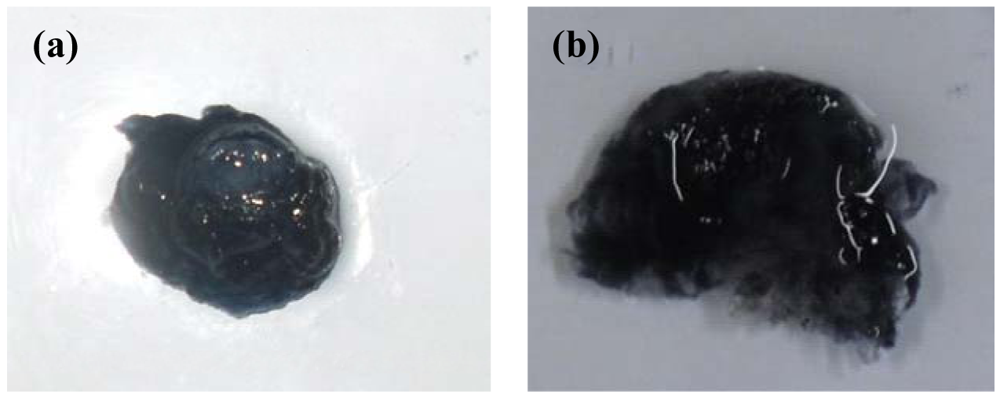
3.7. Determination of the Erosion Rate
The rate of erosion was determined by accurately weighing the ECH prior to exposure to electrical stimulation in the fully dehydrated state. After the mass of the ECH was determined the system was hydrated and actuated via electrical stimulation for four release cycles. The ECH was then allowed to air-dry at room temperature for 48 hours in order to facilitate the removal of water from the ECH system. The ECH which was air-dried in this fashion was then re-weighed and the difference in mass was recorded. The rate of erosion was expressed by
Equation 2.
Where, ER% represents the rate of erosion expressed as a percentage of the original weight, Wo is the weight (g) of the non-eroded ECH and We is the weight (g) of the eroded ECH.
3.8. The influence of Varying Concentration of Polyaniline on the Electro-Conductive Hydrogel
In order to assess the effects of polyaniline (PANi) concentration on the erosion rate of the ECH, two separate ECH formulations were prepared, each comprising 0.8 g of PVA, 0.0689 g of DAA and 100 mg of indomethacin. The only difference was that the first ECH formulation constituted 1 %w/w PANi while the second constituted 3 %w/w PANi. The two formulations were then immersed in 20 mL PBS and a potential difference of 1.2 V was applied for 400 seconds in order to assess the influence of PANi on the extent of matrix erosion.
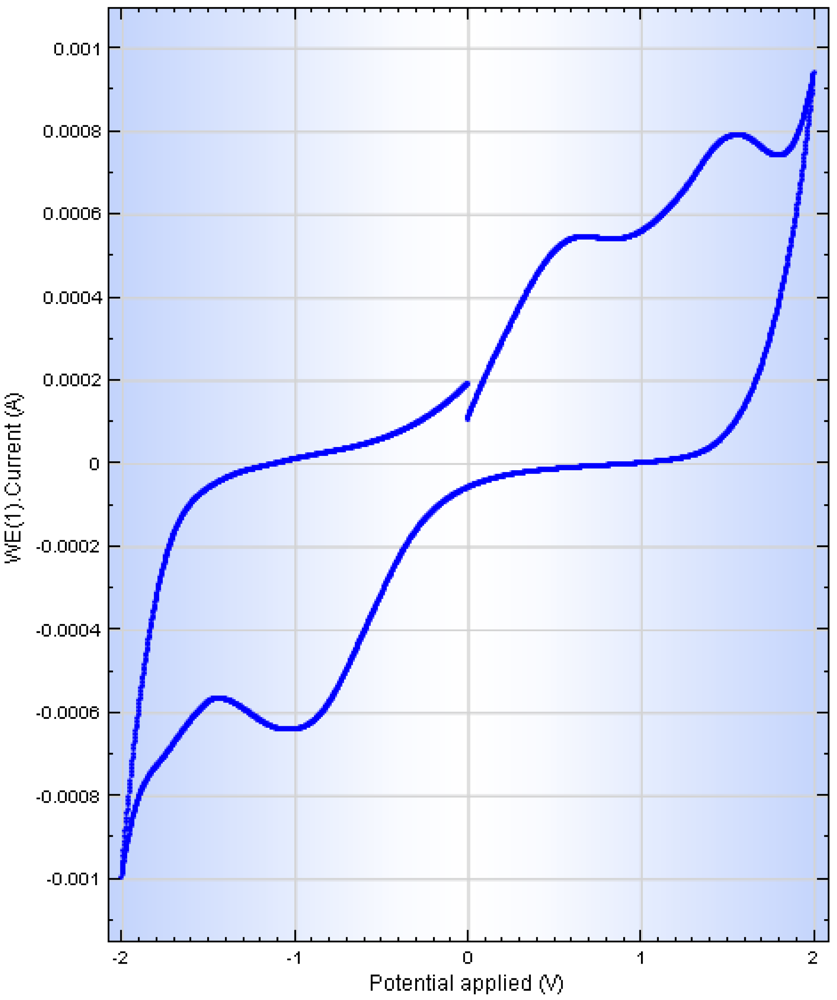
3.9. Cyclic Voltammetry Studies on the Electro-Conductive Hydrogel
Cyclic voltammetry was performed on the ECH in order to assess its electro-activity. PBS (pH 7.4) was employed as the conducting solvent [
55-
57]. Firstly, the ECH were homogenized into the PBS for a period of 5 minutes followed by ultrasonication (Vibra-Cell™, Sonics
® Sonics & Material Inc., Newtown, CT, U.S.) at an amplitude of 70% for a period of 3 minutes. The resultant solution was then purged with nitrogen gas for 3 minutes prior to cyclic voltammetry using a conventional three-electrode system (PGSTAT302N, Autolab, Utrecht, Netherlands) with a saturate Ag/AgCl (3.0 M KCl) as the reference electrode, a platinum wire as the auxiliary electrode and a 5 mm glassy carbon electrode as the working electrode [
58]. The scan rate was 0.1 V/second.
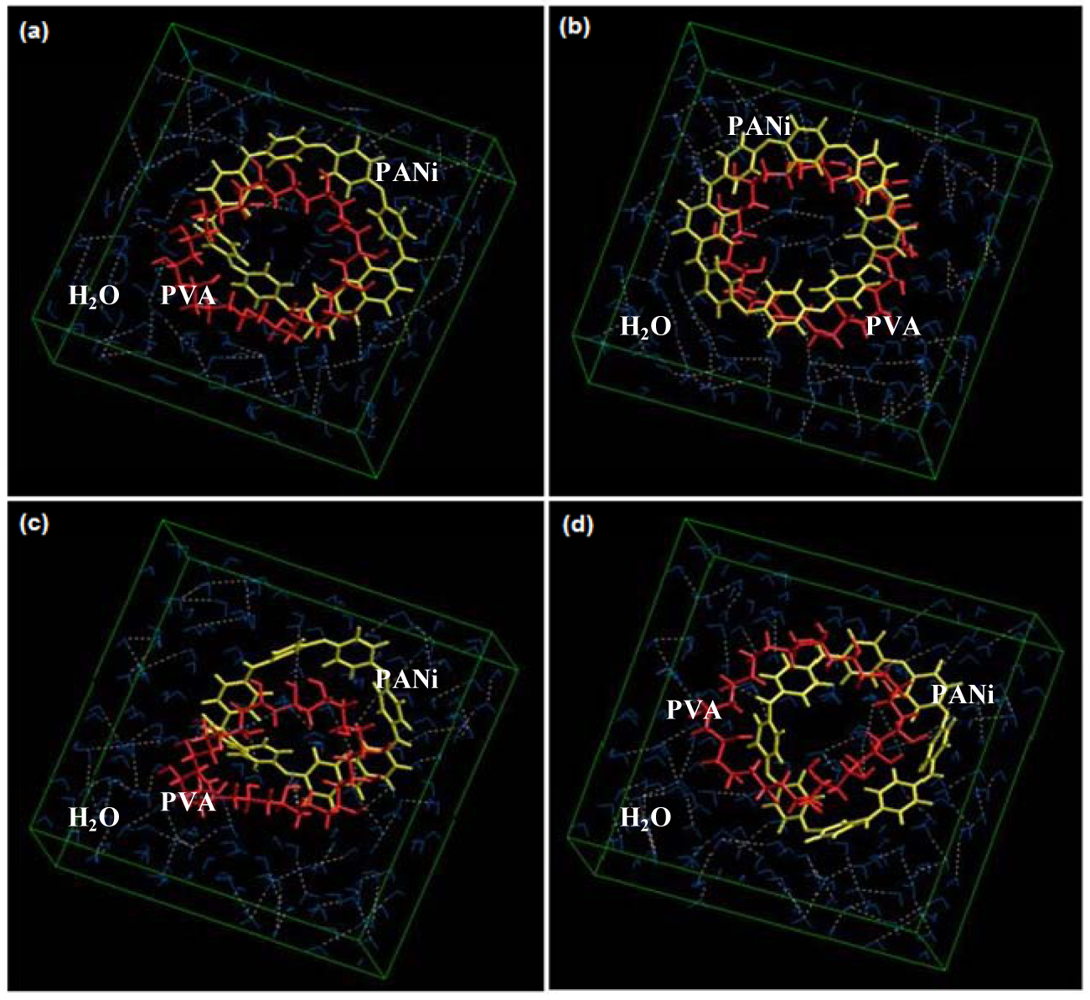
3.10. Molecular Mechanics Simulations
Molecular mechanics (MM) simulations for the solvated system with water as the surrounding fluid were performed using HyperChem™ 8.0.8 Molecular Modeling software (Hypercube Inc., Gainesville, FL, U.S.) and ChemBio3D Ultra 11.0 (CambridgeSoft Corporation, Cambridge, U.K.). The octamer of polyaniline (PANi) and hexadecamer of polyvinyl alcohol (PVA) was generated from standard bond lengths and angles employing the Polymer Builder tool using ChemBio3D Ultra in their syndiotactic stereochemistry as a 3D model. The individual polymer models were initially energy-minimized using MM+ Force Field and the resulting structures were once more energy-minimized using the Amber 3 (Assisted Model Building and Energy Refinements) Force Field. The conformers having the lowest energy were used to create the polymer-polymer complexes (PVA-PANi). All MM simulations were performed under the influence of an external electric field ranging from 0.00–0.05 a.u. for cubic periodic boxes with dimensions of 25 × 12 × 25 Å3 containing one centered PVA-PANi complex with the centre of the cubic box and the remaining free-space filled with water molecules. The same procedure of energy-minimization was repeated to generate the final models: PVA-PANi-0.01 to PVA-PANi-0.05. Full geometrical optimizations were performed for the solvated system employing the Polak-Ribiere Conjugate Gradient method until a Root Mean Square (RMS) gradient of 0.001 kcal/mol was reached. Force Field options in the AMBER (with explicit solvent) were extended to incorporate limits to inner and outer options with the nearest-image periodic boundary conditions. The outer limit was set to 6.46 Å and the inner was at 2.46 Å to ensure that there were no discontinuities in the potential surface.