Block Copolymer Nanostructures for Technology
Abstract
:1. Introduction: Applications Using Block Copolymer Nanostructures

2. Current Status of Density Multiplication Using Guided Assembly of Block Copolymers

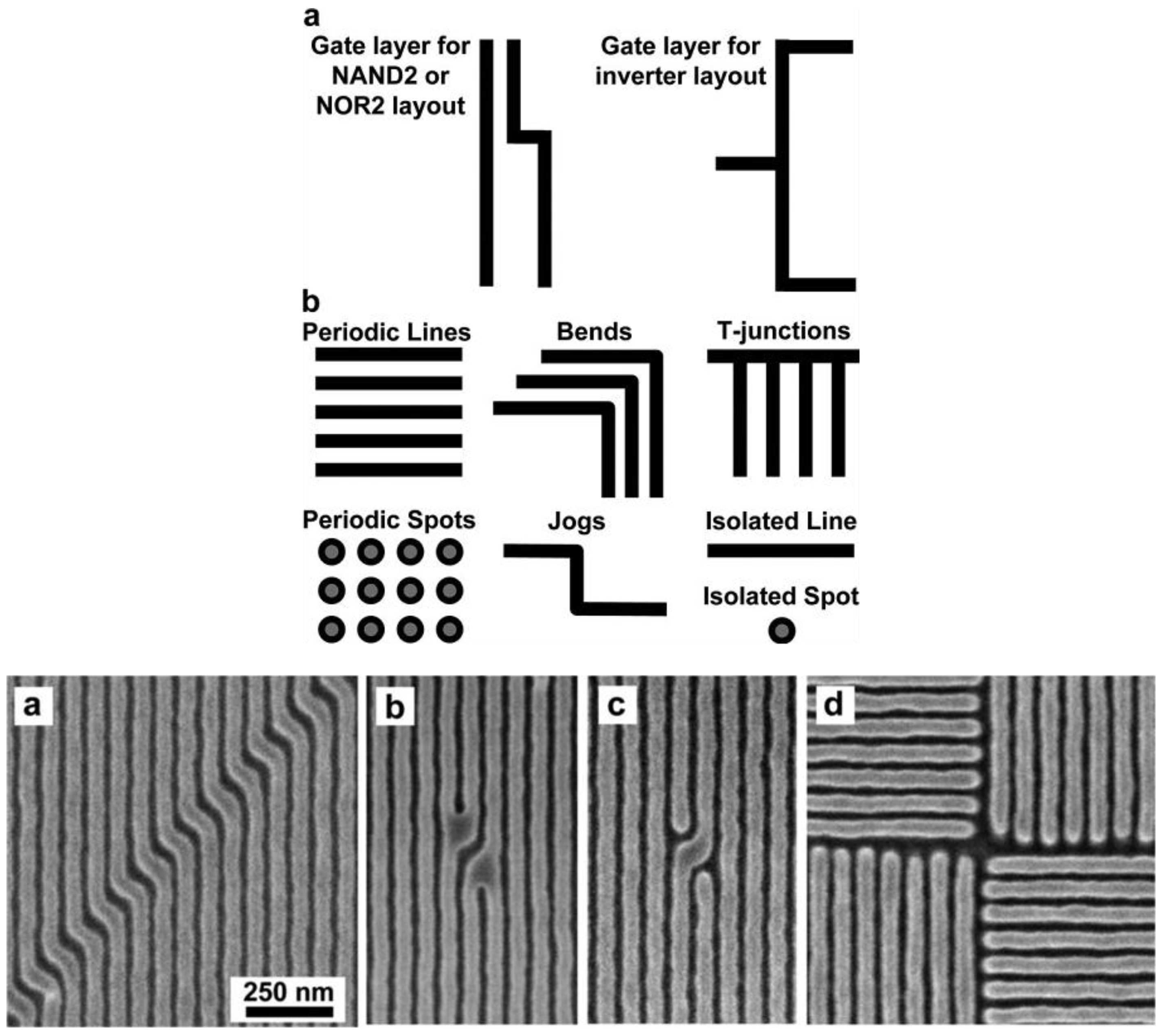
3. Some Challenges and Potential Limitations of BCP Lithography
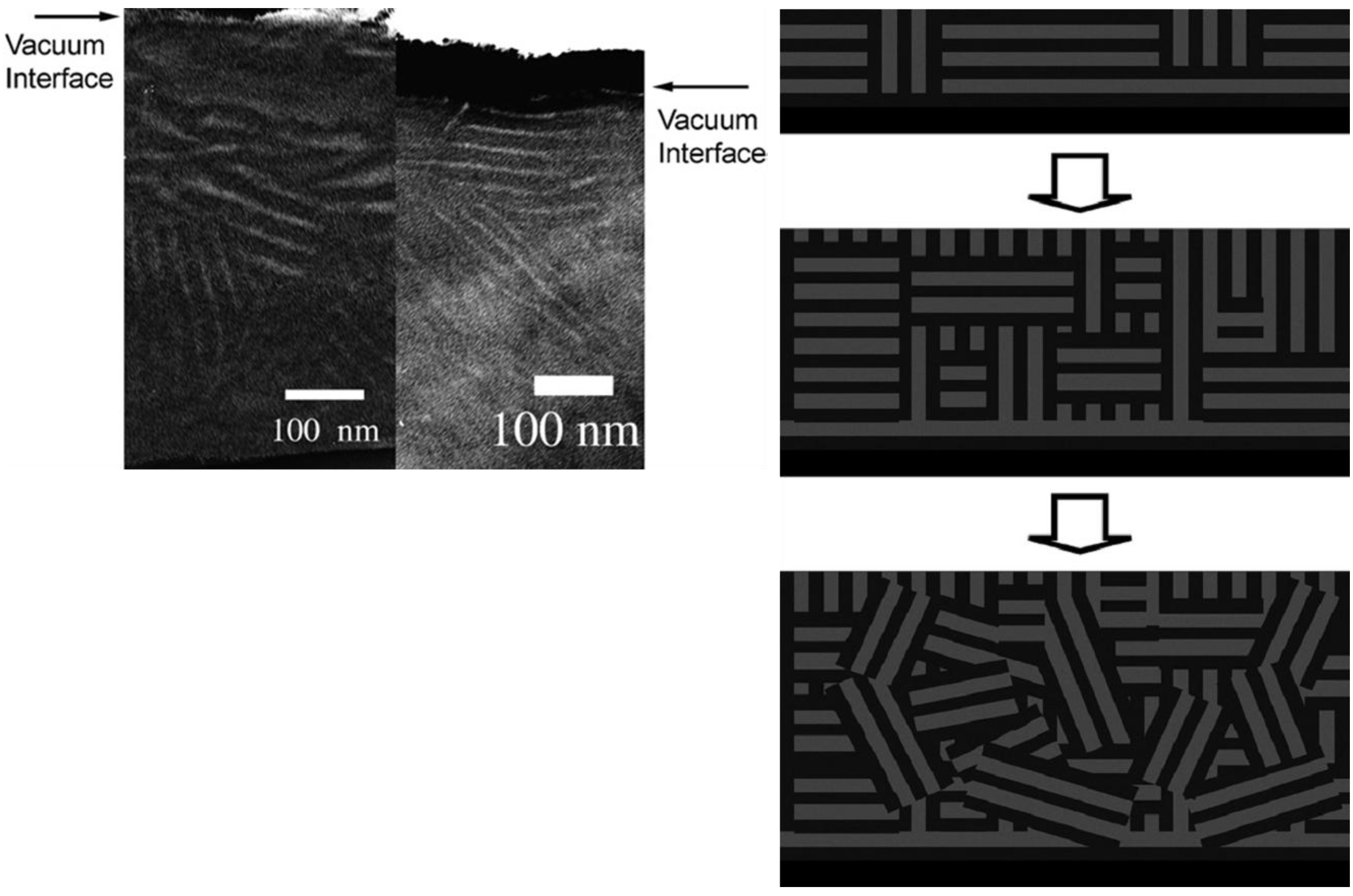
3.1. Pattern Transfer
3.2. Line-Edge Roughness
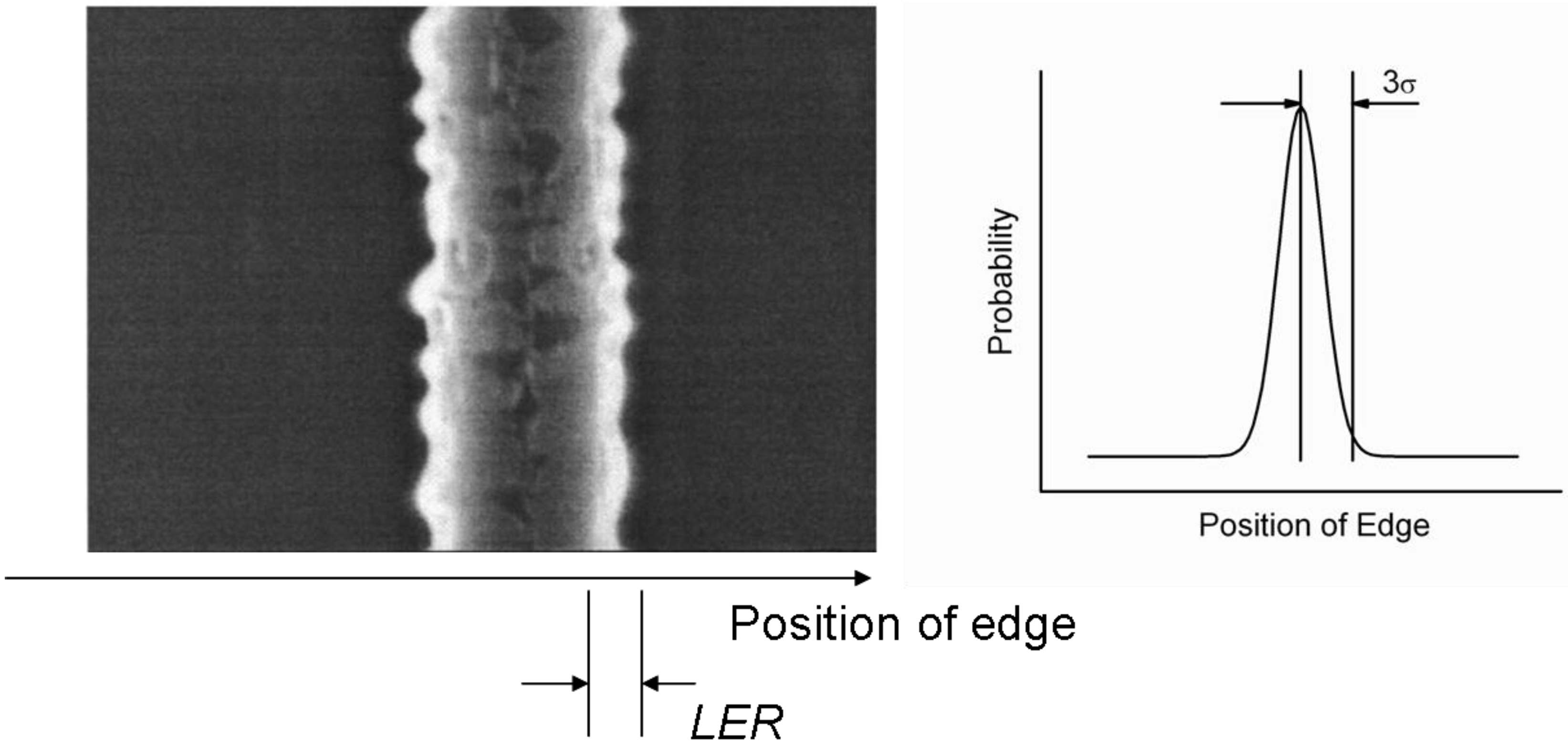
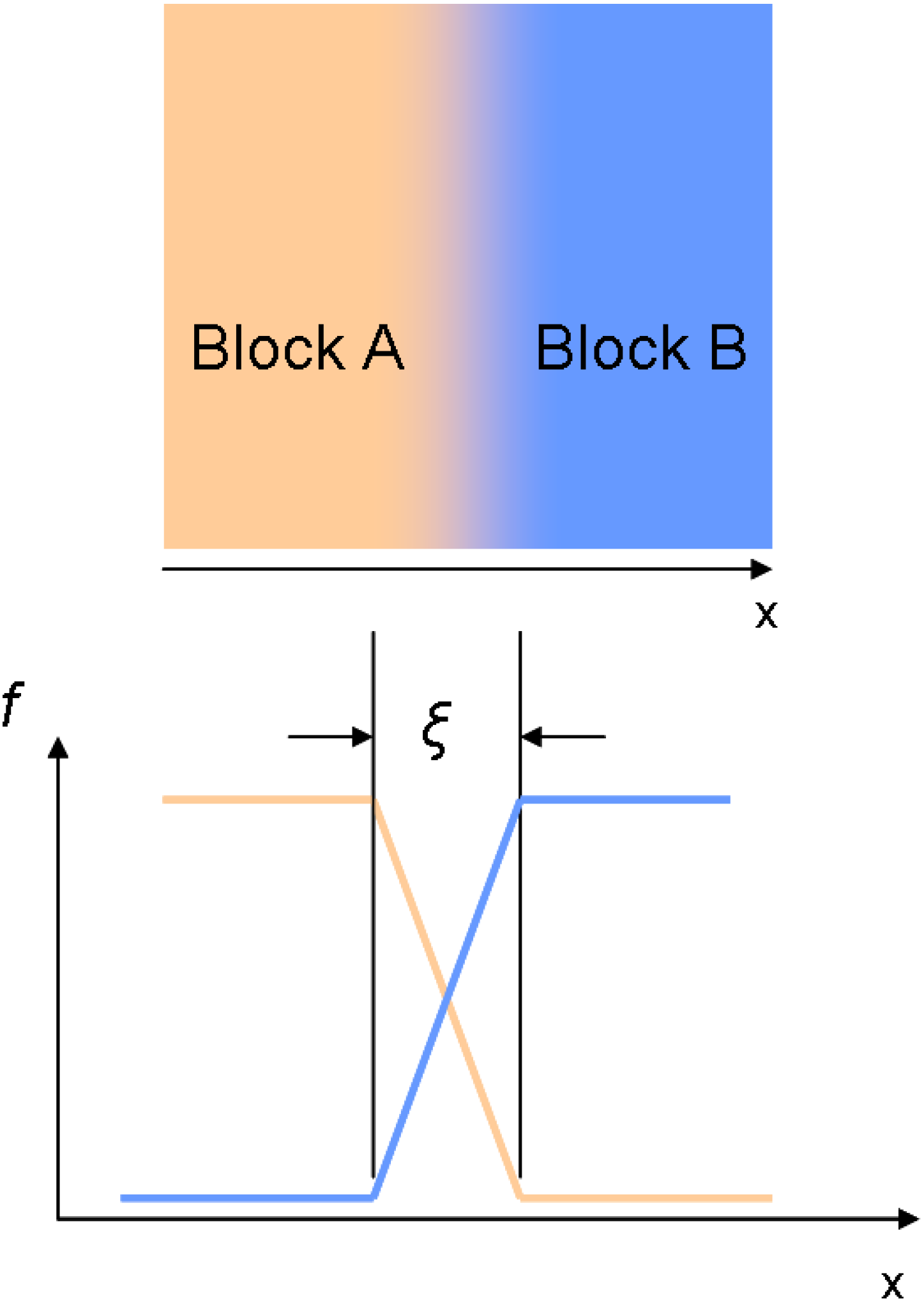
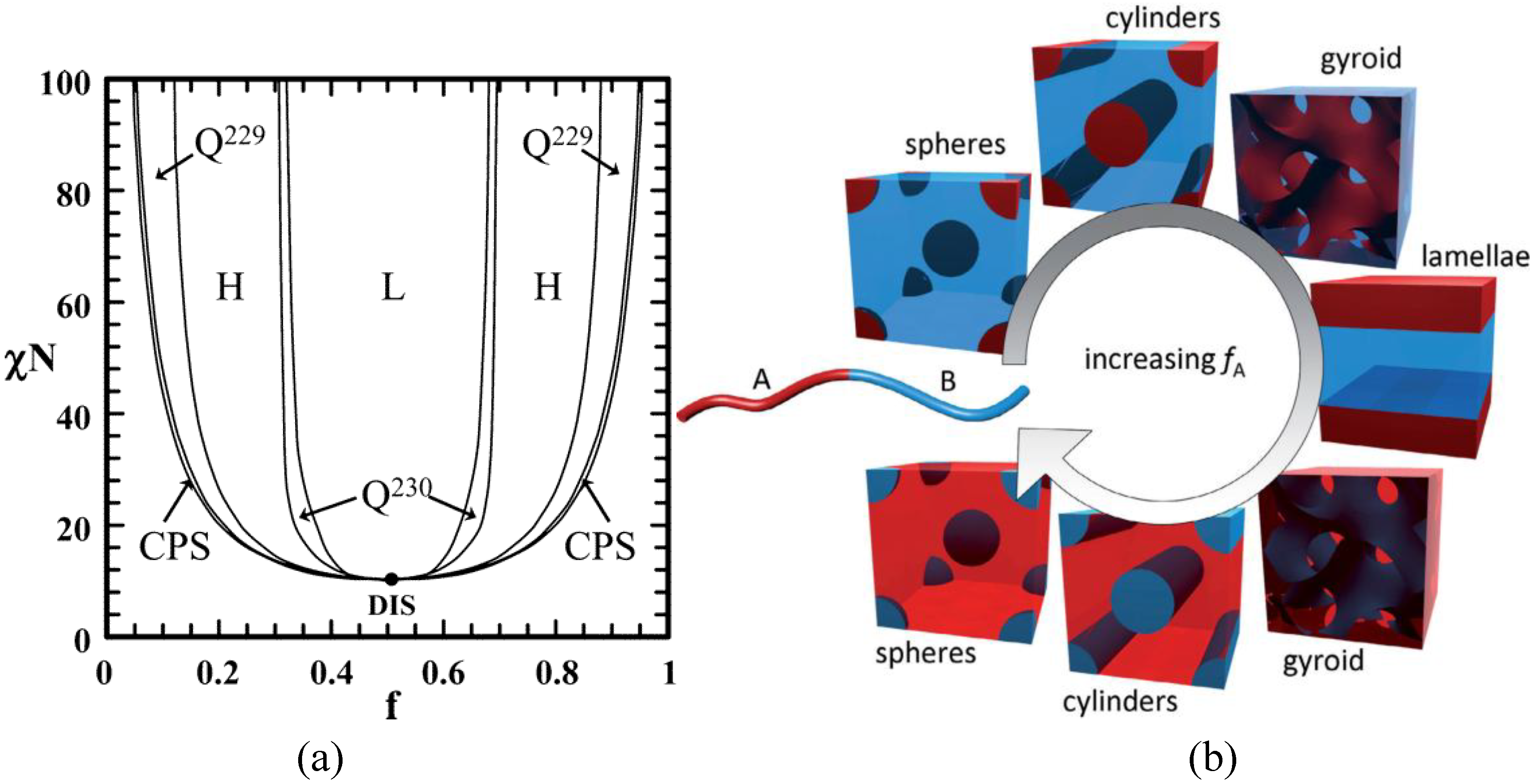
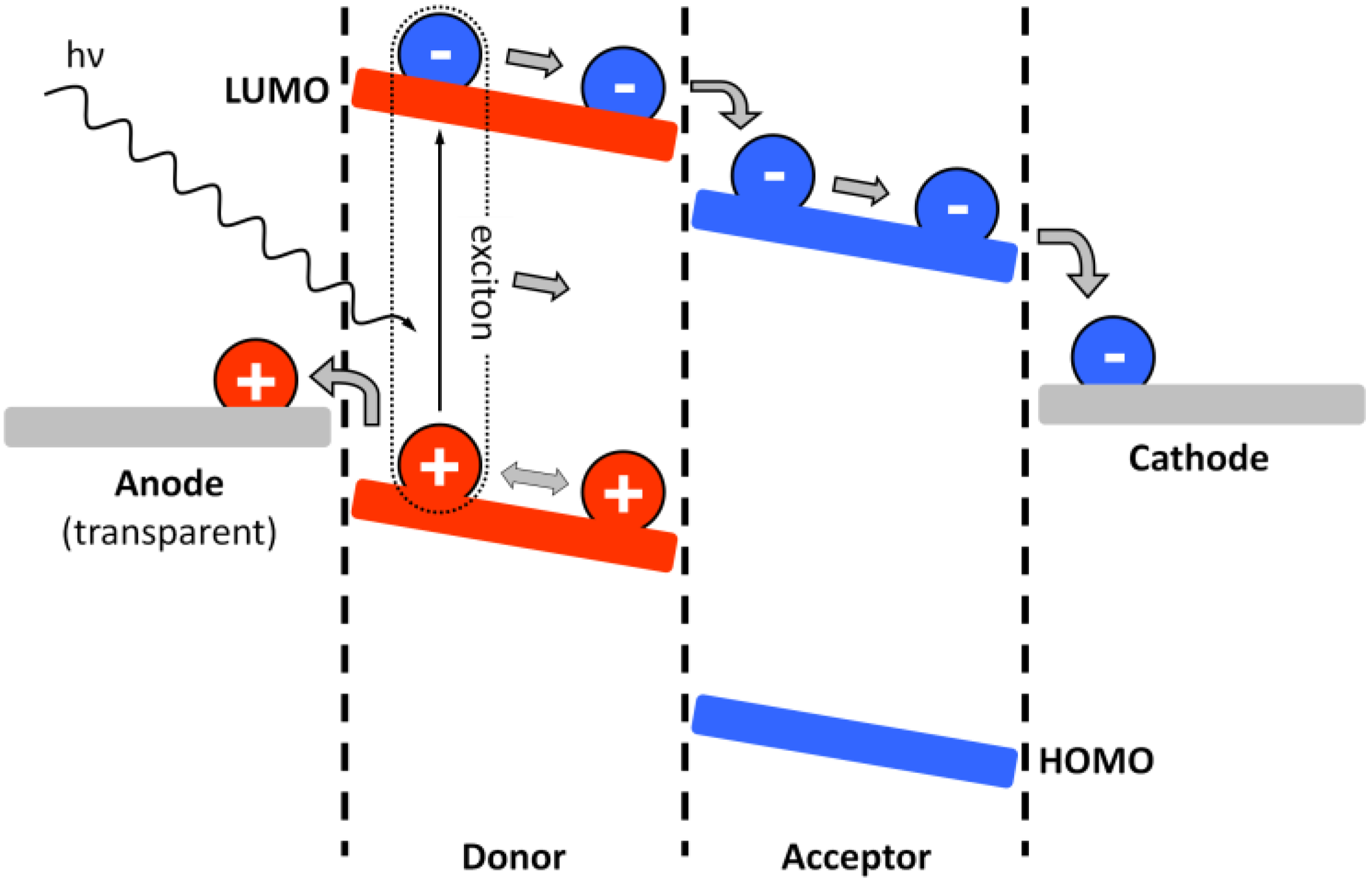
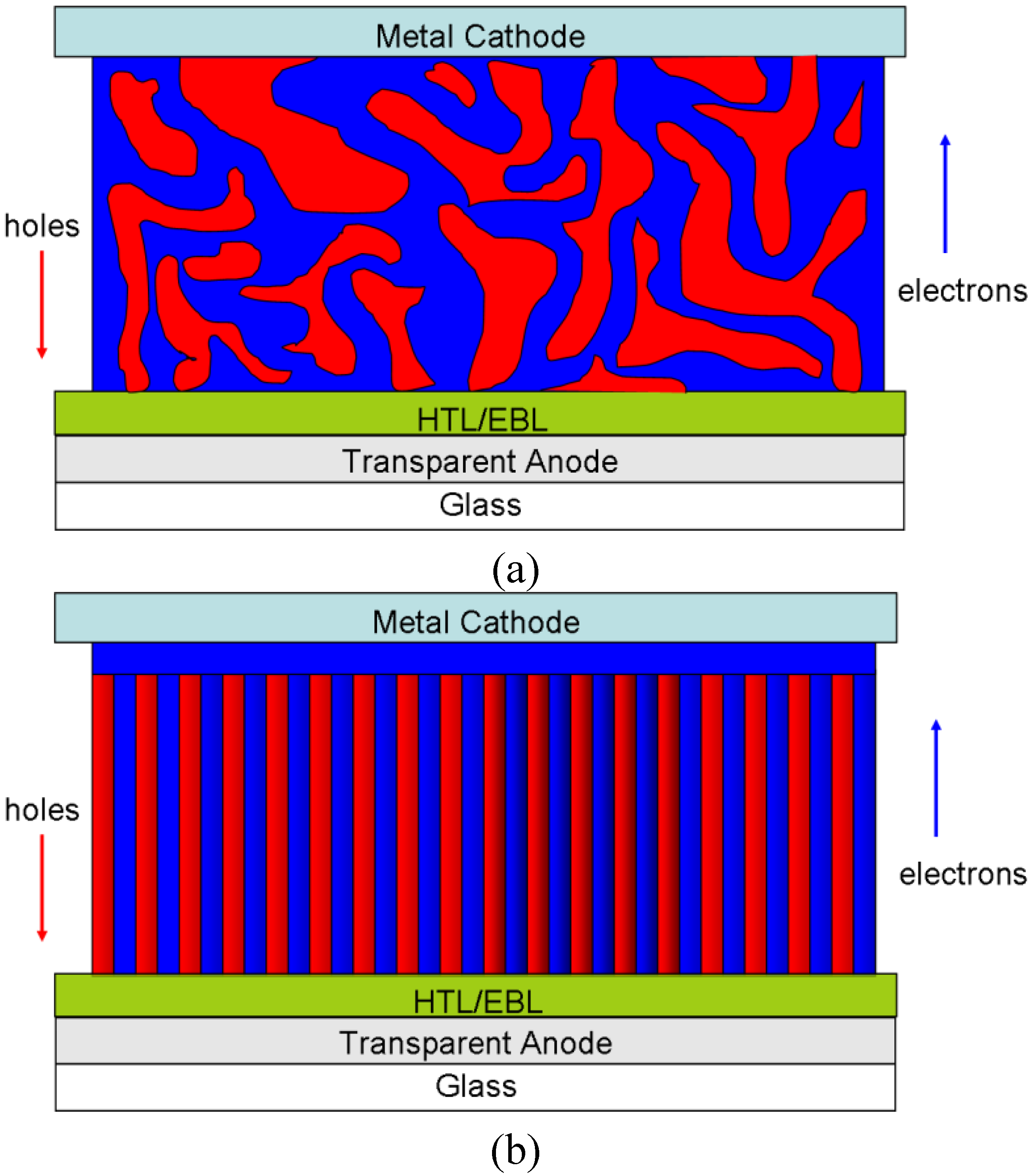
4. Requirements on the Nanostructure of Conjugated BCPs for Photovoltaic Purposes

5. Current Progress Using Donor-Acceptor BCPs
6. Summary and Future Directions
Acknowledgements
References
- Darling, S.B. Directing the self-assembly of block copolymers. Progr. Polym. Sci. 2007, 32, 1152–1204. [Google Scholar] [CrossRef]
- Kim, H.C.; Park, S.M.; Hinsberg, W.D. Block copolymer based nanostructures: Materials, processes, and applications to electronics. Chem. Rev. 2010, 110, 146–177. [Google Scholar] [CrossRef] [PubMed]
- Cochran, E.W.; Garcia-Cervera, C.J.; Fredrickson, G.H. Stability of the gyroid phase in diblock copolymers at strong segregation. Macromolecules 2006, 39, 2449–2451. [Google Scholar] [CrossRef]
- Guarini, K.W.; Black, C.T.; Zhang, Y.; Kim, H.; Sikorski, E.M.; Babich, I.V. Process integration of self-assembled polymer templates into silicon nanofabrication. J. Vac. Sci. Technol. B 2002, 20, 2788–2792. [Google Scholar] [CrossRef]
- Black, C.T.; Ruiz, R.; Breyta, G.; Cheng, J.Y.; Colburn, M.E.; Guarini, K.W.; Kim, H.C.; Zhang, Y. Polymer self assembly in semiconductor microelectronics. IBM J. Res. Dev. 2007, 51, 605–633. [Google Scholar] [CrossRef]
- Hamley, I.W. Nanostructure fabrication using block copolymers. Nano Technol. 2003, 14, R39–R54. [Google Scholar]
- Aizawa, M.; Buriak, J.M. Block copolymer templated chemistry for the formation of metallic nanoparticle arrays on semiconductor surfaces. Chem. Mater. 2007, 19, 5090–5101. [Google Scholar] [CrossRef]
- Shenhar, R.; Norsten, T.B.; Rotello, V.M. Polymer-mediated nanoparticle assembly: Structural control and applications. Adv. Mater. 2005, 17, 657–669. [Google Scholar] [CrossRef]
- Darling, S.B. Mechanism for hierarchical self-assembly of nanoparticles on scaffolds derived from block copolymers. Surf. Sci. 2007, 601, 2555–2561. [Google Scholar] [CrossRef]
- Darling, S.B.; Hoffmann, A. Tuning metal surface diffusion on diblock copolymer films. J. Vac. Sci. Technol. A 2007, 25, 1048–1051. [Google Scholar] [CrossRef]
- Lopes, W.A.; Jaeger, H.M. Hierarchical self-assembly of metal nanostructures on diblock copolymer scaffolds. Nature 2001, 414, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, B.; Cirpan, A.; Russell, T.P. Preparation of metallic line patterns from functional block copolymers. Small 2009, 5, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- The International Technology Roadmap for Semiconductors. Available online: http://www.itrs.net (accessed on 18 October 2010).
- Krebs, F. Polymeric Solar Cells: Materials, Design, Manufacture, 1st ed.; DEStech Publications, Inc.: Lancaster, PA, USA, 2010; pp. 119–169. [Google Scholar]
- Craig, G.S.W.; Nealey, P.F. Directed assembly of block copolymers on lithographically defined surfaces. Proc. SPIE 2010, 7637, 76370L. [Google Scholar]
- Tada, Y.; Akasaka, S.; Takenaka, M.; Yoshida, H.; Ruiz, R.; Dobisz, E.; Hasegawa, H. Nine-fold density multiplication of hcp lattice pattern by directed self-assembly of block copolymer. Polymer 2009, 50, 4250–4256. [Google Scholar] [CrossRef]
- Tada, Y.; Akasaka, S.; Yoshida, H.; Hasegawa, H.; Dobisz, E.; Kercher, D.; Takenaka, M. Directed self-assembly of diblock copolymer thin films on chemically-patterned substrates for defect-free nano-patterning. Macromolecules 2008, 41, 9267–9276. [Google Scholar] [CrossRef]
- Tada, Y.; Akasaka, S.; Chen, F.; Yoshida, H.; Takenaka, M.; Hasegawa, H. Density multiplication by directed self-assembly of block copolymer binary blends. J. Photopolym. Sci. Technol. 2009, 22, 229–233. [Google Scholar] [CrossRef]
- Ruiz, R.; Kang, H.M.; Detcheverry, F.A.; Dobisz, E.; Kercher, D.S.; Albrecht, T.R.; de Pablo, J.J.; Nealey, P.F. Density multiplication and improved lithography by directed block copolymer assembly. Science 2008, 321, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L.; Thomas, C.S.; Craig, G.S.W.; Nealey, P.F. Integration of density multiplication in the formation of device-oriented structures by directed assembly of block copolymer-homopolymer blends. Adv. Funct. Mater. 2010, 20, 1251–1257. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Ross, C.A.; Chan, V.Z.H.; Thomas, E.L.; Lammertink, R.G.H.; Vancso, G.J. Formation of a cobalt magnetic dot array via block copolymer lithography. Adv. Mater. 2001, 13, 1174–1178. [Google Scholar] [CrossRef]
- Ross, C.A.; Smith, H.I.; Savas, T.; Schattenburg, M.; Farhoud, M.; Hwang, M.; Walsh, M.; Abraham, M.C.; Ram, R.J. Fabrication of patterned media for high density magnetic storage. J. Vac. Sci. Technol. B 1999, 17, 3168–3176. [Google Scholar] [CrossRef]
- Bita, I.; Yang, J.K.W.; Jung, Y.S.; Ross, C.A.; Thomas, E.L.; Berggren, K.K. Graphoepitaxy of self-assembled block copolymers on two-dimensional periodic patterned templates. Science 2008, 321, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Solarmer Energy, Inc. Available online: http://www.solarmer.com (accessed on 18 October 2010).
- Botiz, I.; Darling, S.B. Optoelectronics using block copolymers. Mater. Today 2010, 13, 42–51. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Brown, P.J.; Friend, R.H.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.M.W.; Spiering, A.J.H.; Janssen, R.A.J.; Meijer, E.W.; Herwig, P.; de Leeuw, D.M. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999, 401, 685–688. [Google Scholar] [CrossRef]
- Horowitz, G. Organic field-effect transistors. Adv. Mater. 1998, 10, 365–377. [Google Scholar] [CrossRef]
- He, X.M.; Gao, F.; Tu, G.L.; Hasko, D.; Huttner, S.; Steiner, U.; Greenham, N.C.; Friend, R.H.; Huck, W.T.S. Formation of nanopatterned polymer blends in photovoltaic devices. Nano Lett. 2010, 10, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Levenson, M.D.; Viswanathan, N.S.; Simpson, R.A. Improving resolution in photolithography with a phase-shifting mask. IEEE Trans. Electron. Devices 1982, 29, 1828–1836. [Google Scholar] [CrossRef]
- Hinsberg, W.; Wallraff, G.; Larson, C.; Davis, B.; Deline, V.; Raoux, S.; Miller, D.; Houle, F.; Hoffnagle, J.; Sanchez, M.; Rettner, C.; Sundberg, L.; Medeiros, D.; Dammel, R.; Conley, W. Liquid immersion lithography––Evaluation of resist issues. In Liquid immersion lithography––Evaluation of Resist Issues; Sturtevant, J.L., Ed.; SPIE-International Society Optical Engineering: Bellingham, WA, USA, 2004; pp. 21–33. [Google Scholar]
- Lin, B.J. Immersion lithography and its impact on semiconductor manufacturing. In Immersion Lithography and its Impact on Semiconductor Manufacturing, 1st ed.; Smith, B.W., Ed.; SPIE-International Society Optical Engineering: Bellingham, WA, USA, 2004; pp. 46–67. [Google Scholar]
- Byers, J.; Lee, S.; Jeri, K.; Zimmerman, P.; Turr, N.J.; Willson, C.G. Double exposure materials: Simulation study of feasibility. J. Photopolym. Sci. Technol. 2007, 20, 707–717. [Google Scholar] [CrossRef]
- Rockford, L.; Liu, Y.; Mansky, P.; Russell, T.P.; Yoon, M.; Mochrie, S.G.J. Polymers on nanoperiodic, heterogeneous surfaces. Phys. Rev. Lett. 1999, 82, 2602–2605. [Google Scholar] [CrossRef]
- Kim, S.O.; Solak, H.H.; Stoykovich, M.P.; Ferrier, N.J.; de Pablo, J.J.; Nealey, P.F. Epitaxial self-assembly of block copolymers on lithographically defined nanopatterned substrates. Nature 2003, 424, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.; Rettner, C.T.; Sanders, D.P.; Kim, H.C.; Hinsberg, W.D. Dense self-assembly on sparse chemical patterns: Rectifying and multiplying lithographic patterns using block copolymers. Adv. Mater. 2008, 20, 3155–3158. [Google Scholar] [CrossRef]
- Stoykovich, M.P.; Kang, H.; Daoulas, K.C.; Liu, G.; Liu, C.C.; de Pablo, J.J.; Mueller, M.; Nealey, P.F. Directed self-assembly of block copolymers for nanolithography: Fabrication of isolated features and essential integrated circuit geometries. ACS Nano 2007, 1, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Guarini, K.W.; Black, C.T.; Yeuing, S.H.I. Optimization of diblock copolymer thin film self assembly. Adv. Mater. 2002, 14, 1290–1294. [Google Scholar] [CrossRef]
- Yang, J.K.W.; Jung, Y.S.; Chang, J.B.; Mickiewicz, R.A.; Alexander-Katz, A.; Ross, C.A.; Berggren, K.K. Complex self-assembled patterns using sparse commensurate templates with locally varying motifs. Nat. Nano Technol. 2010, 5, 256–260. [Google Scholar] [CrossRef]
- Welander, A.M.; Nealey, P.F.; Cao, H.; Bristol, R. Impact of trench width roughness on the graphoepitaxial assembly of block copolymers. J. Vac. Sci. Technol. B 2008, 26, 2484–2488. [Google Scholar] [CrossRef]
- Kim, S.H.; Misner, M.J.; Russell, T.P. Solvent-induced ordering in thin film diblock copolymer/homopolymer mixtures. Adv. Mater. 2004, 16, 2119–2123. [Google Scholar] [CrossRef]
- Kim, S.H.; Misner, M.J.; Xu, T.; Kimura, M.; Russell, T.P. Highly oriented and ordered arrays from block copolymers via solvent evaporation. Adv. Mater. 2004, 16, 226–231. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.; Xu, J.; Hofmann, T.; Ocko, B.M.; Russell, T.P. Lateral Ordering of cylindrical microdomains under solvent vapor. Macromolecules 2009, 42, 1278–1284. [Google Scholar] [CrossRef]
- Stein, G.E.; Kramer, E.J.; Li, X.; Wang, J. Single-crystal diffraction from two-dimensional block copolymer arrays. Phys. Rev. Lett. 2007, 98. [Google Scholar] [CrossRef] [PubMed]
- Segalman, R.A.; Yokoyama, H.; Kramer, E.J. Graphoepitaxy of spherical domain block copolymer films. Adv. Mater. 2001, 13, 1152–1155. [Google Scholar] [CrossRef]
- Fontana, S.M.; Dadmun, M.D.; Lowndes, D.H. Long-range order of cylinders in diblock copolymer thin films using graphoepitaxy. Thin Solid Films 2010, 518, 2783–2792. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.H.; Xu, J.; Kim, B.; Hong, S.W.; Jeong, U.; Xu, T.; Russell, T.P. Macroscopic 10-Terabit-per-Square- Inch arrays from block copolymers with lateral order. Science 2009, 323, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Chang, J.B.; Verploegen, E.; Berggren, K.K.; Ross, C.A. A path to ultranarrow patterns using self-assembled lithography. Nano Lett. 2010, 10, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.H.; Park, S.M.; Liu, C.C.; Liu, X.S.; Himpsel, F.J.; Nealey, P.F.; Wendt, A.E. Plasma etch removal of poly(methyl methacrylate) in block copolymer lithography. J. Vac. Sci. Technol. B 2008, 26, 1684–1689. [Google Scholar] [CrossRef]
- Hempenius, M.A.; Lammertink, R.G.H.; Peter, M.; Vancso, G.J. Poly(ferrocenylsilanes) as etch barriers in nano and microlithographic applications. Macromol. Sym. 2003, 196, 45–56. [Google Scholar] [CrossRef]
- Chuang, V.P.; Gwyther, J.; Mickiewicz, R.A.; Manners, I.; Ross, C.A. Templated self-assembly of square symmetry arrays from an ABC triblock terpolymer. Nano Lett. 2009, 9, 4364–4369. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.; Nettleton, E.; Darling, S.B. Simple orientational control over cylindrical organic-inorganic block copolymer domains for etch mask applications. Thin Solid Films 2009, 517, 4474–4478. [Google Scholar] [CrossRef]
- Lammertink, R.G.H.; Hempenius, M.A.; van den Enk, J.E.; Chan, V.Z.H.; Thomas, E.L.; Vancso, G.J. Nanostructured thin films of organic-organometallic block copolymers: One-step lithography with poly(ferrocenylsilanes) by reactive ion etching. Adv. Mater. 2000, 12, 98–103. [Google Scholar] [CrossRef]
- Jung, Y.S.; Jung, W.; Ross, C.A. Nanofabricated concentric ring structures by templated self-assembly of a diblock copolymer. Nano Lett. 2008, 8, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Wang, T.C.; Ho, R.M.; Georgopanos, P.; Avgeropoulos, A.; Thomas, E.L. Robust block copolymer mask for nanopatterning polymer films. ACS Nano 2010, 4, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Ross, C.A. Orientation-controlled self-assembled nanolithography using a polystyrene-polydimethylsiloxane block copolymer. Nano Lett. 2007, 7, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.; Palmateer, S.C.; Forte, A.R.; Lyszczarz, T.M. Comparison of metrology methods for quantifying the line edge roughness of patterned features. J. Vac. Sci. Technol. B 1999, 17, 2488–2498. [Google Scholar] [CrossRef]
- Xiong, S.Y.; Bokor, J.; Xiang, Q.; Fisher, P.; Dudley, I.; Rao, P. Study of gate line edge roughness effects in 50 nm bulk MOSFET devices. In Study of Gate Line Edge Roughness Effects in 50 nm Bulk MOSFET Devices, 1st ed.; Herr, D.J.C., Ed.; SPIE––International Society Optical Engineering: Bellingham, WA, USA, 2002; pp. 733–741. [Google Scholar]
- Diaz, C.H.; Tao, H.J.; Ku, Y.C.; Yen, A.; Young, K. An experimentally validated analytical model for gate line-edge roughness (LER) effects on technology scaling. IEEE Electron. Device Lett. 2001, 22, 287–289. [Google Scholar] [CrossRef]
- Yu, S.M.; Zhao, Y.N.; Du, G.; Kang, J.F.; Han, R.Q.; Liu, X.Y. The impact of line edge roughness on the stability of a FinFET SRAM. Semiconduct. Sci. Technol. 2009, 24, 9. [Google Scholar]
- Srinivas, G.; Swope, W.C.; Pitera, J.W. Interfacial fluctuations of block copolymers: A coarse-grain molecular dynamics simulation study. J. Phys. Chem. B 2007, 111, 13734–13742. [Google Scholar] [CrossRef] [PubMed]
- Bates, F.S.; Fredrickson, G.H. Block copolymer thermodynamics––Theory and experiment. Ann. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Ross, C.A.; Jung, Y.S.; Chuang, V.P.; Ilievski, F.; Yang, J.K.W.; Bita, I.; Thomas, E.L.; Smith, H.I.; Berggren, K.K.; Vancso, G.J.; Cheng, J.Y. Si-containing block copolymers for self-assembled nanolithography. J. Vac. Sci. Technol. B 2008, 26, 2489–2494. [Google Scholar] [CrossRef]
- Bosse, A.W.; Lin, E.K.; Jones, R.L.; Karim, A. Interfacial fluctuations in an ideal block copolymer resist. Soft Matter 2009, 5, 4266–4271. [Google Scholar] [CrossRef]
- Stein, G.E.; Liddle, J.A.; Aquila, A.L.; Gullikson, E.M. Measuring the structure of epitaxially assembled block copolymer domains with soft X-ray diffraction. Macromolecules 2010, 43, 433–441. [Google Scholar] [CrossRef]
- Darling, S.B. Block copolymers for photovoltaics. Energ. Environ. Sci. 2009, 2, 1266–1273. [Google Scholar] [CrossRef]
- Segalman, R.A.; McCulloch, B.; Kirmayer, S.; Urban, J.J. Block copolymers for organic optoelectronics. Macromolecules 2009, 42, 9205–9216. [Google Scholar] [CrossRef]
- Lee, M.; Cho, B.K.; Zin, W.C. Supramolecular structures from rod-coil block copolymers. Chem. Rev. 2001, 101, 3869–3892. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.D.; Segalman, R.A. Structure and thermodynamics of weakly segregated rod-coil block copolymers. Macromolecules 2005, 38, 10127–10137. [Google Scholar] [CrossRef]
- Olsen, B.D.; Segalman, R.A. Nonlamellar phases in asymmetric rod-coil block copolymers at increased segregation strengths. Macromolecules 2007, 40, 6922–6929. [Google Scholar] [CrossRef]
- Sun, S.S.; Zhang, C.; Ledbetter, A.; Choi, S.; Seo, K.; Bonner, C.E.; Drees, M.; Sariciftci, N.S. Photovoltaic enhancement of organic solar cells by a bridged donor-acceptor block copolymer approach. Appl. Phys. Lett. 2007, 90, 3. [Google Scholar]
- Shah, M.; Ganesan, V. Correlations between morphologies and photovoltaic properties of rod-coil block copolymers. Macromolecules 2010, 43, 543–552. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Cirpan, A.; Russell, T.P.; Emrick, T. Donor-acceptor poly(thiophene-block-perylene diimide) copolymers: Synthesis and solar cell fabrication. Macromolecules 2009, 42, 1079–1082. [Google Scholar] [CrossRef]
- Olsen, B.D.; Li, X.F.; Wang, J.; Segalman, R.A. Thin film structure of symmetric rod-coil block copolymers. Macromolecules 2007, 40, 3287–3295. [Google Scholar] [CrossRef]
- Olsen, B.D.; Li, X.F.; Wang, J.; Segalman, R.A. Near-surface and internal lamellar structure and orientation in thin films of rod-coil block copolymers. Soft Matter 2009, 5, 182–192. [Google Scholar] [CrossRef]
- Tao, Y.F.; Zohar, H.; Olsen, B.D.; Segalman, R.A. Hierarchical nanostructure control in rod-coil block copolymers with magnetic fields. Nano Lett. 2007, 7, 2742–2746. [Google Scholar] [CrossRef] [PubMed]
- Barrau, S.; Heiser, T.; Richard, F.; Brochon, C.; Ngov, C.; van de Wetering, K.; Hadziioannou, G.; Anokhin, D.V.; Ivanov, D.A. Self-assembling of novel fullerene-grafted donor-acceptor rod-coil block copolymers. Macromolecules 2008, 41, 2701–2710. [Google Scholar] [CrossRef]
- de Boer, B.; Stalmach, U.; van Hutten, P.F.; Melzer, C.; Krasnikov, V.V.; Hadziioannou, G. Supramolecular self-assembly and opto-electronic properties of semiconducting block copolymers. Polymer 2001, 42, 9097–9109. [Google Scholar] [CrossRef]
- Lee, J.U.; Cirpan, A.; Emrick, T.; Russell, T.P.; Jo, W.H. Synthesis and photophysical property of well-defined donor-acceptor diblock copolymer based on regioregular poly(3-hexylthiophene) and fullerene. J. Mater. Chem. 2009, 19, 1483–1489. [Google Scholar] [CrossRef]
- Tao, Y.F.; McCulloch, B.; Kim, S.; Segalman, R.A. The relationship between morphology and performance of donor-acceptor rod-coil block copolymer solar cells. Soft Matter 2009, 5, 4219–4230. [Google Scholar] [CrossRef]
- King, S.; Sommer, M.; Huettner, S.; Thelakkat, M.; Haque, S.A. Charge separation and recombination in self-organizing nanostructured donor-acceptor block copolymer films. J. Mater. Chem. 2009, 19, 5436–5441. [Google Scholar] [CrossRef]
- Sary, N.; Richard, F.; Brochon, C.; Leclerc, N.; Leveque, P.; Audinot, J.N.; Berson, S.; Heiser, T.; Hadziioannou, G.; Mezzenga, R. A new supramolecular route for using rod-coil block copolymers in photovoltaic applications. Adv. Mater. 2010, 22, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Choi, S.; Haliburton, J.; Cleveland, T.; Li, R.; Sun, S.S.; Ledbetter, A.; Bonner, C.E. Design, synthesis, and characterization of a -donor-bridge-acceptor-bridge-type block copolymer via alkoxy- and sulfone-derivatized poly(phenylenevinylenes). Macromolecules 2006, 39, 4317–4326. [Google Scholar] [CrossRef]
- Yang, C. Donor/spacer/acceptor block copolymer containing poly(2,7-carbazole) and perylenetetracarboxydiimide subunits. Macromol. Chem. Phys. 2010, 211, 1446–1451. [Google Scholar] [CrossRef]
- Dante, M.; Yang, C.; Walker, B.; Wudl, F.; Nguyen, T.Q. Self-assembly and charge-transport properties of a polythiophene-fullerene triblock copolymer. Adv. Mater. 2010, 22, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Botiz, I.; Darling, S.B. Self-assembly of poly(3-hexylthiophene)-block-polylactide block copolymer and subsequent incorporation of electron acceptor material. Macromolecules 2009, 42, 8211–8217. [Google Scholar] [CrossRef]
- Boudouris, B.W.; Frisbie, C.D.; Hillmyer, M.A. Nanoporous poly(3-alkylthiophene) thin films generated from block copolymer templates. Macromolecules 2008, 41, 67–75. [Google Scholar] [CrossRef]
- Botiz, I.; Martinson, A.B.F.; Darling, S.B. Minimizing lateral domain collapse in etched poly(3-hexylthiophene)-block-polylactide thin films for improved optoelectronic performance. Langmuir 2010, 26, 8756–8761. [Google Scholar] [CrossRef] [PubMed]
- Crossland, E.J.W.; Kamperman, M.; Nedelcu, M.; Ducati, C.; Wiesner, U.; Smilgies, D.M.; Toombes, G.E.S.; Hillmyer, M.A.; Ludwigs, S.; Steiner, U.; Snaith, H.J. A bicontinuous double gyroid hybrid solar cell. Nano Lett. 2009, 9, 2807–2812. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lee, J.K.; Heeger, A.J.; Wudl, F. Well-defined donor-acceptor rod-coil diblock copolymers based on P3HT containing C-60: the morphology and role as a surfactant in bulk-heterojunction solar cells. J. Mater. Chem. 2009, 19, 5416–5423. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.U.; Jung, J.W.; Emrick, T.; Russell, T.P.; Jo, W.H. Synthesis of C60-end capped P3HT and its application for high performance of P3HT/PCBM bulk heterojunction solar cells. J. Mater. Chem. 2010, 20, 3287–3294. [Google Scholar] [CrossRef]
- Lee, J.U.; Jung, J.W.; Emrick, T.; Russell, T.P.; Jo, W.H. Morphology control of a polythiophene-fullerene bulk heterojunction for enhancement of the high-temperature stability of solar cell performance by a new donor-acceptor diblock copolymer. Nano Technol. 2010, 21, 9. [Google Scholar]
- Sivula, K.; Ball, Z.T.; Watanabe, N.; Frechet, J.M.J. Amphiphilic diblock copolymer compatibilizers and their effect on the morphology and performance of polythiophene: Fullerene solar cells. Adv. Mater. 2006, 18, 206–210. [Google Scholar] [CrossRef]
- Alam, M.M.; Jenekhe, S.A. Nanolayered heterojunctions of donor and acceptor conjugated polymers of interest in light emitting and photovoltaic devices: Photoinduced electron transfer at polythiophene/polyquinoline interfaces. J. Phys. Chem. B 2001, 105, 2479–2482. [Google Scholar] [CrossRef]
- Economopoulos, S.P.; Chochos, C.L.; Gregoriou, V.G.; Kallitsis, J.K.; Barrau, S.; Hadziioannou, G. Novel brush-type copolymers bearing thiophene backbone and side chain quinoline blocks. Synthesis and their use as a compatibilizer in thiophene-quinoline polymer blends. Macromolecules 2007, 40, 921–927. [Google Scholar] [CrossRef]
- Park, S.M.; Park, O.H.; Cheng, J.Y.; Rettner, C.T.; Kim, H.C. Patterning sub-10 nm line patterns from a block copolymer hybrid. Nano Technol. 2008, 19, 6. [Google Scholar]
- Freer, E.M.; Krupp, L.E.; Hinsberg, W.D.; Rice, P.M.; Hedrick, J.L.; Cha, J.N.; Miller, R.D.; Kim, H.C. Oriented mesoporous organosilicate thin films. Nano Lett. 2005, 5, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.; Pitera, J.; Park, O.H.; Flickner, M.; Ruiz, R.; Black, C.T.; Kim, H.C. Rapid directed self assembly of lamellar microdomains from a block copolymer containing hybrid. Appl. Phys. Lett. 2007, 91, 3. [Google Scholar]
- Hirai, T.; Leolukman, M.; Liu, C.C.; Han, E.; Kim, Y.J.; Ishida, Y.; Hayakawa, T.; Kakimoto, M.; Nealey, P.F.; Gopalan, P. One-step direct-patterning template utilizing self-assembly of poss-containing block copolymers. Adv. Mater. 2009, 21, 4334–4338. [Google Scholar] [CrossRef]
- Green, M.A. Solar Cells, Operating Principles, Technology and System Applications, 1st ed.; University of New South Wales: Sydney, Australia, 1998; pp. 88–89. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tseng, Y.-C.; Darling, S.B. Block Copolymer Nanostructures for Technology. Polymers 2010, 2, 470-489. https://doi.org/10.3390/polym2040470
Tseng Y-C, Darling SB. Block Copolymer Nanostructures for Technology. Polymers. 2010; 2(4):470-489. https://doi.org/10.3390/polym2040470
Chicago/Turabian StyleTseng, Yu-Chih, and Seth B. Darling. 2010. "Block Copolymer Nanostructures for Technology" Polymers 2, no. 4: 470-489. https://doi.org/10.3390/polym2040470



