Preparation and Characterization of Date Palm Bio-Oil Modified Phenolic Foam
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Date Palm Bio-Oil
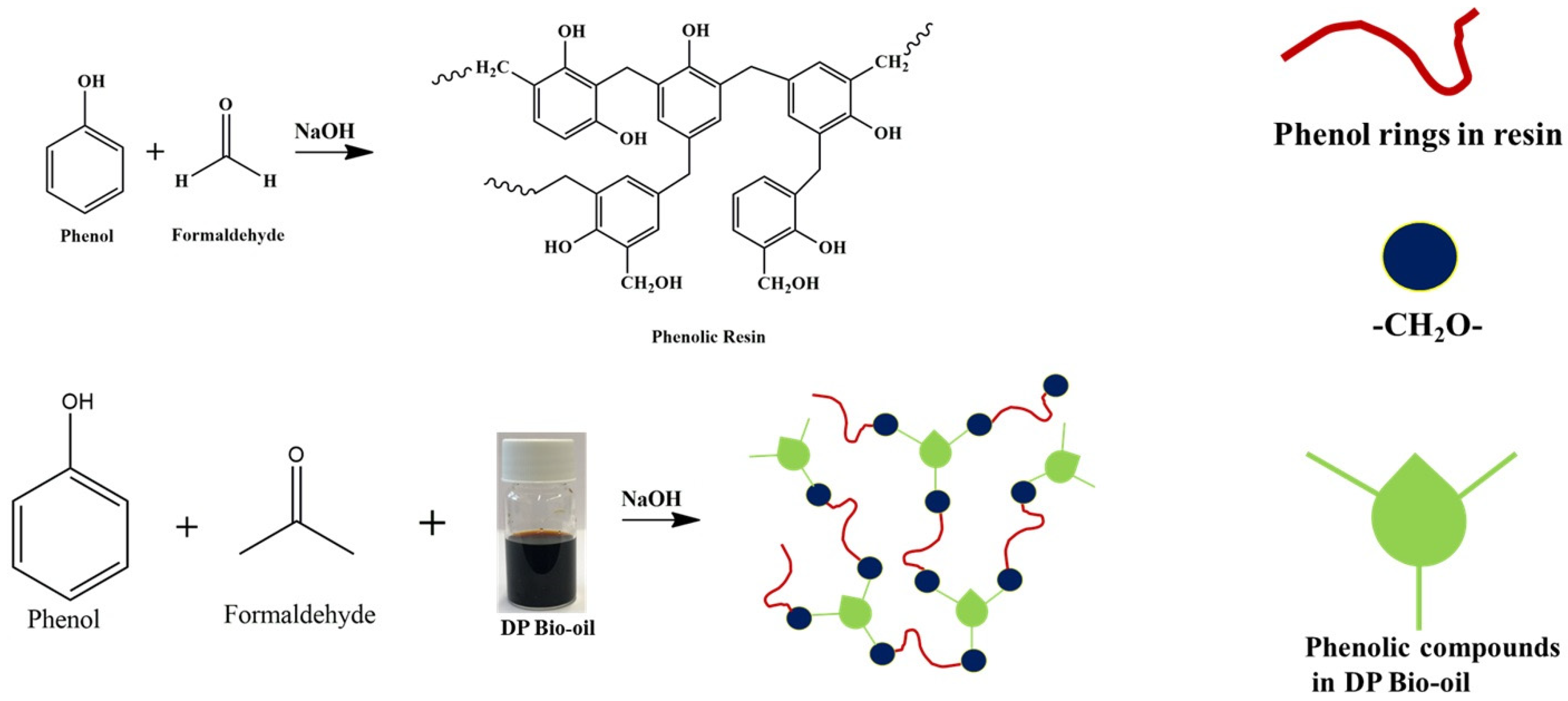
2.3. Synthesis of Phenolic Resin (PR)
2.4. Synthesis of Date Palm Bio-Oil Substituted Phenolic Resin (DPR)
2.5. Resin Characterization
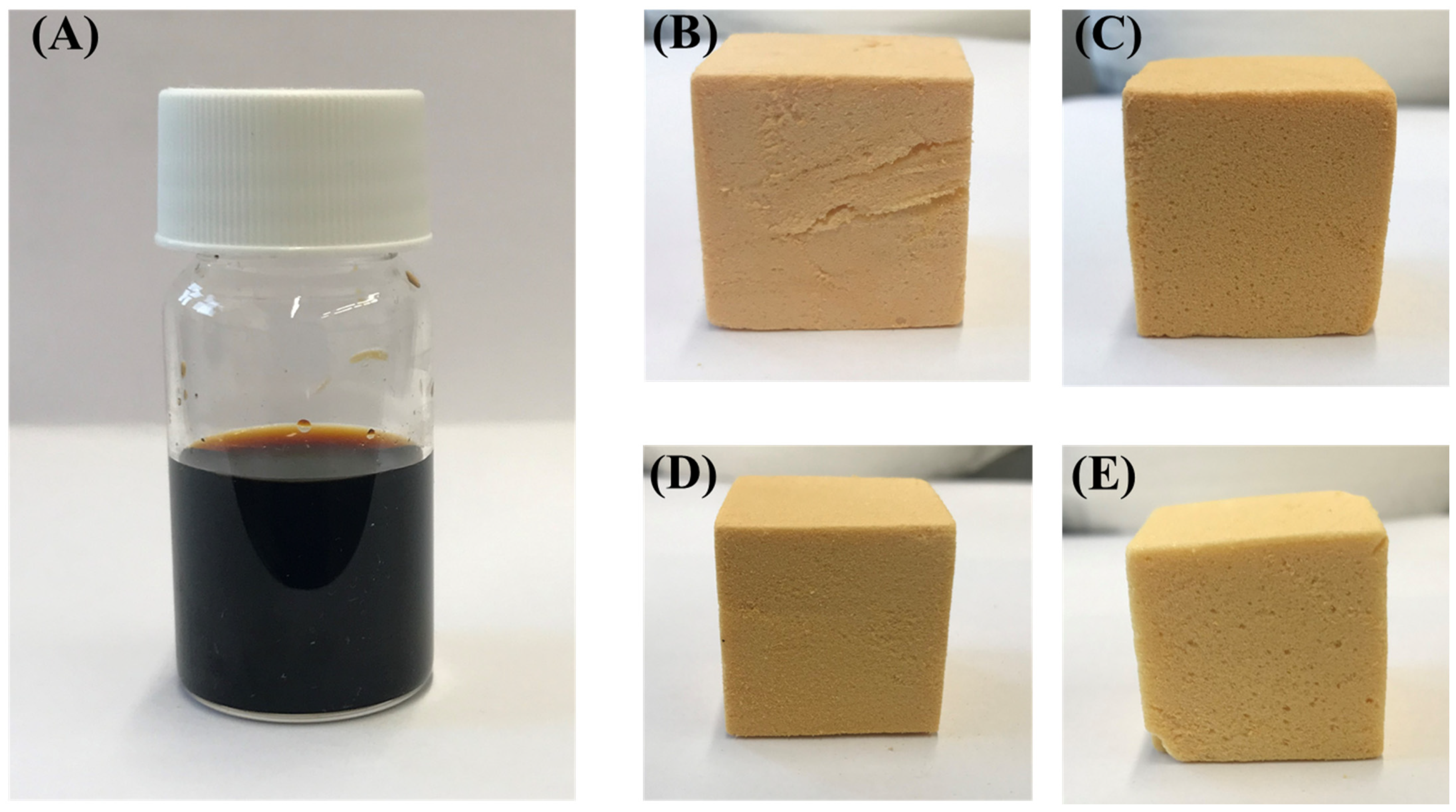
2.6. Synthesis of Date Palm Bio-Oil Substituted Phenolic Foam (DPF)
2.7. Foam Characterization
3. Results and Discussion
3.1. Characterization of Date Palm Bio-Oil, PR and DPR
3.2. Characterization of PF and DPF
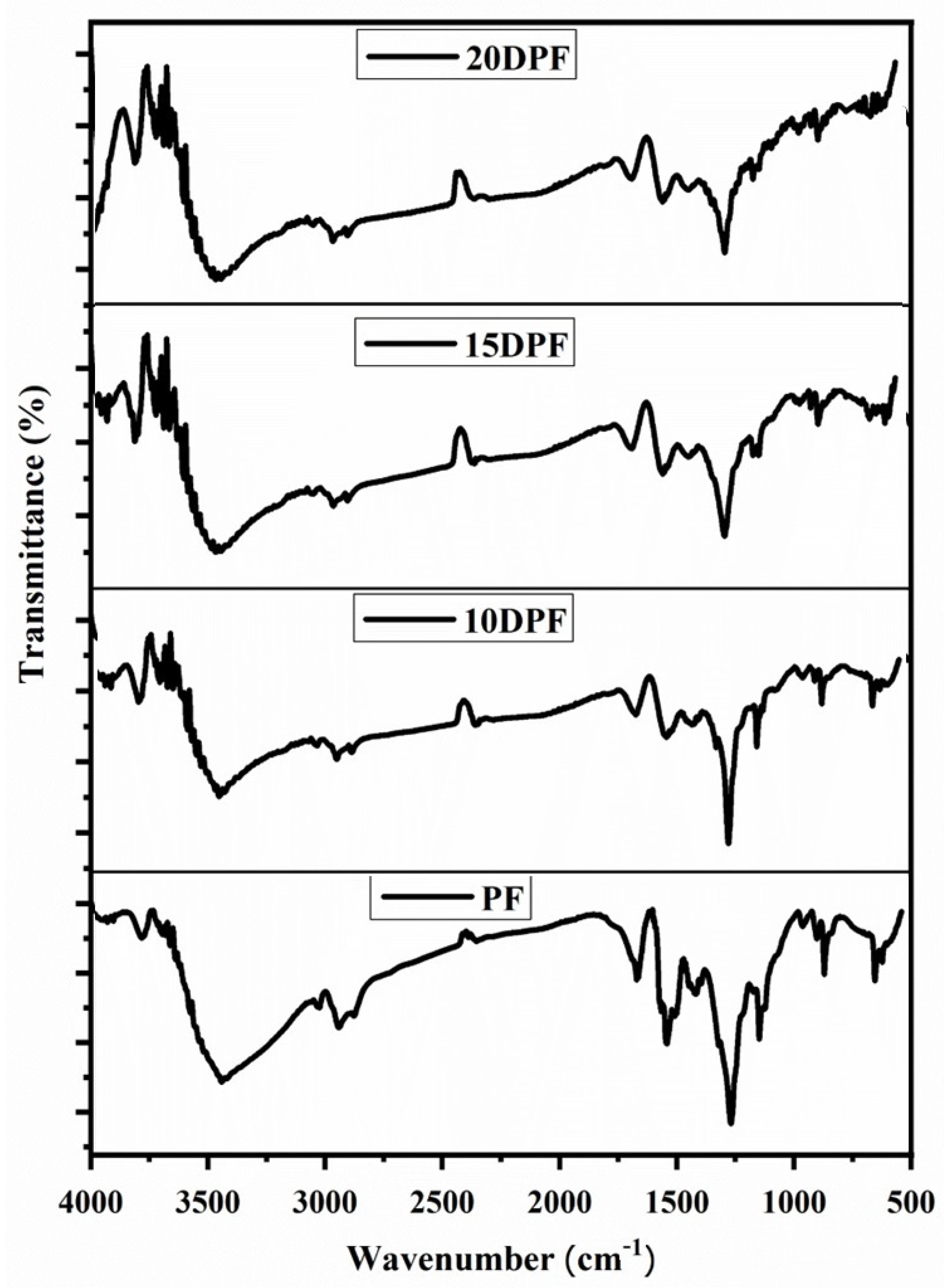
3.2.1. FT-IR Characterization of PF and DPF
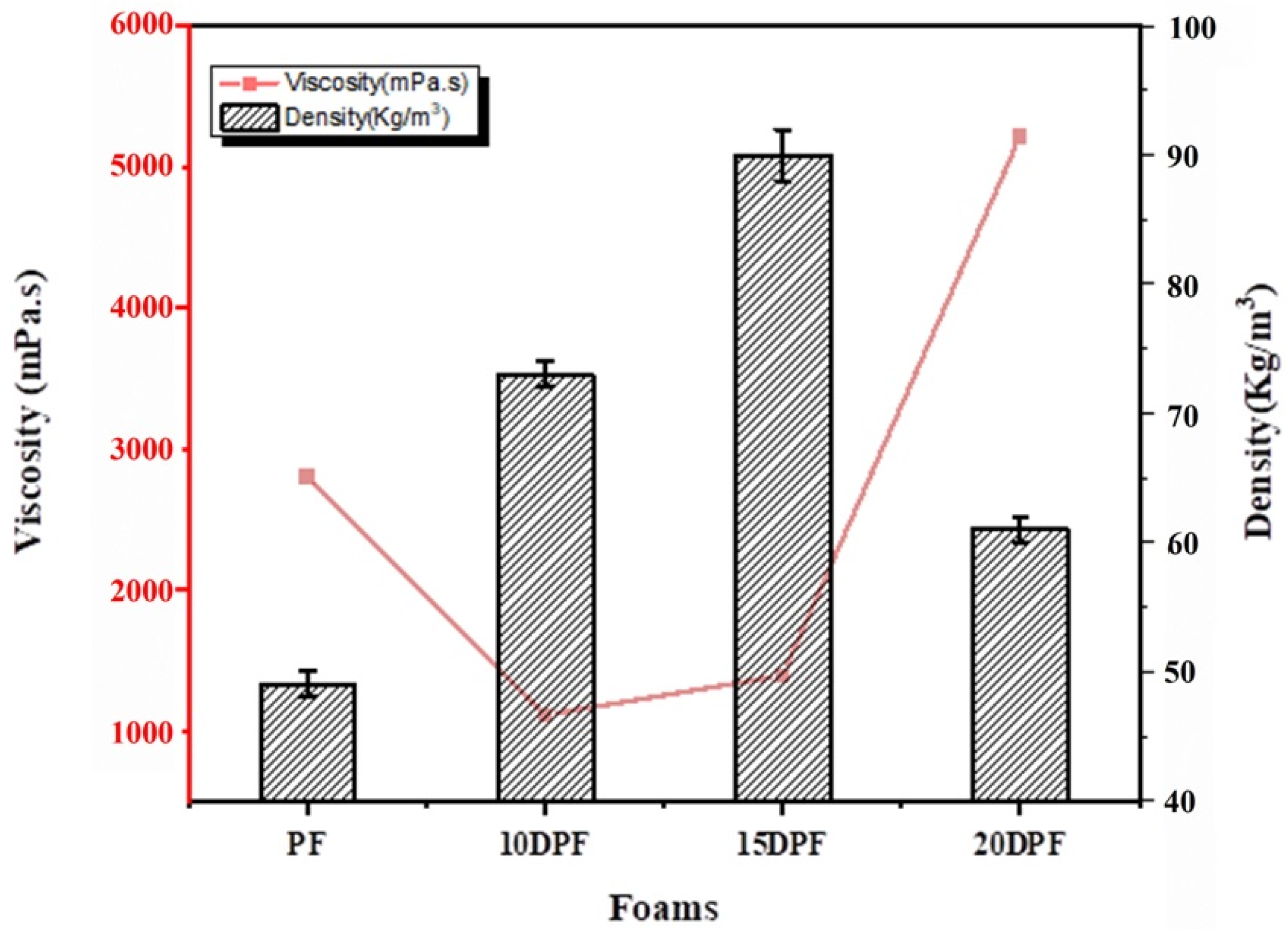
3.2.2. Apparent Density of PF and DPF
3.2.3. Thermal Conductivity PF and DPF
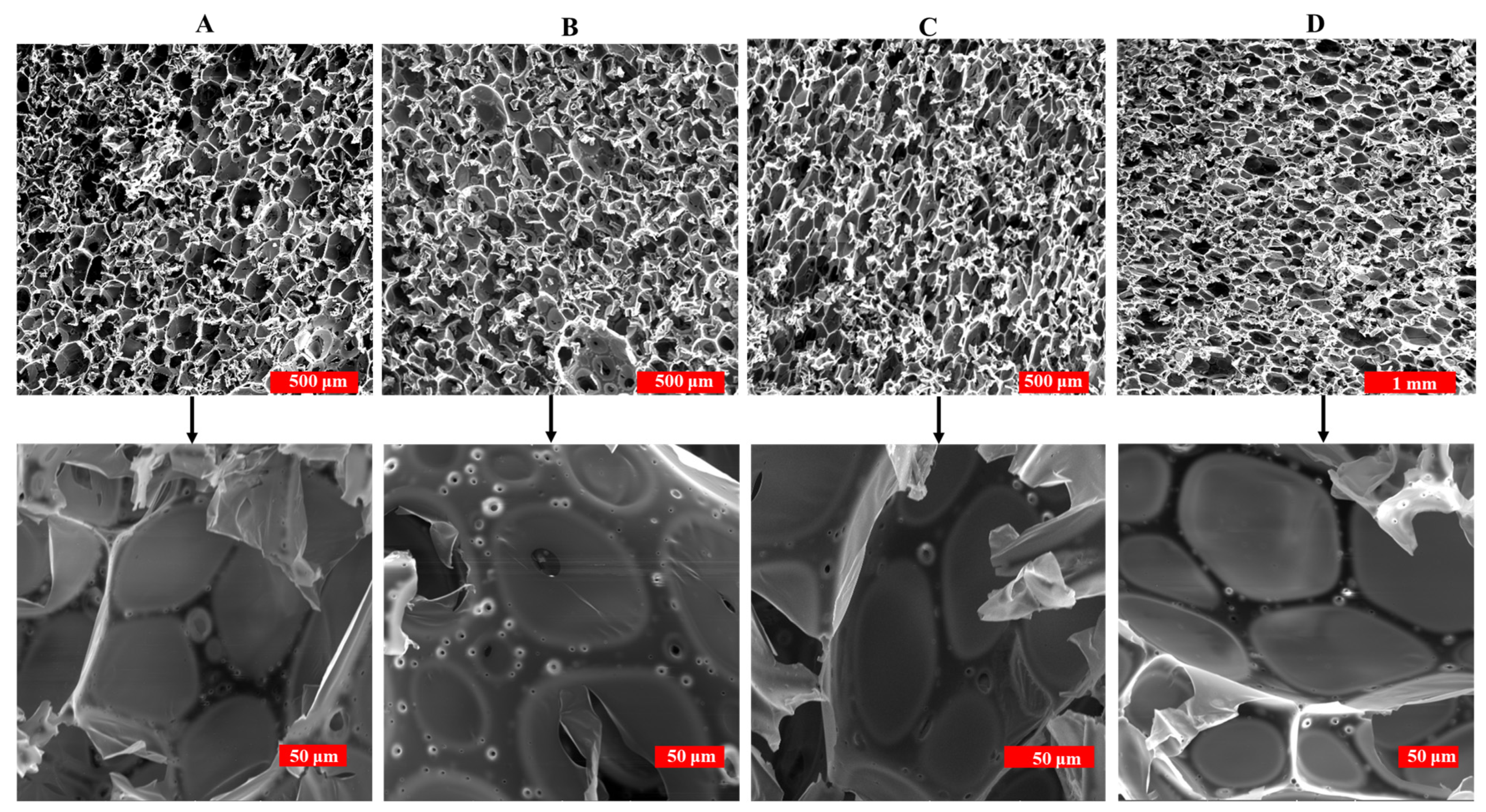
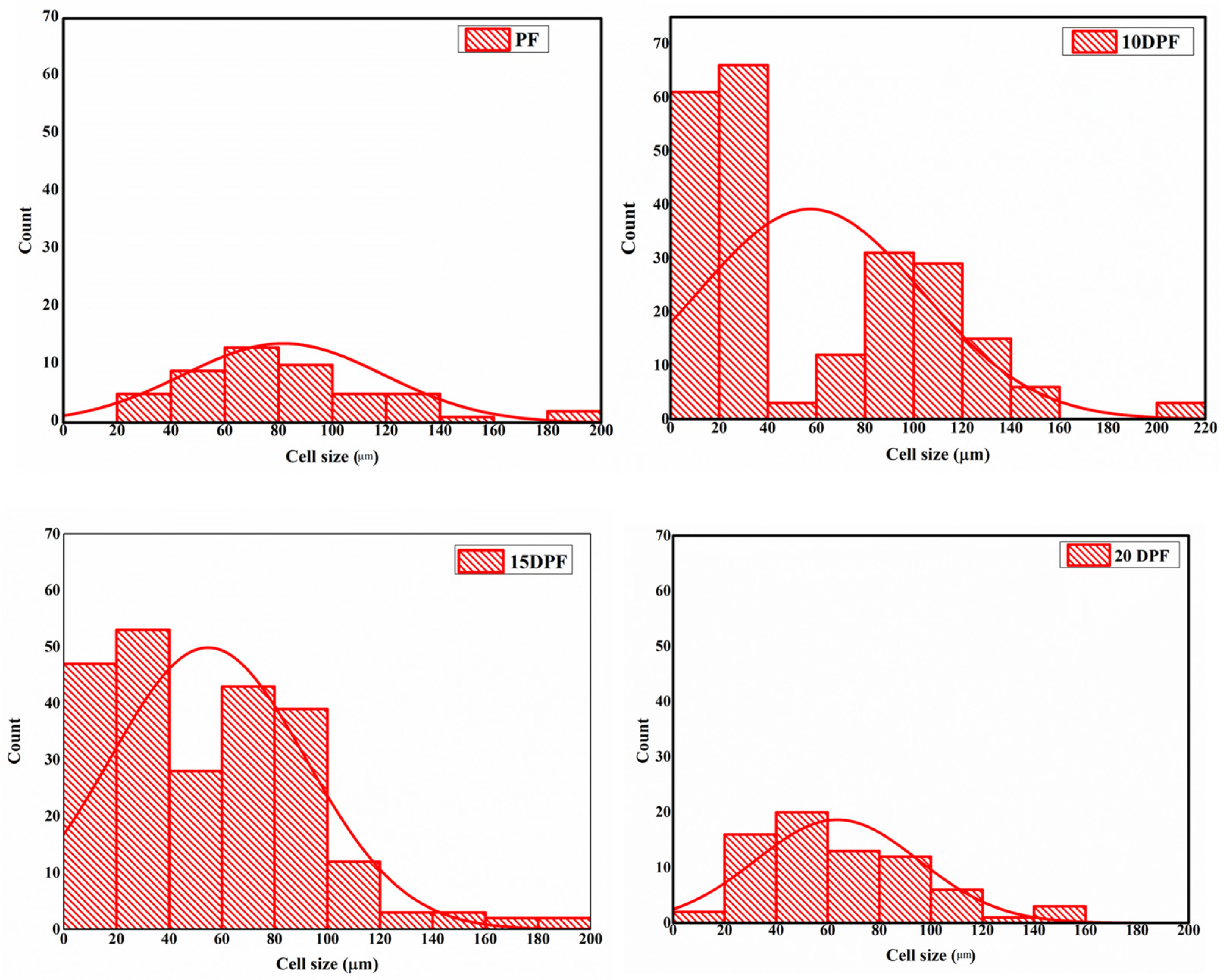
3.2.4. Morphology of PF and DPF
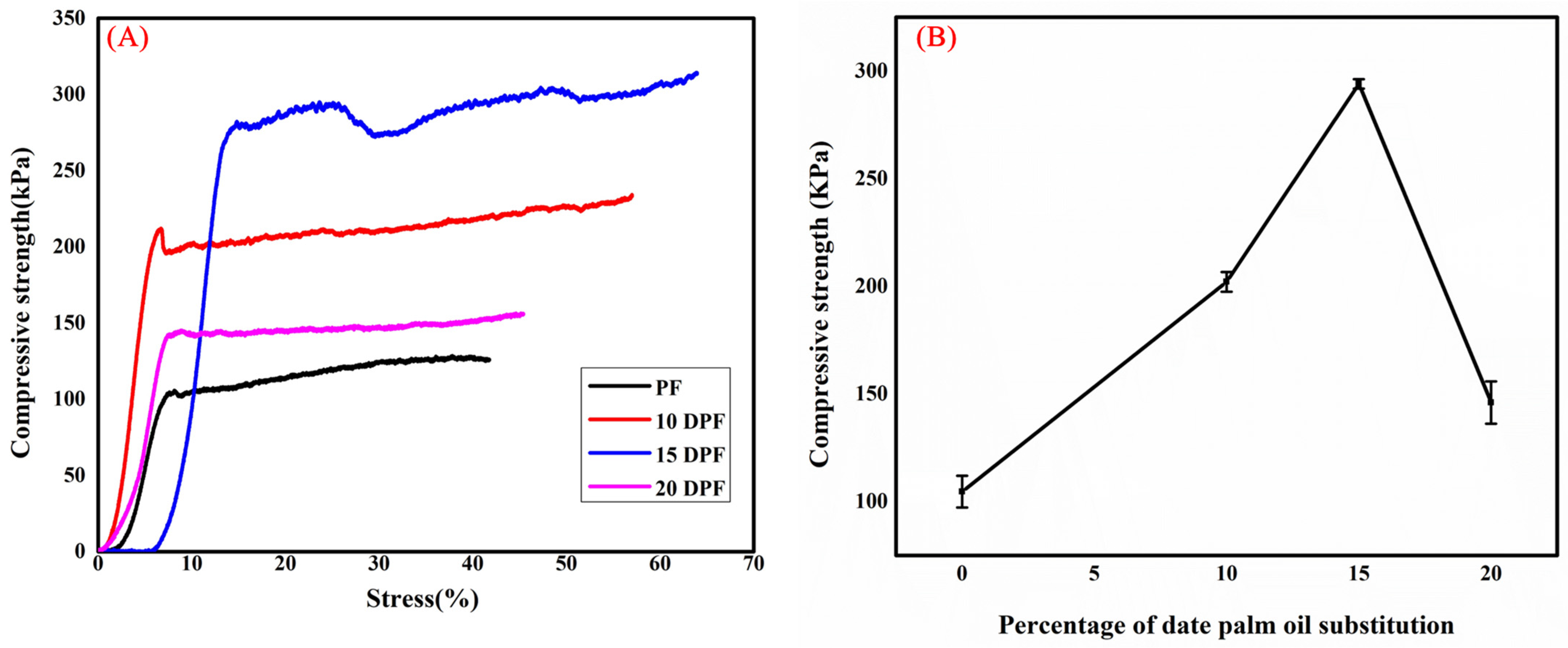
3.2.5. Mechanical Properties of PF and DPF
3.2.6. Thermal Properties of PF and DPF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mougel, C.; Garnier, T.; Cassagnau, P.; Sintes-Zydowicz, N. Phenolic foams: A review of mechanical properties, fire resistance and new trends in phenol substitution. Polymer 2019, 164, 86–117. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, L.; Zhang, H.; Zhai, H.; Ren, H. Research status, industrial application demand and prospects of phenolic resin. RSC Adv. 2019, 9, 28924–28935. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, J.P.; Torero, J.L.; Welch, S. Fire performance of charring closed-cell polymeric insulation materials: Polyisocyanurate and phenolic foam. Fire Mater. 2018, 42, 358–373. [Google Scholar] [CrossRef]
- Yao, J.W.; Hou, Z.M.; Yao, Y.S. Application of phenolic foam plate in the exterior wall thermal insulation. Appl. Mech. Mater. 2012, 174, 1363–1366. [Google Scholar] [CrossRef]
- Sandhya, P.K.; Sreekala, M.S.; Thomas, S. Phenolic-Based Foams: State of the Art, New Challenges, and Opportunities. In Phenolic Based Foams: Preparation, Characterization, and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–14. [Google Scholar]
- Sarika, P.R.; Nancarrow, P.; Ibrahim, T. Comparison of Toughening Effects of Various Additives on Phenolic Foam. ACS Omega 2024, 9, 4695–4704. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, X.; Yu, B.; Yuan, H.; Song, L.; Hu, Y.; Yuen, R.K.K.; Yeoh, G.H. A novel polyurethane prepolymer as toughening agent: Preparation, characterization, and its influence on mechanical and flame retardant properties of phenolic foam. J. Appl. Polym. Sci. 2013, 128, 2720–2728. [Google Scholar] [CrossRef]
- Hu, X.M.; Wang, D.M.; Cheng, W.M.; Zhou, G. Effect of polyethylene glycol on the mechanical property, microstructure, thermal stability, and flame resistance of phenol–urea–formaldehyde foams. J. Mater. Sci. 2014, 49, 1556–1565. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Yuan, H.; Song, L.; Hu, Y.; Yuen, R.K.K. Fire performance and mechanical properties of phenolic foams modified by phosphorus-containing polyethers. J. Polym. Res. 2012, 19, 9831. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Progress in Bio-Based Phenolic Foams: Synthesis, Properties, and Applications. ChemBioEng. Rev. 2021, 8, 612–632. [Google Scholar] [CrossRef]
- Pilato, L. Phenolic Resins: A Century of Progress; Springer: New York, NY, USA, 2010; ISBN 9783642047138. [Google Scholar]
- Klass, D.L. Biomass for Renewable Energy and Fuels. In Encyclopedia of Energy; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Mante, O.D.; Agblevor, F.A. Catalytic conversion of biomass to bio-syncrude oil. Biomass Convers. Biorefinery 2011, 1, 203–215. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Larch tannin-based rigid phenolic foam with high compressive strength, low friability, and low thermal conductivity reinforced by cork powder. Compos. Part B Eng. 2019, 156, 368–377. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.; Pizzi, A.; Laborie, M.-P.; Celzard, A.; Fierro, V. Pine tannin-based rigid foams: Mechanical and thermal properties. Ind. Crop. Prod. 2013, 43, 245–250. [Google Scholar] [CrossRef]
- Bo, C.; Shi, Z.; Hu, L.; Pan, Z.; Hu, Y.; Yang, X.; Jia, P.; Ren, X.; Zhang, M.; Zhou, Y. Cardanol derived P, Si and N based precursors to develop flame retardant phenolic foam. Sci. Rep. 2020, 10, 12082. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, L.; Bo, C.; Shang, Q.; Feng, G.; Jia, P.; Zhang, B.; Zhou, Y. Mechanical property of lignin-modified phenolic foam enhanced by nano-SiO2 via a novel method. Chem. Pap. 2018, 72, 763–767. [Google Scholar] [CrossRef]
- Xu, P.; Yu, Y.; Chang, M.; Chang, J. Preparation and characterization of bio-oil phenolic foam reinforced with montmorillonite. Polymers 2019, 11, 1471. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Jia, P.; Bo, C.; Ren, X.; Hu, L.; Zhou, Y. Preparation and characterization of tung oil toughened modified phenolic foams with enhanced mechanical properties and smoke suppression. J. Renew. Mater. 2020, 8, 535–547. [Google Scholar] [CrossRef]
- Chaouch, M.; Diouf, P.N.; Laghdir, A.; Yin, S. Bio-oil from whole-tree feedstock in resol-type phenolic resins. J. Appl. Polym. Sci. 2013, 131, 1–6. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Xu, P.; Chang, J. Preparation and characterization of phenolic foam modified with bio-oil. Materials 2018, 11, 2228. [Google Scholar] [CrossRef]
- Makkawi, Y.; El Sayed, Y.; Salih, M.; Nancarrow, P.; Banks, S.; Bridgwater, T. Fast pyrolysis of date palm (Phoenix dactylifera) waste in a bubbling fluidized bed reactor. Renew. Energy 2019, 143, 719–730. [Google Scholar] [CrossRef]
- Li, B.; Feng, S.H.; Niasar, H.S.; Zhang, Y.S.; Yuan, Z.S.; Schmidt, J.; Xu, C. Preparation and characterization of bark-derived phenol formaldehyde foams. RSC Adv. 2016, 6, 40975–40981. [Google Scholar] [CrossRef]
- Song, F.; Li, Z.; Jia, P.; Bo, C.; Zhang, M.; Hu, L.; Zhou, Y. Phosphorus-containing tung oil-based siloxane toughened phenolic foam with good mechanical properties, fire performance and low thermal conductivity. Mater. Des. 2020, 192, 108668. [Google Scholar] [CrossRef]
- Jonoobi, M.; Shafie, M.; Shirmohammadli, Y.; Ashori, A.; Zarea-Hosseinabadi, H.; Mekonnen, T. A review on date palm tree: Properties, characterization and its potential applications. J. Renew. Mater. 2019, 7, 1055–1075. [Google Scholar] [CrossRef]
- Idowu, A.T.; Igiehon, O.O.; Adekoya, A.E.; Idowu, S. Dates palm fruits: A review of their nutritional components, bioactivities and functional food applications. AIMS Agric. Food 2020, 5, 734–755. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayad, A.A.; Williams, L.L.; Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O. Date fruit: A review of the chemical and nutritional compounds, functional effects and food application in nutrition bars for athletes. Int. J. Food Sci. Technol. 2021, 56, 1503–1513. [Google Scholar] [CrossRef]
- Hai, A.; Bharath, G.; Rambabu, K.; Kannan, P.; Banat, F.; Taher, H.; Jayaraman, R.; Show, P.L. Pyrolysis of different date palm industrial wastes into high-quality bio-oils: A comparative study. Clean Technol. Environ. Policy 2021, 23, 55–64. [Google Scholar] [CrossRef]
- Tahir, A.H.F.; Al-Obaidy, A.H.M.J.; Mohammed, F.H. Biochar from date palm waste, production, characteristics and use in the treatment of pollutants: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 737, 012171. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the applications of bio-oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Bensidhom, G.; Ben Hassen-Trabelsi, A.; Alper, K.; Sghairoun, M.; Zaafouri, K.; Trabelsi, I. Pyrolysis of Date palm waste in a fixed-bed reactor: Characterization of pyrolytic products. Bioresour. Technol. 2018, 247, 363–369. [Google Scholar] [CrossRef]
- Ateş, F.; Yaşar, B. Utilization of date palm stones for bio-oil and char production using flash and fast pyrolysis. Biomass Convers. Biorefinery 2023, 13, 2907–2919. [Google Scholar] [CrossRef]
- Makkawi, Y.; Khan, M.; Pour, F.H.; Moussa, O.; Mohamed, B.; Alnoman, H.; Elsayed, Y. A comparative analysis of second-generation biofuels and its potentials for large-scale production in arid and semi-arid regions. Fuel 2023, 343, 127893. [Google Scholar] [CrossRef]
- ISO 9397; Plastics—Phenolic Resins—Determination of Free-Formaldehyde Content Method—Hydroxylamine. International Organization for standardization: Geneva, Switzerland, 1995.
- ASTM D4426-01; Standard Test Method for Determination of Percent Nonvolatile Content of Liquid Phenolic Resins Used for Wood Laminating. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D1622; Standard Test Method for Apparent Density of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D1621; Standard Test Method for Compressive Properties of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2016.
- Choi, M.H.; Byun, H.Y.; Chung, I.J. The effect of chain length of flexible diacid on morphology and mechanical property of modified phenolic resin. Polymer 2002, 43, 4437–4444. [Google Scholar] [CrossRef]
- Del Saz-Orozco, B.; Alonso, M.V.; Oliet, M.; Domínguez, J.C.; Rodriguez, F. Effects of formulation variables on density, compressive mechanical properties and morphology of wood flour-reinforced phenolic foams. Compos. Part B Eng. 2014, 56, 546–552. [Google Scholar] [CrossRef]
- Song, S.A.; Oh, H.J.; Kim, B.G.; Kim, S.S. Novel foaming methods to fabricate activated carbon reinforced microcellular phenolic foams. Compos. Sci. Technol. 2013, 76, 45–51. [Google Scholar] [CrossRef]
- de Carvalho, G.; Pimenta, J.A.; dos Santos, W.N.; Frollini, E. Phenolic and lignophenolic closed cells foams: Thermal conductivity and other properties. Polym. Plast. Technol. Eng. 2003, 42, 605–626. [Google Scholar] [CrossRef]
- Oct, P.; Carlson, I.J.D.; Woods, B.; Kifer, E.W.; Wojtyna, J.; James, P. Method for Preparing Phenolic Foams Using Anhydrous Aryl Sulfonic Acid Catalysts. U.S. Patent 4,478,958, 23 October 1984. [Google Scholar]
- Mougel, C.; Garnier, T.; Sintes-Zydowicz, N.; Cassagnau, P. Density Effect on Morphological, Mechanical and Friability Properties of Phenolic Foams. J. Mater. Sci. Eng. Adv. Technol. 2019, 19, 43–78. [Google Scholar] [CrossRef]
- Rickle, G.; Denslow, K. The Effect of Water on Phenolic Foam Cell Structure. J. Cell. Plast. 1988, 24, 70–78. [Google Scholar] [CrossRef]
- Chen, X.; Yu, W.; Ma, L.; Zhou, S.; Liu, X. Mechanical properties and thermal characteristics of diferent-density phenolic foams. J. Therm. Anal. Calorim. 2021, 144, 393–401. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, Y.; Zhang, M.; Liu, R. Characterization and properties of a lignosulfonate-based phenolic foam. BioResources 2012, 7, 554–564. [Google Scholar] [CrossRef]
- Sui, X.; Wang, Z. Flame-retardant and mechanical properties of phenolic foams toughened with polyethylene glycol phosphates. Polym. Adv. Technol. 2013, 24, 593–599. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Li, X.; Zhang, J.; Zhang, X.; Zheng, K.; Fang, F.; Zhou, H.; Tian, X. Effect of multi-walled carbon nanotubes on mechanical, thermal and electrical properties of phenolic foam via in-situ polymerization. Compos. Part A Appl. Sci. Manuf. 2016, 82, 214–225. [Google Scholar] [CrossRef]
- Ozaki, J.; Ohizumi, W.; Oya, A. TG-MS study of poly(vinyl butyral)/phenol-formaldehyde resin blend fiber. Carbon N. Y. 2000, 38, 1515–1519. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The pyrolysis mechanism of phenol formaldehyde resin. Polym. Degrad. Stab. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, W.; Liu, G. Effects of two kinds of THEIC-based charring agents on flame-retardant properties of polylactide. J. Appl. Polym. Sci. 2015, 132, 42086. [Google Scholar] [CrossRef]
- Savov, V.; Valchev, I.; Antov, P.; Yordanov, I.; Popski, Z. Effect of the adhesive system on the properties of fiberboard panels bonded with hydrolysis lignin and phenol-formaldehyde resin. Polymers 2022, 14, 1768. [Google Scholar] [CrossRef]


| Resin | Viscosity @ 25 °C (mPa·s) | Solid Content (%) | pH | Free Formaldehyde Content (%) |
|---|---|---|---|---|
| PR | 2800 | 75.9 ± 0.4 | 10 | 0.178 |
| 10DPR | 1110 | 69.8 ± 0.3 | 9.7 | 0.470 |
| 15DPR | 1390 | 67.9 ± 0.4 | 9.6 | 0.715 |
| 20DPR | 5210 | 75.8 ± 0.2 | 9.6 | 1.18 |
| PF | 10DPF | 15DPF | 20DPF | |
|---|---|---|---|---|
| Resin viscosity (mPa·s) | 2800 | 1110 | 1390 | 5210 |
| Resin (g) | 25 | 25 | 25 | 25 |
| Agnique CSO30 (g) | 1 | 1 | 1 | 1 |
| Hexane (mL) | 4 | 4 | 4 | 4 |
| L-MSA (mL) | 2 | 2 | 2 | 2 |
| Density (kg/m3) | Thermal Conductivity (W/m·K) | Compressive Strength (kPa) | |
|---|---|---|---|
| PF | 49 ± 1 | 0.035 ± 0.001 | 105 ± 7 |
| 10DPF | 73 ± 1 | 0.040 ± 0.001 | 202 ± 5 |
| 15DPF | 90 ± 2 | 0.038 ± 0.002 | 294 ± 2 |
| 20DPF | 61 ± 1 | 0.036 ± 0.002 | 146 ± 10 |
| Density (kg/m3) | Minimum Cell Size (μm) | Maximum Cell Size (μm) | Mean Cell Size (μm) | Minimum Cell Thickness (μm) | Maximum Cell Thickness (μm) | Mean Cell Wall Thickness (μm) | |
|---|---|---|---|---|---|---|---|
| PF | 49 ± 1 | 25 | 188 | 85.9 ± 34 | 4.5 | 39.5 | 12.6 ± 7 |
| 10DPF | 73 ± 1 | 9 | 210 | 57.09 ± 45 | 9.4 | 44.2 | 21.7 ± 7 |
| 15DPF | 90 ± 2 | 9 | 188 | 54.4 ± 36 | 5 | 45.6 | 28.6 ± 11 |
| 20DPF | 61 ± 1 | 18 | 150 | 64.5 ± 32 | 9.8 | 31.6 | 22.3 ± 6 |
| T-5% (°C) | Tmax (°C) | Residual Weight (%) | ||||
|---|---|---|---|---|---|---|
| 200 °C | 400 °C | 600 °C | 800 °C | |||
| PF | 124.7 | 564.2 | 91.6 | 81.9 | 52.2 | 29.1 |
| 10DPF | 180.9 | 587.4 | 93. | 84.6 | 56.5 | 32.4 |
| 15DPF | 192.1 | 588.9 | 94.4 | 84.1 | 55.7 | 30.6 |
| 20DPF | 147.3 | 567. | 92.5 | 83.4 | 54.1 | 30.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarika, P.R.; Nancarrow, P.; Makkawi, Y.; Ibrahim, T.H. Preparation and Characterization of Date Palm Bio-Oil Modified Phenolic Foam. Polymers 2024, 16, 955. https://doi.org/10.3390/polym16070955
Sarika PR, Nancarrow P, Makkawi Y, Ibrahim TH. Preparation and Characterization of Date Palm Bio-Oil Modified Phenolic Foam. Polymers. 2024; 16(7):955. https://doi.org/10.3390/polym16070955
Chicago/Turabian StyleSarika, Paprayil Reghunadh, Paul Nancarrow, Yassir Makkawi, and Taleb H. Ibrahim. 2024. "Preparation and Characterization of Date Palm Bio-Oil Modified Phenolic Foam" Polymers 16, no. 7: 955. https://doi.org/10.3390/polym16070955






