Biaxial Orientation of PLA/PBAT/Thermoplastic Cereal Flour Sheets: Structure–Processing–Property Relationships
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Blend Compounding
2.3. Biaxial Stretching of Cast Sheets
3. Results and Discussions
3.1. Preliminary Study
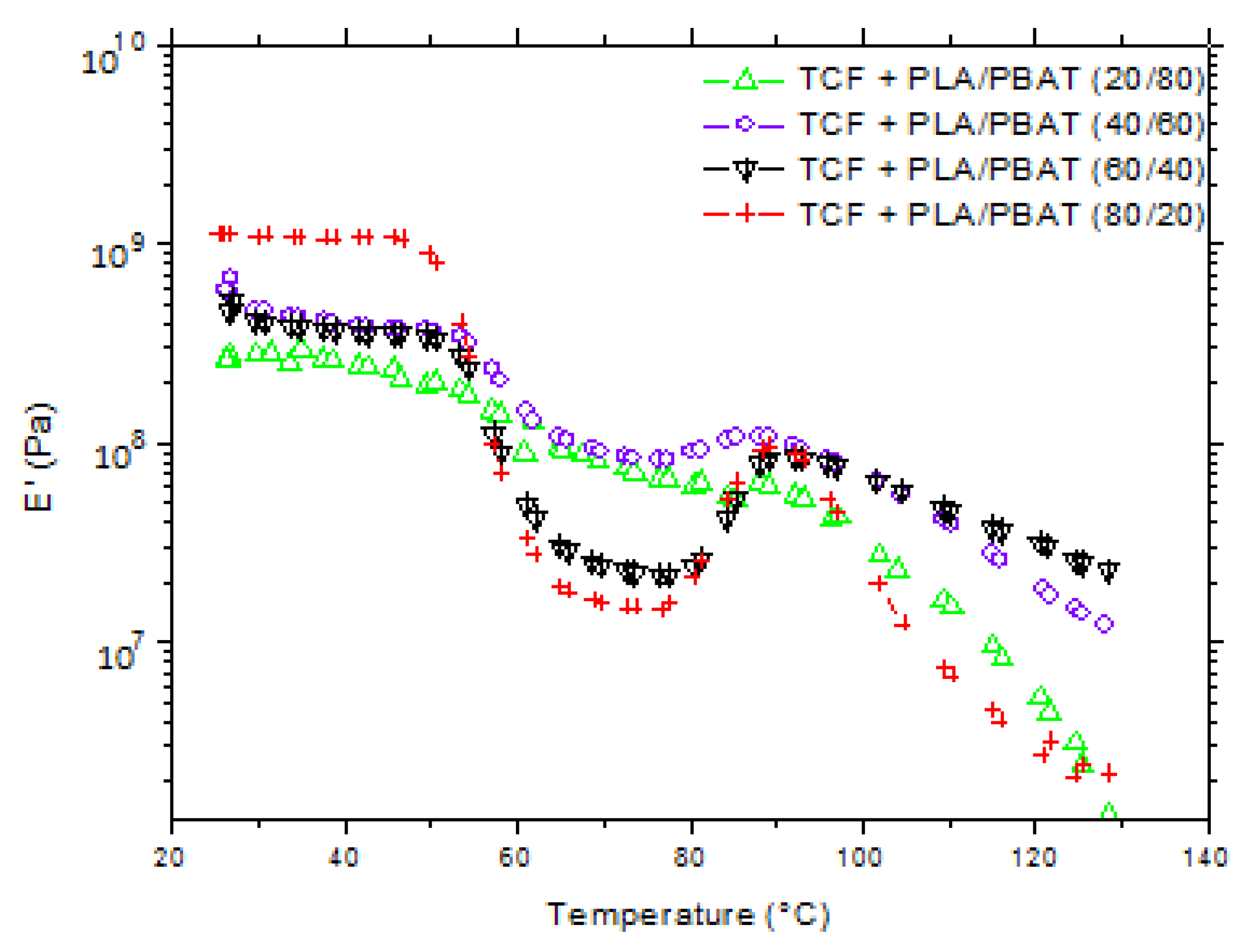
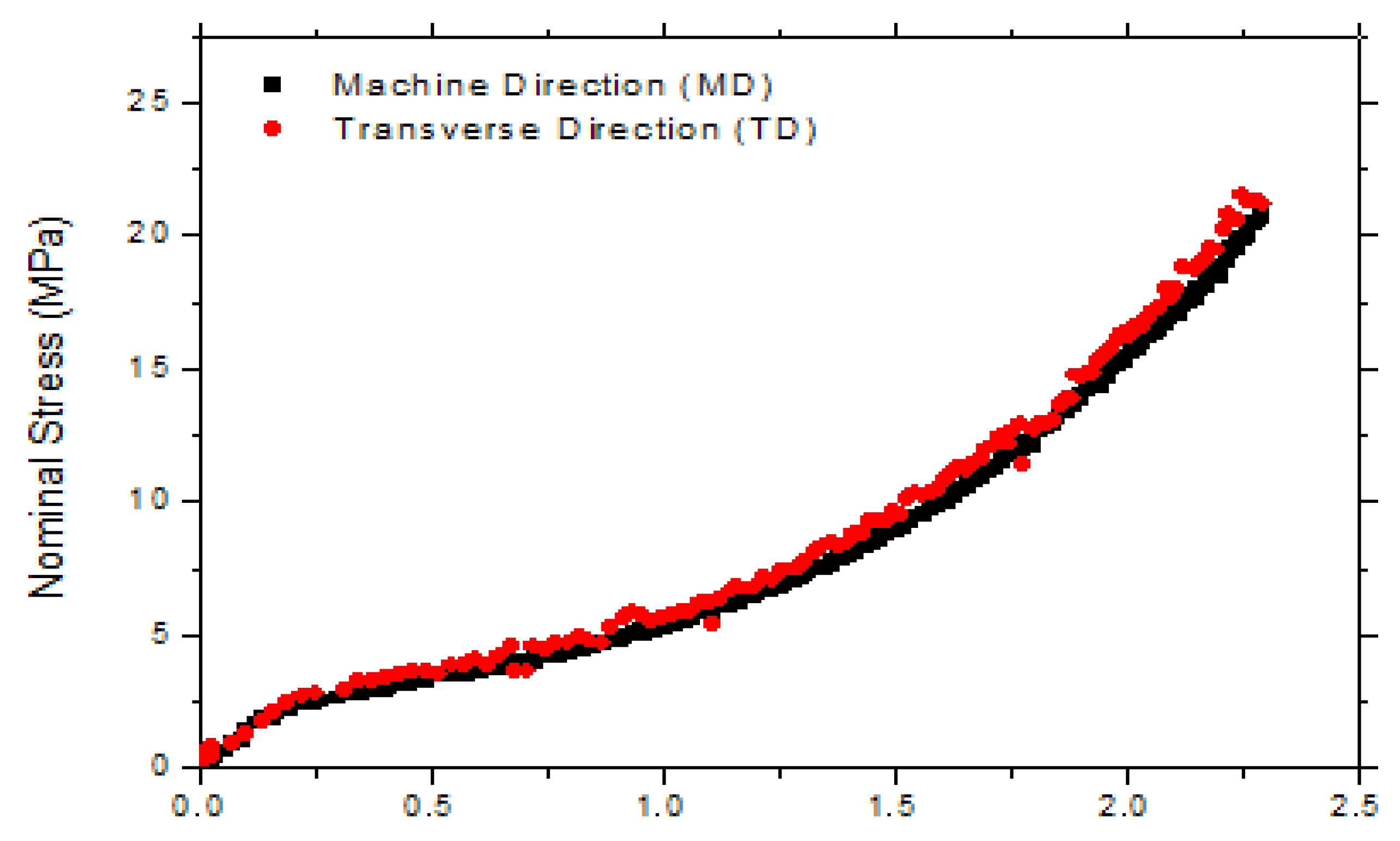
3.2. Properties of the Bi-Axially Stretched Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef]
- Rocca-Smith, J.R.; Pasquarelli, R.; Lagorce-Tachon, A.; Rousseau, J.; Fontaine, S.; Aguié-Béghin, V.; Debeaufort, F.; Karbowiak, T. Toward sustainable PLA-based multilayer complexes with improved barrier properties. ACS Sustain. Chem. Eng. 2019, 7, 3759–3771. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, S.; Sun, J.; Ren, Y.; Wang, S.; Wang, X.; Wang, W.; Li, H.; Fei, B.; Gu, X.; et al. A new strategy to prepare fully bio-based poly (lactic acid) composite with high flame retardancy, UV resistance, and rapid degradation in soil. Chem. Eng. J. 2022, 428, 131979. [Google Scholar] [CrossRef]
- Mirabal, A.S.; Scholz, L.; Carus, M. Bio-based polymers in the World: Capacities, Production and Applications: Status Quo and Trends towards 2020. Nova Inst. 2013, 11, 154–159. [Google Scholar]
- Oguz, O.; Candau, N.; Citak, M.K.; Cetin, F.N.; Avaz Seven, S.; Menceloglu, Y.Z. A sustainable approach to produce stiff, super-tough, and heat-resistant poly(lactic acid)-based green materials. ACS Sustain. Chem. Eng. 2019, 7, 7869–7877. [Google Scholar] [CrossRef]
- Walha, F.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Biosourced blends based on poly(lactic acid) and polyamide 11: Structure–properties relationships and enhancement of film blowing processability. Adv. Polym. Technol. 2018, 37, 2061–2074. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technology for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Y.; Luo, B.; Cui, Y.; Shu, S.; Chen, W.; Wang, L. Effect of Styrene-Maleic Anhydride Copolymer on Properties of PBST/PLA Blends. Polymers 2023, 15, 952. [Google Scholar] [CrossRef]
- Babaei, A.; Haji Abdolrasouli, M.; Rostami, A. Polylactic acid/polycaprolactone bionanocomposites containing zinc oxide nanoparticles: Structure, characterization and cytotoxicity assay. J. Thermoplast. Compos. Mater. 2022, OnlineFirst. [Google Scholar] [CrossRef]
- Sempere-Torregrosa, J.; Ferri, J.M.; Rosa-Ramirez, H.; Pavon, C.; Samper, M.D. Effect of Epoxidized and Maleinized Corn Oil on Properties of Polylactic Acid (PLA) and Polyhydroxybutyrate (PHB) Blend. Polymers 2022, 14, 4205. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Biosourced Poly (lactic acid)/polyamide-11 Blends: Effect of an Elastomer on the Morphology and Mechanical Properties. Molecules 2022, 27, 6819. [Google Scholar] [CrossRef] [PubMed]
- Rasselet, D.; Caro-Bretelle, A.-S.; Taguet, A.; Lopez-Cuesta, J.-M. Reactive Compatibilization of PLA/PA11 Blends and Their Application in Additive Manufacturing. Materials 2019, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y. Biodegradation behavior of poly (butylene adipate-co-terephthalate)(PBAT), poly (lactic acid)(PLA), and their blend in freshwater with sediment. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications- A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, J.; Yang, X.; Xiao, P. Morphology and properties of biodegradable poly (lactic acid)/poly(butylene adipate-co-terephthalate) blends with different viscosity ratio. Polym. Test. 2017, 60, 58–67. [Google Scholar] [CrossRef]
- Gu, S.Y.; Zhang, K.; Ren, J.; Zhan, H. Melt rheology of polylactide/poly(butylene adipate-co-terephthalate) blends. Carbohydr. Polym. 2008, 74, 79–85. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Bronco, S.; Chinea, C. The effect of free radical reactions on structure and properties of poly(lactic acid)(PLA) based blends. Polym. Degrad. Stab. 2010, 95, 332–341. [Google Scholar] [CrossRef]
- Masahiro, N.; Hiroki, I.; Hiroki, W.; Norio, F.; Hiroaki, I. Effect of Dialkyl Peroxide Blending on Tensile Properties of PLA/PBAT Polymer Alloys. Eng. Trans. 2012, 60, 171–184. [Google Scholar]
- Coltelli, M.B.; Toncelli, C.; Ciardelli, F.; Bronco, S. Compatible blends of biorelated polyesters through catalytic transesterification in the melt. Polym. Degrad. Stab. 2011, 96, 982–990. [Google Scholar] [CrossRef]
- Girdthep, S.; Worajittiphon, P.; Molly, R.; Leejarkpai, T.; Punyodom, W. Formulation and characterization of compatibilized poly(lactic acid)-based blends and their nanocomposites with silver-loaded kaolinite. Polym. Int. 2015, 64, 203–211. [Google Scholar] [CrossRef]
- Dong, W.; Zou, B.; Ma, P.; Liu, W.; Zhou, X.; Shi, D.; Ni, Z.; Chen, M. Influence of phthalic anhydride and bioxazoline on the mechanical and morphological properties of biodegradable poly(lactic acid)/poly[(butylene adipate)-co-terephthalate] blends. Polym. Int. 2013, 62, 1783–1790. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Maggiore, I.D.; Bertoldo, M.; Signori, F.; Bronco, S.; Ciardelli, F. Poly(lactic acid) properties as a consequence of poly(butylene adipate-co-terephthalate blending and acetyl tributyl citrate plasticization. J. App. Polym. Sci. 2008, 110, 1250–1262. [Google Scholar] [CrossRef]
- Quero, E.; Muller, A.J.; Signori, F.; Coltelli, M.B.; Bronco, S. Isothermal cold-crystallization of PLA/PBAT blends with and without the addition of acetyl tributyl citrate. Macromol. Chem. Phys. 2012, 213, 36–48. [Google Scholar] [CrossRef]
- Pluta, M. Melt compounding of polylactide/organoclay: Structure and properties of nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3392–3405. [Google Scholar] [CrossRef]
- Nofar, M.; Heuzey, M.C.; Carreau, P.J.; Kamal, M.R. Nanoparticle Interactions and Molecular Relaxation in PLA/PBAT/Nanoclay Blends. Exp. Results 2020, 1, E47. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Ferri, J.M.; Dominici, F.; Boronat, T.; Sanchez-Nacher, L.; Balart, R.; Torre, L. Manufacturing and compatibilization of PLA/PBAT binary blends by cottonseed oil-based derivatives. Express Polym. Lett. 2018, 12, 808–823. [Google Scholar] [CrossRef]
- Jiang, W.; Qiao, X.; Tang, Z.; Sun, K. Preparation and properties of thermoplastic acetylated starch. Acta Polym. Sin. 2006, 6, 97–101. [Google Scholar] [CrossRef]
- Martin, O.; Averous, L. Poly (lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Wang, N.; Yu, J.; Ma, X. Preparation and characterization of thermoplastic starch/PLA blends by one-step reactive extrusion. Polym. Int. 2007, 56, 1440–1447. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Morphology and properties of compatibilized polylactide/thermoplastic starch blends. Polymer 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Zhang, J.F.; Sun, X. Mechanical and thermal properties of poly(lactic acid)/starch blends with dioctyl maleate. J. Appl. Polym. Sci. 2004, 94, 1697–1704. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, X.; Liu, Q.; Hrymak, A. The Effect of Polymeric Chain Extenders on Physical Properties of Thermoplastic Starch and Polylactic Acid Blends. J. Polym. Environ. 2012, 20, 315–325. [Google Scholar] [CrossRef]
- Sarazin, P.; Li, G.; Orts, W.J.; Favis, B.D. Binary and ternary blends of polylactide, polycaprolactone and thermoplastic starch. Polymer 2008, 49, 599–609. [Google Scholar] [CrossRef]
- Phetwarotai, W.; Duangdao, A. Reactive Compatibilization of Polylactide, Thermoplastic Starch and Poly(butylene adipate-co-terephthalate) Biodegradable Ternary Blend Films. Mater. Sci. Forum 2011, 695, 178–181. [Google Scholar] [CrossRef]
- Yoksan, R.; Dang, K.M.; Boontanimitr, A.; Chirachanchai, S. Relationship between microstructure and performances of simultaneous biaxially stretched films based on thermoplastic starch and biodegradable polyesters. Int. J. Biol. Macromol. 2021, 190, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chapleau, N.; Huneault, M.; Li, H. Biaxial Orientation of Polylactide/Thermoplastic Starch Blends. Int. Polym. Process. 2007, 22, 8. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A.; Billon, N.; Combeaud, C. Effect of the simultaneous biaxial stretching on the structural and mechanical properties of PLA, PBAT and their blends at rubbery state. Eur. Polym. J. 2015, 68, 288–301. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Rheological, morphological and interfacial properties of compatibilized PLA/PBAT blends. Rheol. Acta 2014, 53, 501–517. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Reactive extrusion of PLA, PBAT with a multi-functional epoxide: Physico-chemical and rheological properties. Eur. Polym. J. 2014, 58, 90–102. [Google Scholar] [CrossRef]
- Katanyoota, P.; Jariyasakoolroj, P.; Sane, A. Mechanical and barrier properties of simultaneous biaxially stretched polylactic acid/thermoplastic starch/poly(butylene adipate-co-terephthalate) films. Polym. Bull. 2022, 80, 5219–5237. [Google Scholar] [CrossRef]
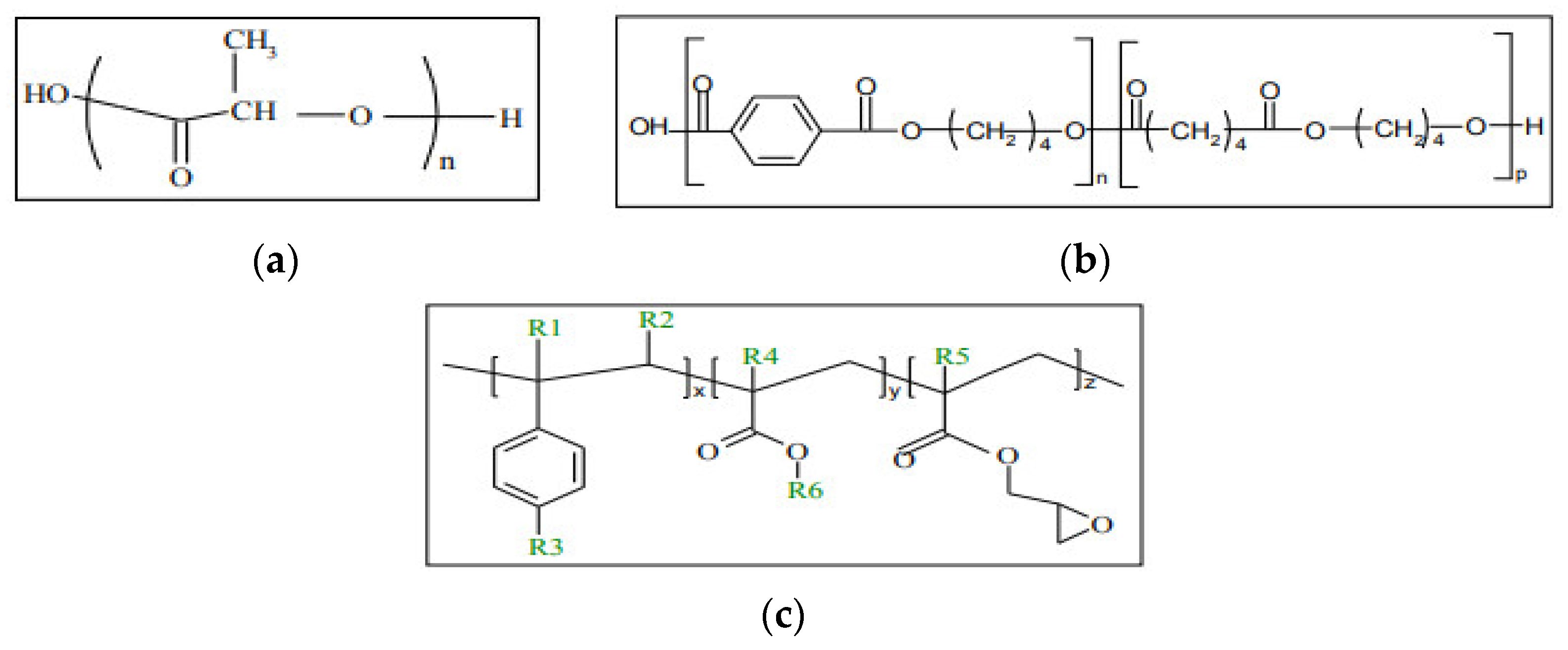



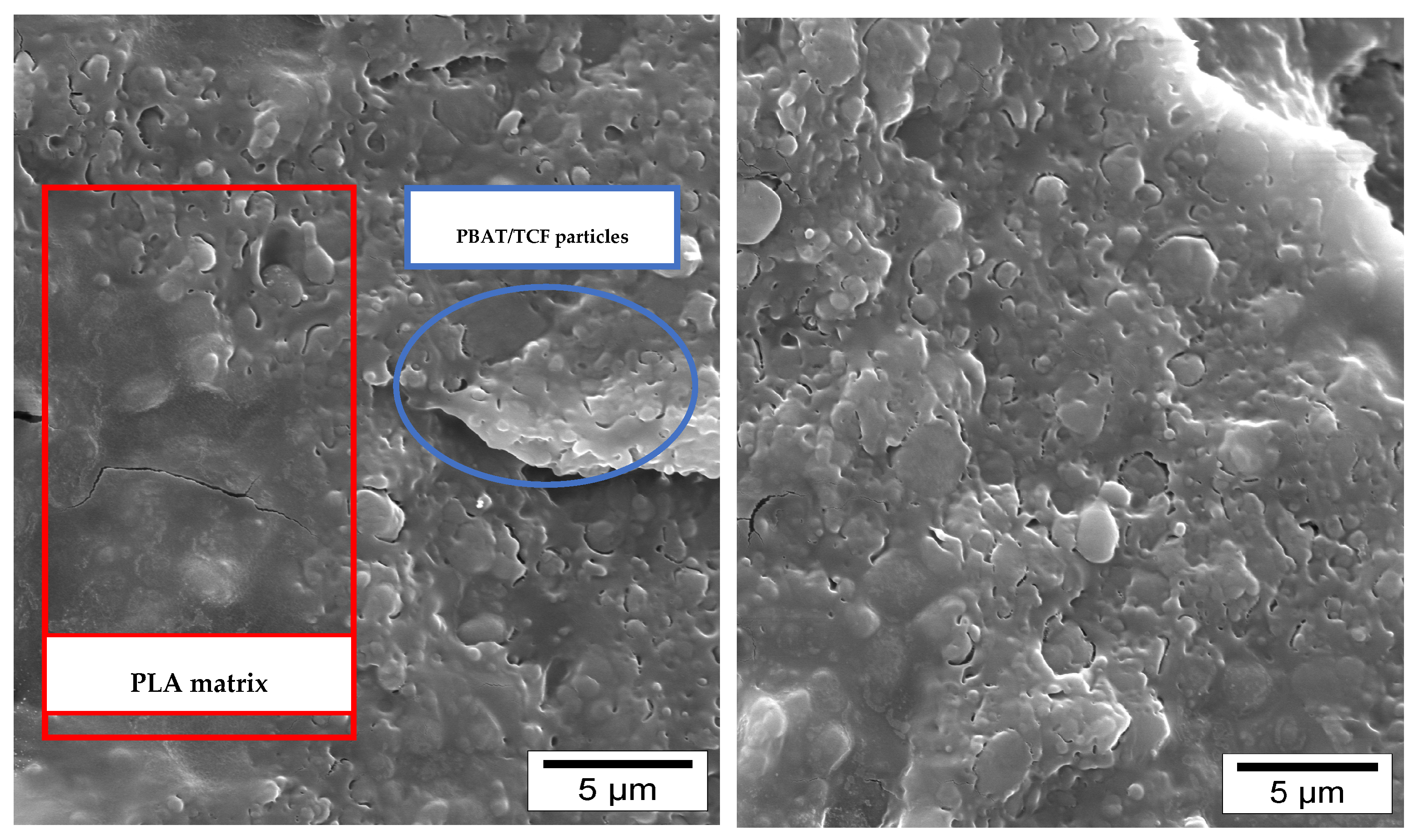
| (a) Samples | % wt Joncryl in the Blend | % wt TCF in the Blend | PLA/PBAT Ratio in the Blend |
|---|---|---|---|
| TCF + PLA/PBAT (0/100) | 0 | 33% | 0/100 |
| TCF + PLA/PBAT (20/80) | 0 | 33% | 20/80 |
| TCF + PLA/PBAT (40/60) | 0 | 33% | 40/60 |
| TCF + PLA/PBAT (60/40) | 0 | 33% | 60/40 |
| TCF + PLA/PBAT (80/20) | 0 | 33% | 80/20 |
| TCF + PLA/PBAT (100/0) | 0 | 33% | 100/0 |
| (b) Samples | % wt Joncryl in the Blend | % wt TCF in the Blend | PLA/PBAT Ratio in the Blend |
| TCF + PLA/PBAT (20/80) | 0.5 | 33% | 20/80 |
| TCF + PLA/PBAT (40/60) | 0.5 | 33% | 40/60 |
| TCF + PLA/PBAT (60/40) | 0.5 | 33% | 60/40 |
| TCF + PLA/PBAT (80/20) | 0.5 | 33% | 80/20 |
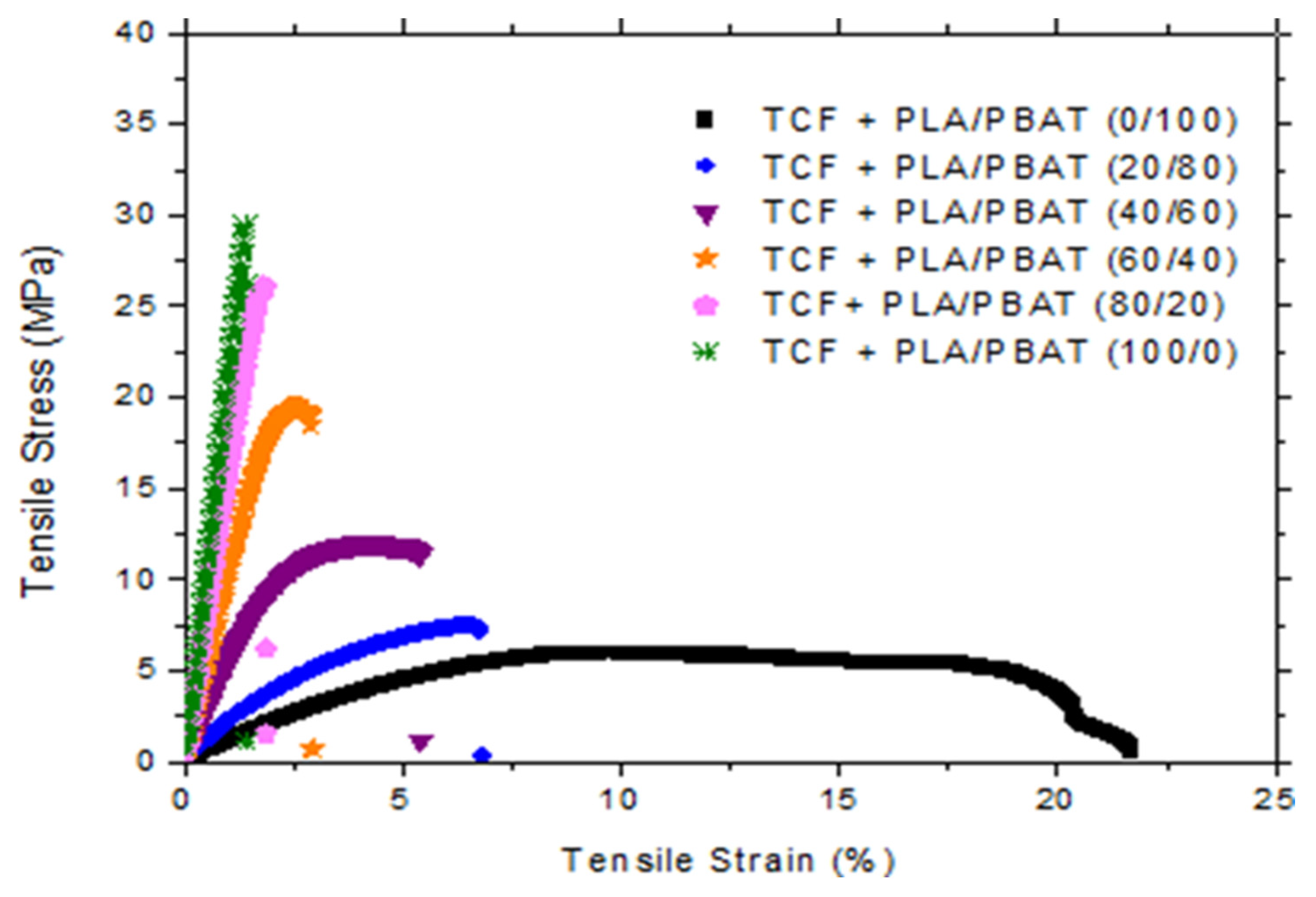
| PLA/PBAT Ratio into PLA/PBAT/TCF Blends | Elastic Modulus (MPa) | Yield Stress (MPa) | Elongation at Break (%) |
|---|---|---|---|
| 0/100 | 96.5 ± 6.8 | 7.4 ± 1.1 | 14.5 ± 4 |
| 20/80 | 184 ± 24 | 8 ± 1 | 9 ± 2 |
| 40/60 | 452 ± 52.4 | 11.5 ± 0.75 | 5.7 ± 1.2 |
| 60/40 | 1029 ± 47 | 19 ± 1.35 | 2.4 ± 0.4 |
| 80/20 | 1508 ± 97.5 | 25 ± 3.8 | 1.8 ± 0.25 |
| 100/0 | 2103 ± 79 | 29 ± 3 | 1.2 ± 0.2 |
| Samples | Composition |
|---|---|
| PLA | 100 % wt PLA |
| PBAT | 100 % wt PBAT |
| TCF+PLA/PBAT (60/40) | 33 % wt TCF in the blend + PLA/PBAT (60/40) |
| TCF+PLA/PBAT/Joncryl (60/40/0.5) | 33 % wt TCF in the blend+ PLA/PBAT (60/40) + 0.5 % wt Joncryl |
| TCF+PLA/PBAT/Joncryl (60/40/0.9) | 33 % wt TCF in the blend+ PLA/PBAT (60/40) + 0.9 % wt Joncryl |
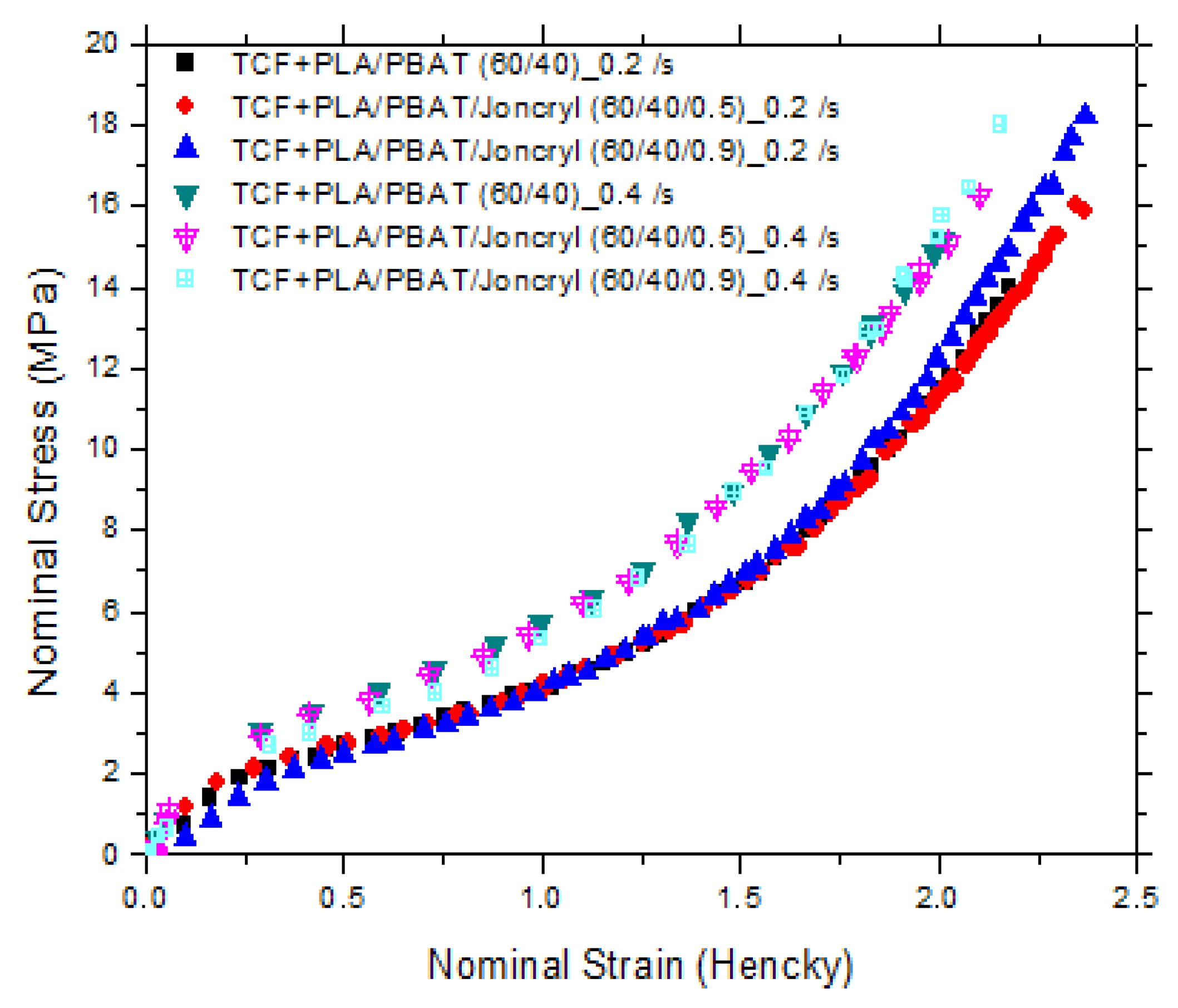
| Samples (=0.2 s−1) | Maximum Stress before Break (MPa) | Uniaxial Strain Hardening Parameter (SHP) or Strain Limit (Hencky) | Samples (= 0.4 s−1) | Maximum Stress before Break (MPa) | Uniaxial Strain Hardening Parameter (SHP) or Strain Limit (Hencky) |
|---|---|---|---|---|---|
| PLA | 35 | 1.5 | PLA | 35 | 1.7 |
| PBAT | 18 | 2.25 | PBAT | 18 | 2.25 |
| TCF + PLA/PBAT (60/40) | 14 | 1.7 | TCF + PLA/PBAT (60/40) | 15 | 1.4 |
| TCF + PLA/PBAT/Joncryl (60/40/0.5) | 16 | 1.75 | TCF+PLA/PBAT/Joncryl (60/40/0.5) | 17 | 1.4 |
| TCF + PLA/PBAT/Joncryl (60/40/0.9) | 19 | 1.65 | TCF + PLA/PBAT/Joncryl (60/40/0.9) | 19 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaouadi, N.; Al-Itry, R.; Maazouz, A.; Lamnawar, K. Biaxial Orientation of PLA/PBAT/Thermoplastic Cereal Flour Sheets: Structure–Processing–Property Relationships. Polymers 2023, 15, 2068. https://doi.org/10.3390/polym15092068
Jaouadi N, Al-Itry R, Maazouz A, Lamnawar K. Biaxial Orientation of PLA/PBAT/Thermoplastic Cereal Flour Sheets: Structure–Processing–Property Relationships. Polymers. 2023; 15(9):2068. https://doi.org/10.3390/polym15092068
Chicago/Turabian StyleJaouadi, Nour, Racha Al-Itry, Abderrahim Maazouz, and Khalid Lamnawar. 2023. "Biaxial Orientation of PLA/PBAT/Thermoplastic Cereal Flour Sheets: Structure–Processing–Property Relationships" Polymers 15, no. 9: 2068. https://doi.org/10.3390/polym15092068








