Influence of Acid-Modified Clinoptilolite on the Self-Adhesive Properties of Silicone Pressure-Sensitive Adhesives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Filler Modification
2.2.2. Preparation of One-Side Si-PSA Tapes
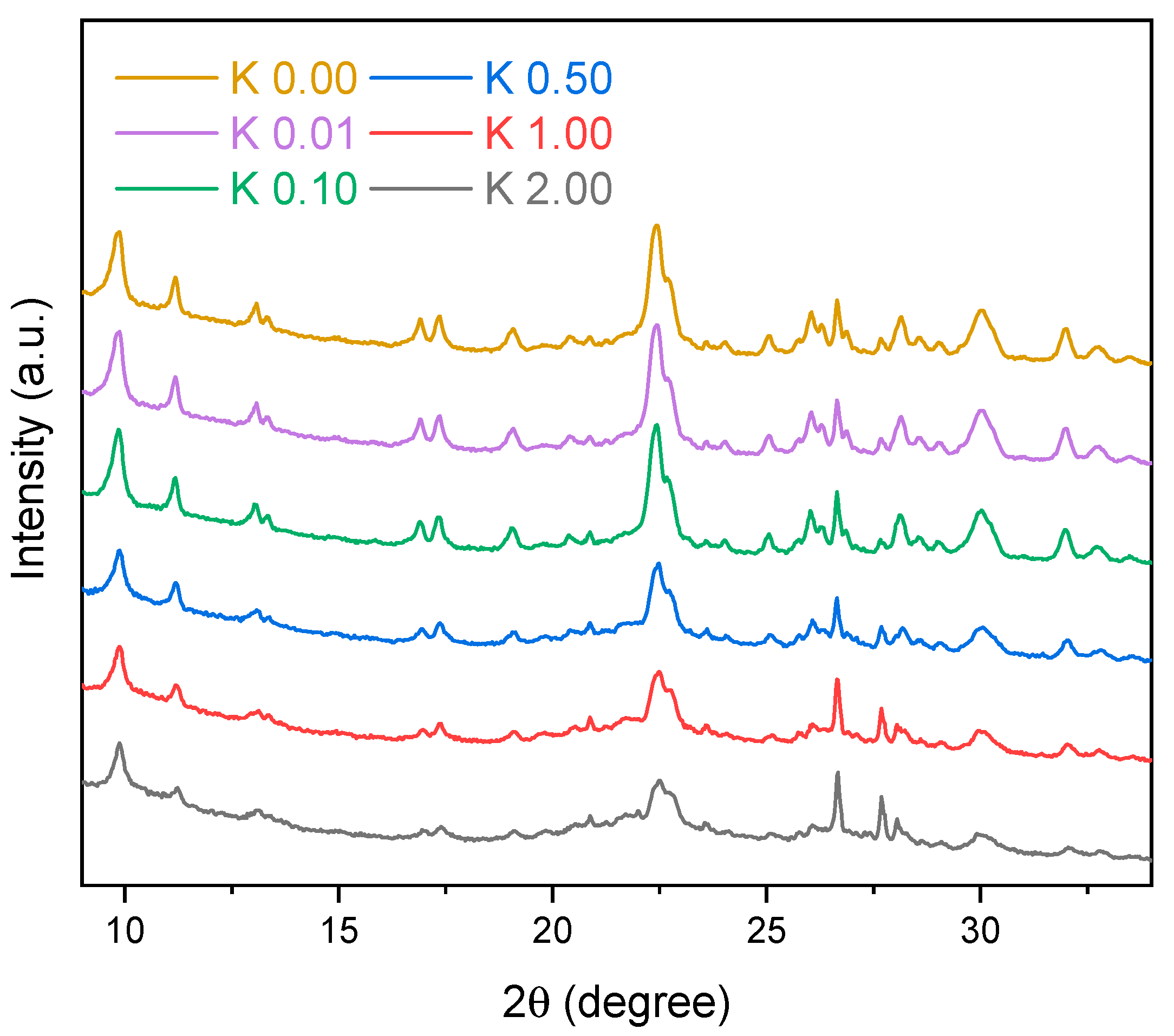
2.2.3. X-ray Diffraction (XRD)
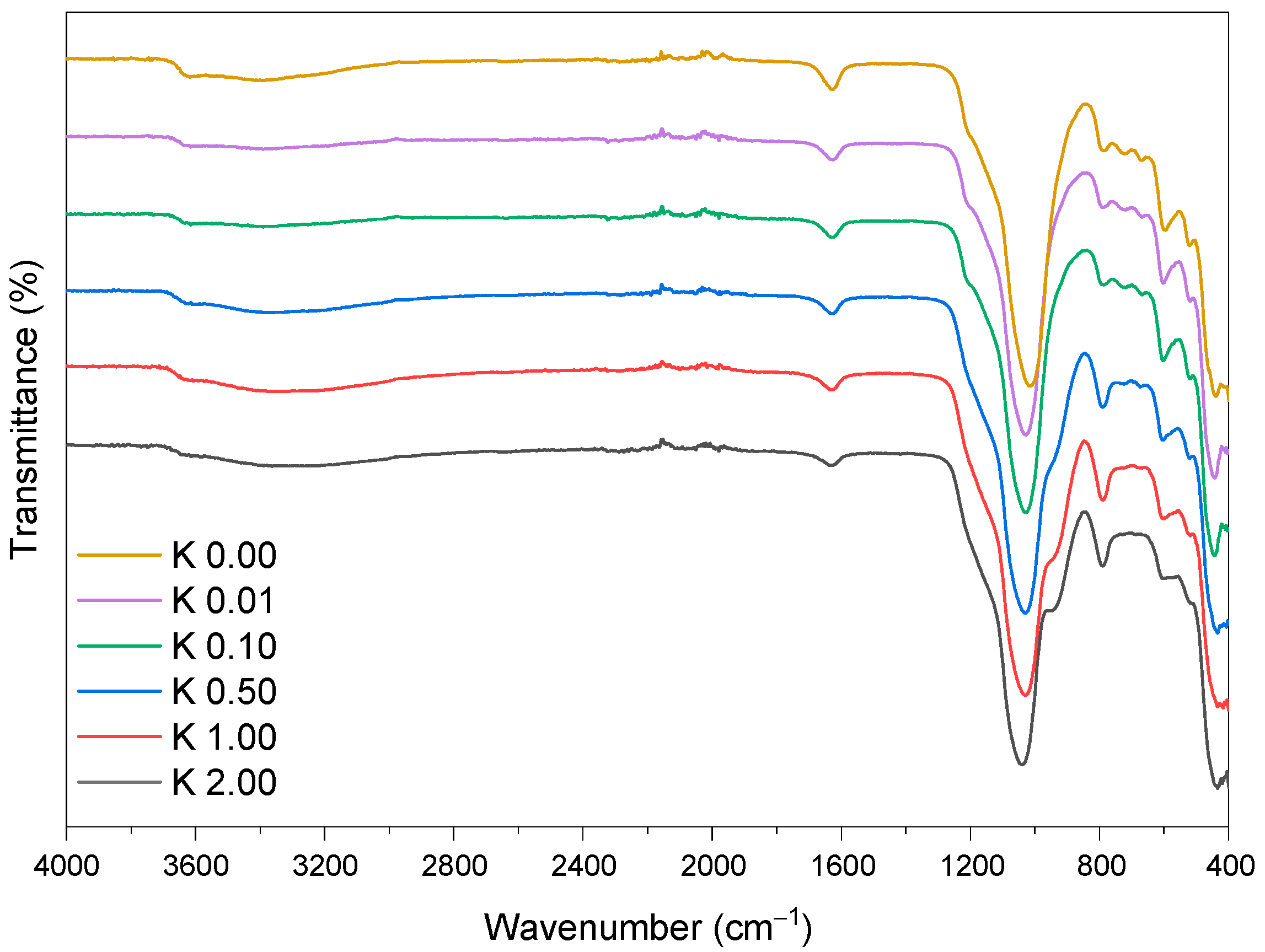
2.2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.5. Pot Life
2.2.6. Tack
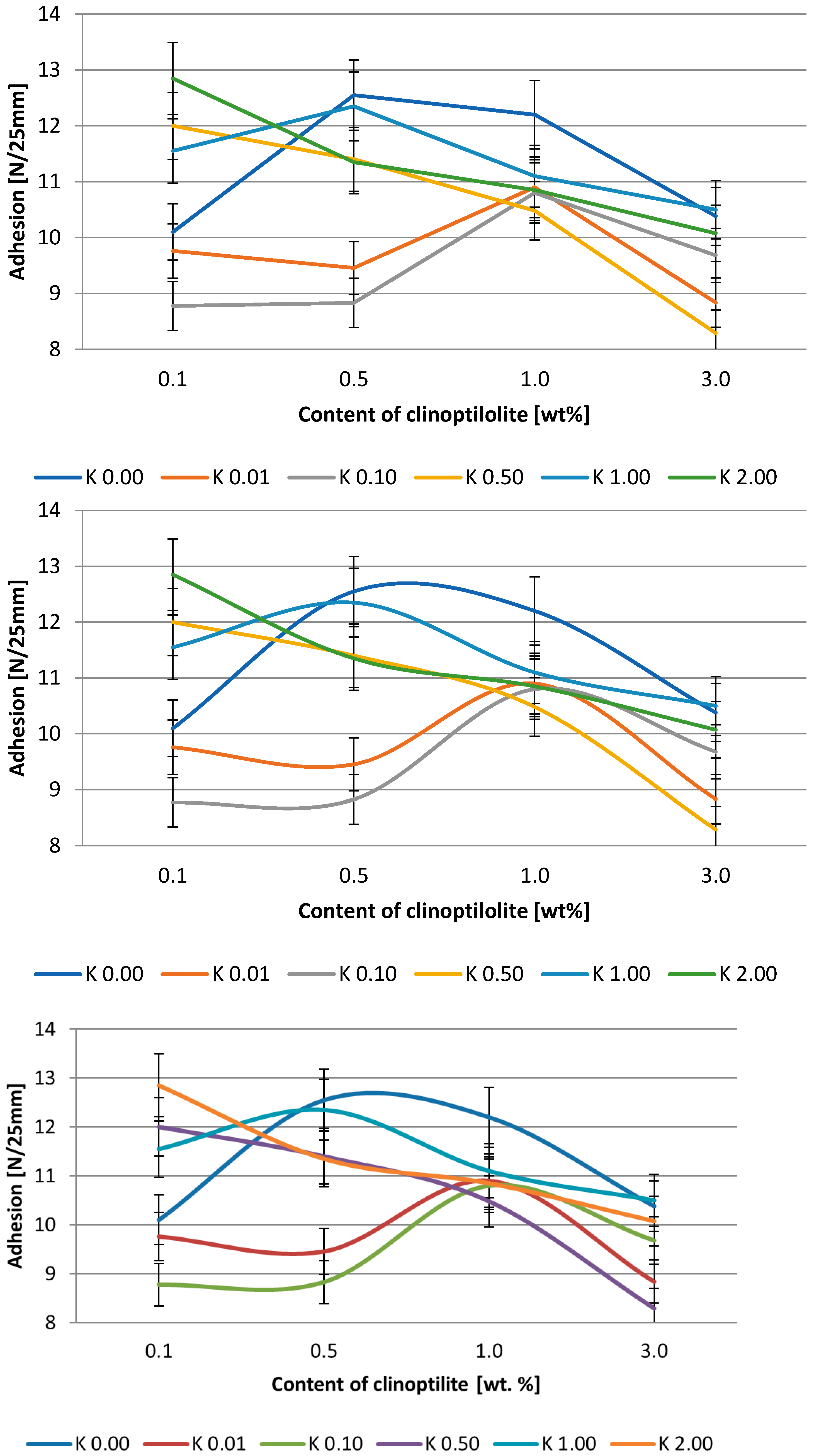
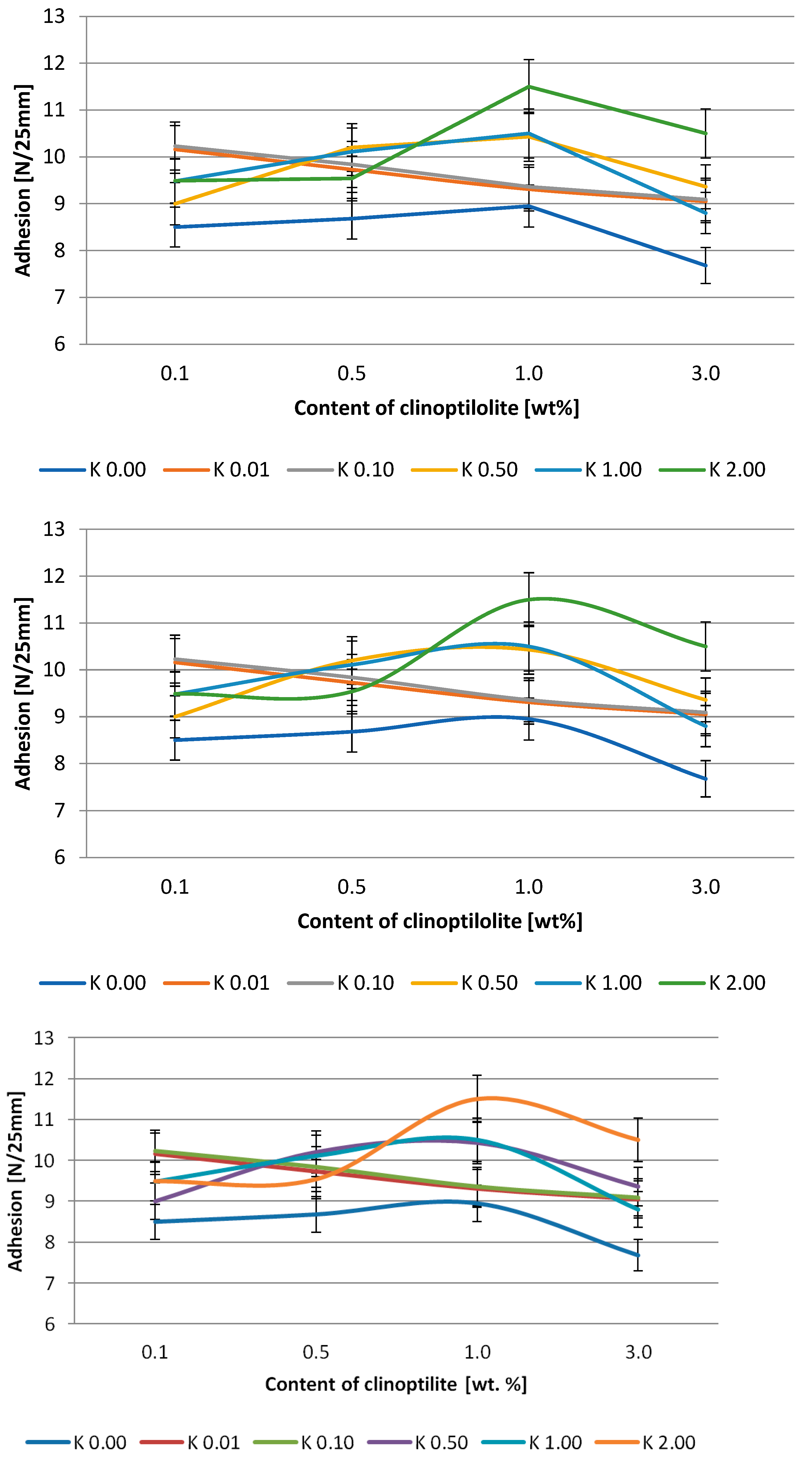
2.2.7. Adhesion
2.2.8. Cohesion
2.2.9. Shrinkage
2.2.10. SAFT Test
3. Results and Discussion
3.1. Clinoptilolite Characterization
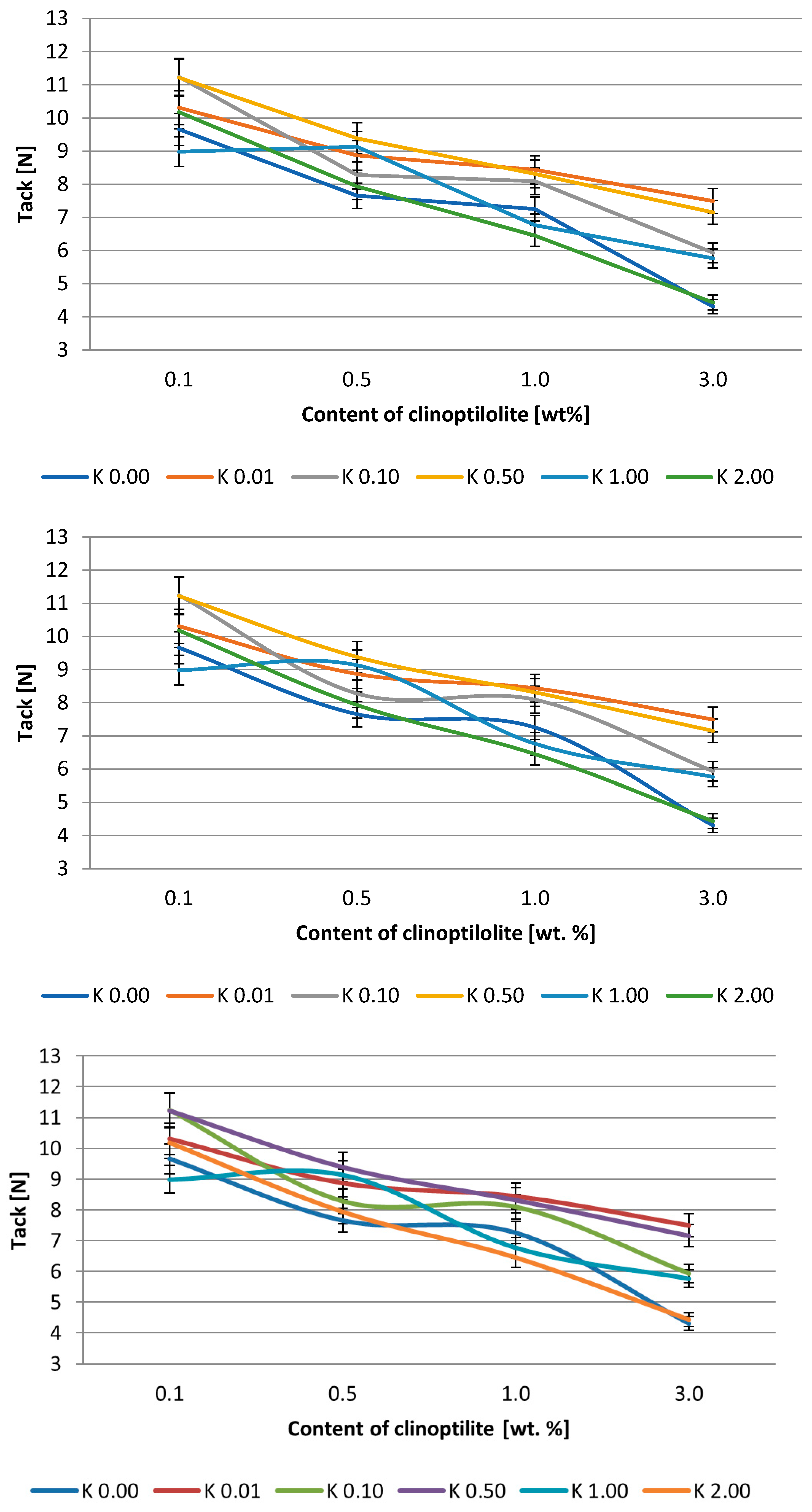
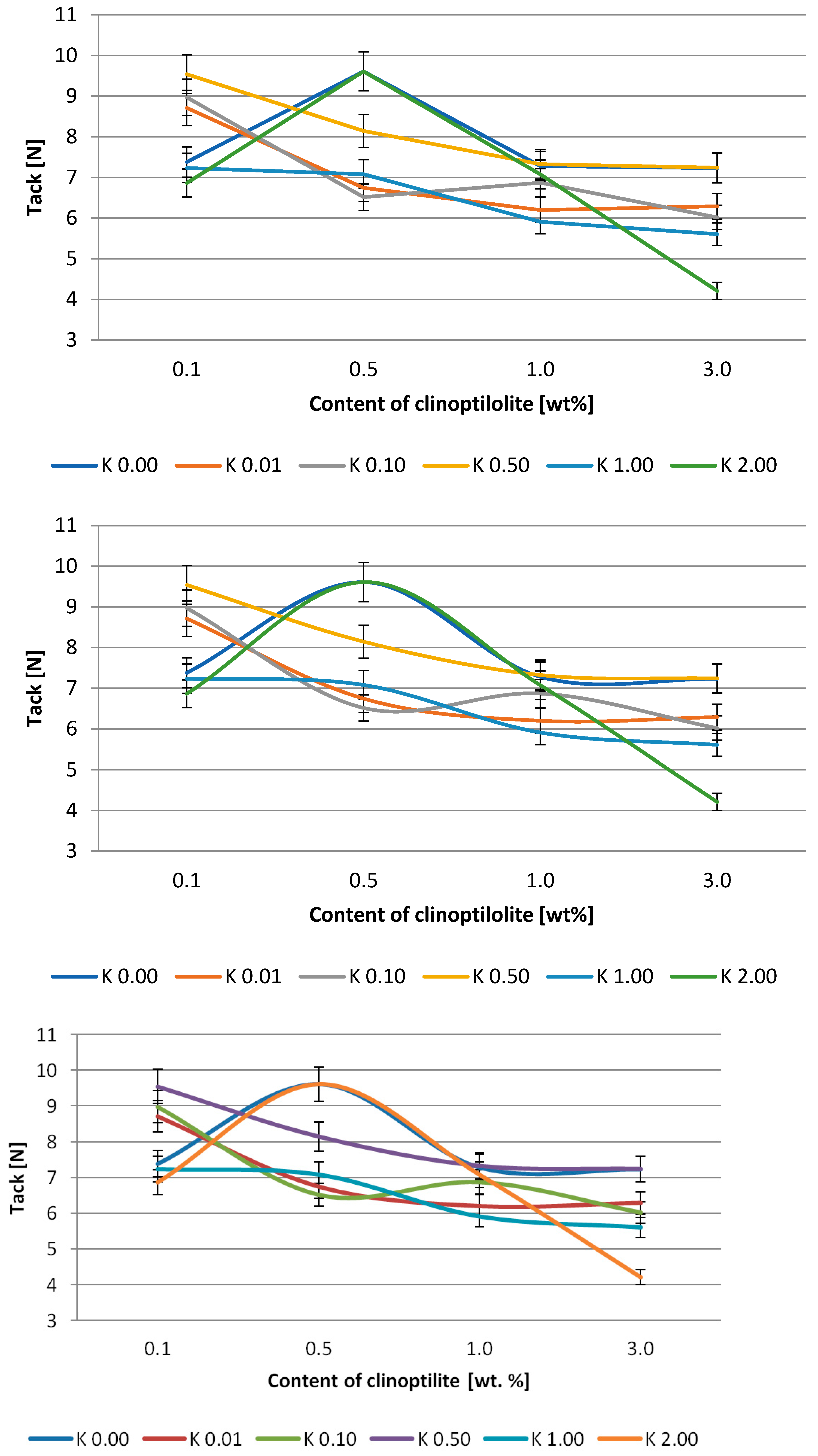
3.2. Silicone Pressure-Sensitive Adhesives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czech, Z.; Kowalczyk, A. Pressure-Sensitive Adhesives for Medical Applications. In Wide Spectra of Quality Control; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Benedek, I. Pressure-Sensitive Adhesives and Applications; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780429215308. [Google Scholar]
- Antosik, A.K. Influence of organic crosslinkers on physical properties of pres- sure-sensitive adhesives based on silicones—Wpływ organicznych środków sieciujących na właściwości fizyczne klejów samoprzylepnych na bazie silikonów. J. Technol. Exploit. Mech. Eng. Poland 2015, 1, 96–106. [Google Scholar]
- Antosik, A.K.; Raganska, P.; Czech, Z. Thermal crosslinking of silicone pressure-sensitive adhesives using organic peroxides. Polimery 2014, 59, 792–797. [Google Scholar] [CrossRef]
- Liu, J.-L.; Xia, R. A unified analysis of a micro-beam, droplet and CNT ring adhered on a substrate: Calculation of variation with movable boundaries. Acta Mech. Sin. 2013, 29, 62–72. [Google Scholar] [CrossRef]
- Dasht Bozorg, B.; Banga, A.K. Effect of Different Pressure-Sensitive Adhesives on Performance Parameters of Matrix-Type Transdermal Delivery Systems. Pharmaceutics 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, S.; Sachdeva, S.; Goswami, T. Role of pressure-sensitive adhesives in transdermal drug delivery systems. Ther. Deliv. 2016, 7, 33–48. [Google Scholar] [CrossRef]
- Anderson, G.L.; Stanley, S.D.; Young, G.L.; Brown, R.A.; Evans, K.B.; Wurth, L.A. The Effects of Silicone Contamination on Bond Performance of Various Bond Systems. J. Adhes. 2010, 86, 1159–1177. [Google Scholar] [CrossRef] [Green Version]
- Czech, Z.; Kowalczyk, A.; Kabatc, J.; Świderska, J. UV-crosslinkable acrylic pressure-sensitive adhesives for industrial application. Polym. Bull. 2012, 69, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Czech, Z.; Butwin, A. New Developments in the Area of Solvent-Borne Acrylic Pressure-Sensitive Adhesives. J. Adhes. Sci. Technol. 2009, 23, 1689–1707. [Google Scholar] [CrossRef]
- Mapari, S.; Mestry, S.; Mhaske, S.T. Developments in pressure-sensitive adhesives: A review. Polym. Bull. 2021, 78, 4075–4108. [Google Scholar] [CrossRef]
- Creton, C. Pressure-Sensitive Adhesives: An Introductory Course. MRS Bull. 2003, 28, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Marghalani, H.Y. Sorption and solubility characteristics of self-adhesive resin cements. Dent. Mater. 2012, 28, e187–e198. [Google Scholar] [CrossRef] [PubMed]
- Czech, Z. Development in the area of UV-crosslinkable solvent-based pressure-sensitive adhesives with excellent shrinkage resistance. Eur. Polym. J. 2004, 40, 2221–2227. [Google Scholar] [CrossRef]
- Czech, Z.; Martysz, D. UV-crosslinkable solvent-based pressure-sensitive adhesives with very low shrinkage. Int. J. Adhes. Adhes. 2004, 24, 533–534. [Google Scholar] [CrossRef]
- Czech, Z. Crosslinking of pressure sensitive adhesive based on water-borne acrylate. Polym. Int. 2003, 52, 347–357. [Google Scholar] [CrossRef]
- Tolia, G.; Kevin Li, S. Study of drug release and tablet characteristics of silicone adhesive matrix tablets. Eur. J. Pharm. Biopharm. 2012, 82, 518–525. [Google Scholar] [CrossRef]
- Lin, S.B.; Durfee, L.D.; Ekeland, R.A.; McVie, J.; Schalau, G.K. Recent advances in silicone pressure-sensitive adhesives. J. Adhes. Sci. Technol. 2007, 21, 605–623. [Google Scholar] [CrossRef]
- He, M.; Zhang, Q.Y.; Guo, J.Y. Synthesis and Characterization of Silicone Based Pressure Sensitive Adhesive. Adv. Mater. Res. 2011, 306–307, 1773–1778. [Google Scholar] [CrossRef]
- Giese-Hinz, J.; Kothe, C.; Louter, C.; Weller, B. Mechanical and chemical analysis of structural silicone adhesives with the influence of artificial aging. Int. J. Adhes. Adhes. 2022, 117, 103019. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Weller, B.; Kothe, M.; Nicklisch, F.; Schadow, T.; Tasche, S.; Vogt, I.; Wünsch, J. Kleben im konstruktiven Glasbau. In Stahlbau Kalender 2011; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 585–646. [Google Scholar]
- Ho, K.Y.; Dodou, K. Rheological studies on pressure-sensitive silicone adhesives and drug-in-adhesive layers as a means to characterise adhesive performance. Int. J. Pharm. 2007, 333, 24–33. [Google Scholar] [CrossRef]
- Antosik, A. Effect of the kaolin nanoparticles on physical properties of silicone pressure-sensitive adhesives Wpływ nanocząstek kaolinu na fizyczne właściwości silikonowych klejów samoprzylepnych. Przem. Chem. 2016, 1, 73–75. [Google Scholar] [CrossRef]
- Mondale, K.D.; Carland, R.M.; Aplan, F.F. The comparative ion exchange capacities of natural sedimentary and synthetic zeolites. Miner. Eng. 1995, 8, 535–548. [Google Scholar] [CrossRef]
- Rashed, M.N.; Palanisamy, P.N. Introductory Chapter: Adsorption and Ion Exchange Properties of Zeolites for Treatment of Polluted Water. In Zeolites and Their Applications; InTech: London, UK, 2018. [Google Scholar]
- Biogazu, O.; Techniczne, C.; Transactions, T.; Techniczne, C.; Kwaśny, J.; Balcerzak, W.; Rezka, P. Application of zeolites for the adsorptive biogas desulfurization. Tech. Trans. 2015, 3-Ś, 39–45. [Google Scholar] [CrossRef]
- Rakitskaya, T.L.; Kiose, T.A.; Golubchik, K.O.; Ennan, A.A.; Volkova, V.Y. Acid-modified clinoptilolite as a support for palladium–copper complexes catalyzing carbon monoxide oxidation with air oxygen. Chem. Cent. J. 2017, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadirbekov, K.A.; Zhambakin, D.K.; Kadirbekov, A.K.; Imanbekov, K.I.; Shakirova, A.K.; Bayakhmetova, Z.K. An influence of modifying acids on clinoptilolite composition and structure. J. Chem. Technol. Metall. 2019, 54, 999–1008. [Google Scholar]
- Miądlicki, P.; Wróblewska, A.; Kiełbasa, K.; Koren, Z.C.; Michalkiewicz, B. Sulfuric acid modified clinoptilolite as a solid green catalyst for solvent-free α-pinene isomerization process. Microporous Mesoporous Mater. 2021, 324, 111266. [Google Scholar] [CrossRef]
- Si, J.; Li, Y.; Yu, X. Curing behavior and mechanical properties of an eco-friendly cold-mixed epoxy asphalt. Mater. Struct. 2019, 52, 81. [Google Scholar] [CrossRef]
- Peláez, J.W.; Ortega, J.A.; Builes, D.H. Scale-up of functional properties of pressure-sensitive adhesive from laboratory to industrial plant production: From monomers to label. J. Adhes. Sci. Technol. 2015, 29, 1873–1883. [Google Scholar] [CrossRef]
- Kajtna, J.; Krajnc, M. Solventless UV crosslinkable acrylic pressure sensitive adhesives. Int. J. Adhes. Adhes. 2011, 31, 822–831. [Google Scholar] [CrossRef]
- Kajtna, J.; Golob, J.; Krajnc, M. The effect of polymer molecular weight and crosslinking reactions on the adhesion properties of microsphere water-based acrylic pressure-sensitive adhesives. Int. J. Adhes. Adhes. 2009, 29, 186–194. [Google Scholar] [CrossRef]
- Kajtna, J.; Krajnc, M. UV crosslinkable microsphere pressure sensitive adhesives—Influence on adhesive properties. Int. J. Adhes. Adhes. 2011, 31, 29–35. [Google Scholar] [CrossRef]
- Czech, Z.; Wojciechowicz, M. The crosslinking reaction of acrylic PSA using chelate metal acetylacetonates. Eur. Polym. J. 2006, 42, 2153–2160. [Google Scholar] [CrossRef]
- Do, H.-S.; Park, Y.-J.; Kim, H.-J. Preparation and adhesion performance of UV-crosslinkable acrylic pressure sensitive adhesives. J. Adhes. Sci. Technol. 2006, 20, 1529–1545. [Google Scholar] [CrossRef]
- Antosik, A.K.; Bednarczyk, P.; Czech, Z. Aging of silicone pressure-sensitive adhesives. Polym. Bull. 2018, 75, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Antosik, A.K.; Weisbrodt, M.; Mozelewska, K.; Czech, Z.; Piątek-Hnat, M. Impact of environmental conditions on silicone pressure-sensitive adhesives. Polym. Bull. 2020, 77, 6625–6639. [Google Scholar] [CrossRef]
- Liu, J.; Xia, R.; Zhou, X. A new look on wetting models: Continuum analysis. Sci. China Physics, Mech. Astron. 2012, 55, 2158–2166. [Google Scholar] [CrossRef]
- Favvas, E.P.; Tsanaktsidis, C.G.; Sapalidis, A.A.; Tzilantonis, G.T.; Papageorgiou, S.K.; Mitropoulos, A.C. Clinoptilolite, a natural zeolite material: Structural characterization and performance evaluation on its dehydration properties of hydrocarbon-based fuels. Microporous Mesoporous Mater. 2016, 225, 385–391. [Google Scholar] [CrossRef]
- Ramishvili, T.; Tsitsishvili, V.; Chedia, R.; Sanaia, E.; Gabunia, V.; Kokiashvili, N. Preparation of Ultradispersed Crystallites of Modified Natural Clinoptilolite with the Use of Ultrasound and Its Application as a Catalyst in the Synthesis of Methyl Salicylate. Am. J. Nano Res. Appl. 2017, 5, 26–32. [Google Scholar] [CrossRef]
- Ruiz-Serrano, D.; Flores-Acosta, M.; Conde-Barajas, E.; Ramírez-Rosales, D.; Yáñez-Limón, J.M.; Ramírez-Bon, R. Study by XPS of different conditioning processes to improve the cation exchange in clinoptilolite. J. Mol. Struct. 2010, 980, 149–155. [Google Scholar] [CrossRef]
- Czech, Z.; Kowalczyk, A.; Shao, L.; Cheng, X.-Q.; Quan, S.; Bai, Y.-P. Novel acrylic pressure-sensitive adhesive (PSA) containing silver particles. J. Adhes. Sci. Technol. 2013, 27, 1446–1454. [Google Scholar] [CrossRef]
- Sanghvi, M.R.; Tambare, O.H.; More, A.P. Performance of various fillers in adhesives applications: A review. Polym. Bull. 2022, 79, 10491–10553. [Google Scholar] [CrossRef]
- Walker, S.; Lingle, E.; Troxler, N.; Wallin, T.; Healy, K.; Mengüç, Y.; Davidson, J.R. Predicting interfacial layer adhesion strength in 3D printable silicone. Addit. Manuf. 2021, 47, 102320. [Google Scholar] [CrossRef]
- Poh, B.T.; Chow, S.K. Effect of zinc oxide on the viscosity, tack, and peel strength of ENR 25-based pressure-sensitive adhesives. J. Appl. Polym. Sci. 2007, 106, 333–337. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Moini Jazani, O.; Navarchian, A.H.; Saeb, M.R. High-performance epoxy-based adhesives reinforced with alumina and silica for carbon fiber composite/steel bonded joints. J. Reinf. Plast. Compos. 2016, 35, 1685–1695. [Google Scholar] [CrossRef]
- de Buyl, F. Silicone sealants and structural adhesives. Int. J. Adhes. Adhes. 2001, 21, 411–422. [Google Scholar] [CrossRef]
- Wójcik-Bania, M.; Matusik, J. The Effect of Surfactant-Modified Montmorillonite on the Cross-Linking Efficiency of Polysiloxanes. Materials 2021, 14, 2623. [Google Scholar] [CrossRef]
| Filler | Treatment | Acronym |
|---|---|---|
| Clinoptilolite | Natural, unmodified | K 0.00 |
| 0.01 M H2SO4 | K 0.01 | |
| 0.1 M H2SO4 | K 0.10 | |
| 0.5 M H2SO4 | K 0.50 | |
| 1.0 M H2SO4 | K 1.00 | |
| 2.0 M H2SO4 | K 2.00 |
| Resin Acronym | Tack [N] | Adhesion [N/25 mm] | Cohesion [h] | SAFT [°C] | |
|---|---|---|---|---|---|
| 20 °C | 70 °C | ||||
| Q2-7358 | 6.9 | 10.2 | >72 | >72 | 147 |
| Q2-7355 | 4.8 | 7.2 | >72 | >72 | 158 |
| Filler Content [wt%] | Viscosity [Pa·s] | |||||
|---|---|---|---|---|---|---|
| K 0.00 | K 0.01 | K 0.10 | K 0.50 | K 1.00 | K 2.00 | |
| After 1 day | ||||||
| 0.1 | 15.2 | 15.3 | 15.2 | 15.8 | 15.4 | 15.8 |
| 0.5 | 15.2 | 16.5 | 16.6 | 15.4 | 15.8 | 16.4 |
| 1.0 | 15.8 | 16.3 | 16.1 | 16.1 | 16.4 | 17.0 |
| 3.0 | 16.9 | 16.9 | 16.8 | 16.8 | 17.1 | 18.2 |
| After 2 days | ||||||
| 0.1 | 16.5 | 16.5 | 16.4 | 16.5 | 16.2 | 16.7 |
| 0.5 | 16.6 | 17.4 | 17.9 | 16.8 | 16.7 | 17.5 |
| 1.0 | 16.6 | 17.1 | 17.0 | 16.7 | 18 | 18.2 |
| 3.0 | 17.1 | 17.6 | 18.4 | 18.0 | 18.6 | 18.3 |
| After 7 days | ||||||
| 0.1 | 29.4 | 29.8 | 28.8 | 28.8 | 28.4 | 27.3 |
| 0.5 | 30.7 | 31.4 | 30.8 | 29.9 | 30.3 | 29.9 |
| 1.0 | 31.6 | 31.8 | 31.6 | 29.2 | 31.8 | 32.0 |
| 3.0 | 30.7 | 31.4 | 35.0 | 30.8 | 35.3 | 33.7 |
| Filler Content [wt%] | Viscosity [Pa∙s] | |||||
|---|---|---|---|---|---|---|
| K 0.00 | K 0.01 | K 0.10 | K 0.50 | K 1.00 | K 2.00 | |
| After 1 day | ||||||
| 0.1 | 2.1 | 2.3 | 1.8 | 8.0 | 2.8 | 1.2 |
| 0.5 | 5.7 | 6.2 | 5.0 | 22.1 | 7.8 | 3.4 |
| 1.0 | 4.9 | 5.3 | 4.3 | 18.9 | 6.7 | 2.9 |
| 3.0 | 4.8 | 5.2 | 4.2 | 18.6 | 6.6 | 2.8 |
| After 2 days | ||||||
| 0.1 | 31.1 | 35.6 | 28.0 | 35.3 | 47.6 | 8.5 |
| 0.5 | 34.1 | 39.1 | 30.7 | 38.7 | 52.1 | 9.3 |
| 1.0 | 30.5 | 34.6 | 27.2 | 34.3 | 46.1 | 8.2 |
| 3.0 | 29.1 | 33.4 | 26.2 | 33.1 | 44.5 | 7.9 |
| After 7 days | ||||||
| 0.1 | 615.5 | 728.0 | 656.6 | 705.3 | 643.1 | 519.8 |
| 0.5 | 593.5 | 702.0 | 633.2 | 680.2 | 620.2 | 501.3 |
| 1.0 | 514.9 | 609.0 | 549.3 | 590.1 | 538.0 | 434.9 |
| 3.0 | 623.7 | 737.7 | 665.4 | 714.8 | 651.7 | 526.8 |
| Filler Content [wt%] | Cohesion [h] | |||||
|---|---|---|---|---|---|---|
| K 0.00 | K 0.01 | K 0.10 | K 0.50 | K 1.00 | K 2.00 | |
| 20 °C | ||||||
| 0.1 | 24.6 | 20.1 | 40.3 | 45.1 | >72 | >72 |
| 0.5 | >72 | >72 | >72 | >72 | >72 | >72 |
| 1.0 | >72 | >72 | >72 | >72 | >72 | >72 |
| 3.0 | >72 | >72 | >72 | >72 | >72 | >72 |
| 70 °C | ||||||
| 0.1 | 0.5 | 0.8 | 1.3 | 1.5 | 14.2 | 20.4 |
| 0.5 | >72 | >72 | >72 | >72 | >72 | >72 |
| 1.0 | >72 | >72 | >72 | >72 | >72 | >72 |
| 3.0 | 14.2 | 9.2 | 10.4 | 56.2 | >72 | 51.0 |
| Filler Content [wt%] | Cohesion [h] | |||||
|---|---|---|---|---|---|---|
| K 0.00 | K 0.01 | K 0.10 | K 0.50 | K 1.00 | K 2.00 | |
| 20 °C | ||||||
| 0.1 | >72 | >72 | >72 | >72 | >72 | >72 |
| 0.5 | >72 | >72 | >72 | >72 | >72 | >72 |
| 1.0 | >72 | >72 | >72 | >72 | >72 | >72 |
| 3.0 | >72 | >72 | >72 | >72 | >72 | >72 |
| 70 °C | ||||||
| 0.1 | >72 | >72 | >72 | >72 | >72 | >72 |
| 0.5 | >72 | >72 | >72 | >72 | >72 | >72 |
| 1.0 | >72 | >72 | >72 | >72 | >72 | >72 |
| 3.0 | >72 | >72 | >72 | >72 | >72 | 8.7 |
| Filler Content [wt%] | SAFT [°C] | |||||
|---|---|---|---|---|---|---|
| K 0.00 | K 0.01 | K 0.10 | K 0.50 | K 1.00 | K 2.00 | |
| Q2-7358 | ||||||
| 0.1 | 89 | 81 | 98 | 95 | 166 | 150 |
| 0.5 | 217 | >225 | >225 | >225 | 210 | 211 |
| 1.0 | 163 | 210 | 201 | 198 | 200 | 187 |
| 3.0 | 159 | 201 | 200 | 197 | 166 | 148 |
| Q2-7355 | ||||||
| 0.1 | >225 | 219 | 219 | 217 | 217 | 216 |
| 0.5 | 219 | >225 | >225 | 210 | 210 | 210 |
| 1.0 | 213 | 219 | 219 | 211 | 211 | 210 |
| 3.0 | 210 | 211 | 217 | 217 | 206 | 209 |
| K 0.00 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler Content[wt%] | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.200 | 0.240 | 0.270 | 0.300 | 0.320 | 0.360 | 0.400 | 0.450 | 0.470 | 0.490 | 0.510 | 0.510 |
| 0.5 | 0.170 | 0.203 | 0.232 | 0.289 | 0.299 | 0.316 | 0.395 | 0.400 | 0.437 | 0.437 | 0.437 | 0.437 |
| 1.0 | 0.087 | 0.295 | 0.324 | 0.379 | 0.398 | 0.413 | 0.452 | 0.452 | 0.452 | 0.452 | 0.452 | 0.452 |
| 3.0 | 0.136 | 0.225 | 0.227 | 0.267 | 0.286 | 0.306 | 0.346 | 0.367 | 0.380 | 0.380 | 0.380 | 0.380 |
| K 0.01 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler content[wt%] | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.180 | 0.240 | 0.280 | 0.300 | 0.340 | 0.370 | 0.400 | 0.430 | 0.440 | 0.450 | 0.470 | 0.470 |
| 0.5 | 0.089 | 0.202 | 0.231 | 0.289 | 0.208 | 0.317 | 0.394 | 0.410 | 0.440 | 0.442 | 0.442 | 0.442 |
| 1.0 | 0.087 | 0.300 | 0.326 | 0.370 | 0.398 | 0.400 | 0.470 | 0.473 | 0.473 | 0.473 | 0.473 | 0.473 |
| 3.0 | 0.110 | 0.211 | 0.221 | 0.232 | 0.254 | 0.298 | 0.326 | 0.367 | 0.380 | 0.380 | 0.380 | 0.380 |
| K 0.10 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler content[wt%] | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.098 | 0.239 | 0.270 | 0.334 | 0.376 | 0.399 | 0.518 | 0.536 | 0.536 | 0.536 | 0.536 | 0.536 |
| 0.5 | 0.085 | 0.208 | 0.235 | 0.290 | 0.327 | 0.347 | 0.450 | 0.466 | 0.466 | 0.466 | 0.466 | 0.466 |
| 1.0 | 0.090 | 0.194 | 0.210 | 0.224 | 0.288 | 0.311 | 0.334 | 0.354 | 0.366 | 0.372 | 0.399 | 0.399 |
| 3.0 | 0.099 | 0.162 | 0.220 | 0.276 | 0.322 | 0.387 | 0.389 | 0.407 | 0.428 | 0.428 | 0.428 | 0.428 |
| K 0.50 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler content (pph) | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.109 | 0.251 | 0.282 | 0.345 | 0.388 | 0.411 | 0.529 | 0.547 | 0.547 | 0.547 | 0.547 | 0.547 |
| 0.5 | 0.095 | 0.218 | 0.245 | 0.300 | 0.337 | 0.357 | 0.460 | 0.476 | 0.476 | 0.476 | 0.476 | 0.476 |
| 1.0 | 0.100 | 0.204 | 0.220 | 0.234 | 0.298 | 0.321 | 0.344 | 0.364 | 0.376 | 0.382 | 0.409 | 0.409 |
| 3.0 | 0.109 | 0.172 | 0.230 | 0.286 | 0.332 | 0.397 | 0.399 | 0.417 | 0.438 | 0.438 | 0.438 | 0.438 |
| K 1.00 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler content[wt%] | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.120 | 0.261 | 0.292 | 0.355 | 0.398 | 0.421 | 0.539 | 0.558 | 0.558 | 0.558 | 0.558 | 0.558 |
| 0.5 | 0.104 | 0.227 | 0.254 | 0.309 | 0.346 | 0.366 | 0.469 | 0.485 | 0.485 | 0.485 | 0.485 | 0.485 |
| 1.0 | 0.090 | 0.194 | 0.210 | 0.224 | 0.288 | 0.311 | 0.334 | 0.354 | 0.366 | 0.372 | 0.399 | 0.399 |
| 3.0 | 0.119 | 0.182 | 0.240 | 0.296 | 0.342 | 0.407 | 0.409 | 0.427 | 0.448 | 0.448 | 0.448 | 0.448 |
| K 2.00 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler content[wt%] | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.121 | 0.274 | 0.305 | 0.368 | 0.411 | 0.434 | 0.552 | 0.570 | 0.570 | 0.570 | 0.570 | 0.570 |
| 0.5 | 0.105 | 0.238 | 0.265 | 0.320 | 0.357 | 0.377 | 0.480 | 0.496 | 0.496 | 0.496 | 0.496 | 0.496 |
| 1.0 | 0.085 | 0.189 | 0.205 | 0.219 | 0.283 | 0.306 | 0.329 | 0.349 | 0.361 | 0.367 | 0.394 | 0.394 |
| 3.0 | 0.089 | 0.152 | 0.210 | 0.266 | 0.312 | 0.377 | 0.379 | 0.397 | 0.418 | 0.418 | 0.418 | 0.418 |
| K 0.00 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler Content[wt%] | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.407 | 0.413 | 0.477 | 0.512 | 0.557 | 0.614 | 0.641 | 0.697 | 0.713 | 0.754 | 0.754 | 0.754 |
| 0.5 | 0.307 | 0.311 | 0.324 | 0.379 | 0.418 | 0.449 | 0.486 | 0.513 | 0.584 | 0.631 | 0.669 | 0.669 |
| 1.0 | 0.065 | 0.069 | 0.072 | 0.075 | 0.078 | 0.082 | 0.089 | 0.091 | 0.097 | 0.104 | 0.112 | 0.131 |
| 3.0 | 0.050 | 0.054 | 0.056 | 0.059 | 0.061 | 0.068 | 0.071 | 0.077 | 0.081 | 0.086 | 0.092 | 0.097 |
| K 0.01 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler content[wt%]) | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.418 | 0.424 | 0.488 | 0.523 | 0.568 | 0.625 | 0.652 | 0.708 | 0.724 | 0.765 | 0.765 | 0.765 |
| 0.5 | 0.318 | 0.322 | 0.335 | 0.390 | 0.429 | 0.460 | 0.497 | 0.524 | 0.595 | 0.642 | 0.680 | 0.680 |
| 1.0 | 0.076 | 0.080 | 0.083 | 0.086 | 0.089 | 0.093 | 0.100 | 0.102 | 0.108 | 0.115 | 0.123 | 0.142 |
| 3.0 | 0.061 | 0.065 | 0.067 | 0.070 | 0.072 | 0.079 | 0.082 | 0.088 | 0.092 | 0.097 | 0.103 | 0.108 |
| K 0.10 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler content (pph) | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.411 | 0.417 | 0.481 | 0.516 | 0.561 | 0.618 | 0.645 | 0.701 | 0.717 | 0.758 | 0.758 | 0.758 |
| 0.5 | 0.311 | 0.315 | 0.328 | 0.383 | 0.422 | 0.453 | 0.490 | 0.517 | 0.588 | 0.635 | 0.673 | 0.673 |
| 1.0 | 0.069 | 0.073 | 0.076 | 0.079 | 0.082 | 0.086 | 0.093 | 0.095 | 0.101 | 0.108 | 0.116 | 0.135 |
| 3.0 | 0.054 | 0.058 | 0.060 | 0.063 | 0.065 | 0.072 | 0.075 | 0.081 | 0.085 | 0.090 | 0.096 | 0.101 |
| K 0.50 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler content[wt%] | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.418 | 0.424 | 0.488 | 0.523 | 0.568 | 0.625 | 0.652 | 0.708 | 0.724 | 0.765 | 0.765 | 0.765 |
| 0.5 | 0.318 | 0.322 | 0.335 | 0.390 | 0.429 | 0.460 | 0.497 | 0.524 | 0.595 | 0.642 | 0.680 | 0.680 |
| 1.0 | 0.076 | 0.080 | 0.083 | 0.086 | 0.089 | 0.093 | 0.100 | 0.102 | 0.108 | 0.115 | 0.123 | 0.142 |
| 3.0 | 0.061 | 0.065 | 0.067 | 0.070 | 0.072 | 0.079 | 0.082 | 0.088 | 0.092 | 0.097 | 0.103 | 0.108 |
| K 1.00 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler content[wt%] | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.293 | 0.310 | 0.370 | 0.396 | 0.430 | 0.491 | 0.512 | 0.603 | 0.613 | 0.705 | 0.705 | 0.705 |
| 0.5 | 0.222 | 0.250 | 0.273 | 0.311 | 0.390 | 0.411 | 0.496 | 0.550 | 0.610 | 0.647 | 0.647 | 0.647 |
| 1.0 | 0.077 | 0.080 | 0.084 | 0.090 | 0.095 | 0.096 | 0.100 | 0.105 | 0.112 | 0.134 | 0.156 | 0.156 |
| 3.0 | 0.043 | 0.050 | 0.052 | 0.054 | 0.061 | 0.063 | 0.069 | 0.073 | 0.077 | 0.083 | 0.090 | 0.096 |
| K 2.00 | ||||||||||||
| Shrinkage (%) | ||||||||||||
| Filler content[wt%] | 10 min | 30 min | 1 h | 3 h | 8 h | 24 h | 2 Days | 3 Days | 4 Days | 5 Days | 6 Days | 7 Days |
| 0.1 | 0.223 | 0.265 | 0.283 | 0.330 | 0.397 | 0.418 | 0.461 | 0.532 | 0.610 | 0.647 | 0.647 | 0.647 |
| 0.5 | 0.210 | 0.242 | 0.250 | 0.283 | 0.278 | 0.311 | 0.333 | 0.353 | 0.377 | 0.417 | 0.471 | 0.471 |
| 1.0 | 0.019 | 0.120 | 0.142 | 0.156 | 0.196 | 0.217 | 0.255 | 0.306 | 0.313 | 0.339 | 0.348 | 0.348 |
| 3.0 | 0.039 | 0.041 | 0.044 | 0.046 | 0.050 | 0.055 | 0.057 | 0.060 | 0.063 | 0.066 | 0.069 | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antosik, A.K.; Musik, M.; Miądlicki, P.; Weisbrodt, M.; Wilpiszewska, K. Influence of Acid-Modified Clinoptilolite on the Self-Adhesive Properties of Silicone Pressure-Sensitive Adhesives. Polymers 2023, 15, 707. https://doi.org/10.3390/polym15030707
Antosik AK, Musik M, Miądlicki P, Weisbrodt M, Wilpiszewska K. Influence of Acid-Modified Clinoptilolite on the Self-Adhesive Properties of Silicone Pressure-Sensitive Adhesives. Polymers. 2023; 15(3):707. https://doi.org/10.3390/polym15030707
Chicago/Turabian StyleAntosik, Adrian Krzysztof, Marlena Musik, Piotr Miądlicki, Mateusz Weisbrodt, and Katarzyna Wilpiszewska. 2023. "Influence of Acid-Modified Clinoptilolite on the Self-Adhesive Properties of Silicone Pressure-Sensitive Adhesives" Polymers 15, no. 3: 707. https://doi.org/10.3390/polym15030707





