Reinforcing a Thermoplastic Starch/Poly(butylene adipate-co-terephthalate) Composite Foam with Polyethylene Glycol under Supercritical Carbon Dioxide
Abstract
:1. Introduction
2. Experimental Preparation
2.1. Materials and Procedure
2.2. Surface and Functional Group Modification of TPS
2.3. The (SA-PEG/TPS)/PBAT Biodegradable Polyester Composite Mixing
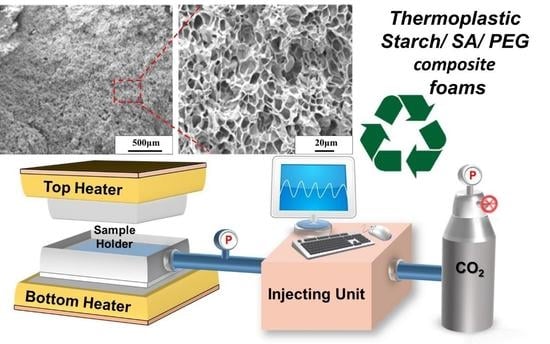
2.4. The (SA-PEG/TPS)/PBAT Composite Foam Test Piece and Supercritical Foaming Experiment
2.5. The (SA-PEG/TPS)/PBAT Composite Foam Appearance and Internal Structure SEM Analysis
3. Results and Discussion
3.1. FTIR Study of (P/TPS) and (SP/TPS)
3.2. The Foam Density of (P/TPS)/PBAT
3.2.1. The Density of Foamed PBAT
3.2.2. The Composite Foam Density of (PEG/TPS)/PBAT
3.2.3. The Foam Density of (SA-PEG/TPS)/PBAT Composite
3.2.4. The Density of Composite Foams at Different Foaming Temperatures and PBAT Ratios (PEG/TPS)
3.2.5. The Effect of Foaming Temperature on the Density of SA-Modified Composite Foams with Different PBAT Ratios (SA-PEG/TPS)
3.3. External and Internal Structure of (PEG/TPS)/PBAT Composite Foam
3.4. External and Internal Structure of (SA-PEG/TPS)/PBAT Composite Foam
3.5. Tensile Properties and Elongation at Break of TPS/PBAT and SA-Modified (SA-PEG/TPS)/PBAT Composite Foam
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, C.-J.; Lu, S.-T.; Kuo, C.-C.; Liang, F.-C.; Chen, B.-Y.; Chu, C.-C. Pyrene or rhodamine derivative–modified surfaces of electrospun nanofibrous chemosensors for colorimetric and fluorescent determination of Cu2+, Hg2+, and pH. React. Funct. Polym. 2016, 108, 137–147. [Google Scholar] [CrossRef]
- Venkatesan, M.; Veeramuthu, L.; Liang, F.-C.; Chen, W.-C.; Cho, C.-J.; Chen, C.-W.; Chen, J.-Y.; Yan, Y.; Chang, S.-H.; Kuo, C.-C. Evolution of electrospun nanofibers fluorescent and colorimetric sensors for environmental toxicants, pH, temperature, and cancer cells—A review with insights on applications. Chem. Eng. J. 2020, 397, 125431. [Google Scholar] [CrossRef]
- Cho, C.-J.; Chen, S.-Y.; Kuo, C.-C.; Veeramuthu, L.; Au-Duong, A.-N.; Chiu, Y.-C.; Chang, S.-H. Morphology and optoelectronic characteristics of organic field-effect transistors based on blends of polylactic acid and poly(3-hexylthiophene). Polym. J. 2018, 50, 975–987. [Google Scholar] [CrossRef]
- Venkatesan, M.; Chen, W.-C.; Cho, C.-J.; Veeramuthu, L.; Chen, L.-G.; Li, K.-Y.; Tsai, M.-L.; Lai, Y.-C.; Lee, W.-Y.; Chen, W.-C.; et al. Enhanced piezoelectric and photocatalytic performance of flexible energy harvester based on CsZn0.75Pb0.25I3/CNC–PVDF composite nanofibers. Chem. Eng. J. 2022, 433, 133620. [Google Scholar] [CrossRef]
- Lu, W.C.; Chuang, F.S.; Venkatesan, M.; Cho, C.J.; Chen, P.Y.; Tzeng, Y.R.; Yu, Y.Y.; Rwei, S.P.; Kuo, C.C. Synthesis of Water Resistance and Moisture-Permeable Nanofiber Using Sodium Alginate-Functionalized Waterborne Polyurethane. Polymers 2020, 12, 2882. [Google Scholar] [CrossRef]
- Chan, H.W.; Cho, C.J.; Hsu, K.H.; He, C.L.; Kuo, C.C.; Chu, C.C.; Chen, Y.H.; Chen, C.W.; Rwei, S.P. Smart Wearable Textiles with Breathable Properties and Repeatable Shaping in In Vitro Orthopedic Support from a Novel Biomass Thermoplastic Copolyester. Macromol. Mater. Eng. 2019, 304, 1900103. [Google Scholar] [CrossRef]
- Motloung, M.P.; Ojijo, V.; Bandyopadhyay, J.; Ray, S.S. Cellulose Nanostructure-Based Biodegradable Nanocomposite Foams: A Brief Overview on the Recent Advancements and Perspectives. Polymers 2019, 11, 1270. [Google Scholar] [CrossRef] [Green Version]
- Boonprasertpoh, A.; Pentrakoon, D.; Junkasem, J. Effect of PBAT on physical, morphological, and mechanical properties of PBS/PBAT foam. Cell. Polym. 2019, 39, 31–41. [Google Scholar] [CrossRef]
- Combrzynski, M.; Matwijczuk, A.; Wojtowicz, A.; Oniszczuk, T.; Karcz, D.; Szponar, J.; Niemczynowicz, A.; Bober, D.; Mitrus, M.; Kupryaniuk, K.; et al. Potato Starch Utilization in Ecological Loose-Fill Packaging Materials-Sustainability and Characterization. Materials 2020, 13, 1390. [Google Scholar] [CrossRef] [Green Version]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.; Amaraweera, S.M.; Fernando, N.M.L.; Wanninayaka, D.B.; Manamperi, A.; Kulatunga, A.K.; Rajapaksha, S.M.; Dassanayake, R.S.; Fernando, C.A.N.; et al. Compatibilization of Starch/Synthetic Biodegradable Polymer Blends for Packaging Applications: A Review. J. Compos. Sci. 2021, 5, 300. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Wang, W.; Gong, P.; Yang, Q.; Park, C.B.; Li, G. Ultra-fast degradable PBAT/PBS foams of high performance in compression and thermal insulation made from environment-friendly supercritical foaming. J. Supercrit. Fluids 2022, 181, 105512. [Google Scholar] [CrossRef]
- Lourdin, D.; Della Valle, G.; Colonna, P. Influence of amylose content on starch films and foams. Carbohydr. Polym. 1995, 27, 261–270. [Google Scholar] [CrossRef]
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Parcq, J.; Favrelle, A.; Bonnet, F.; Zinck, P. Modification of starch by graft copolymerization. Starch-Stärke 2018, 70, 1600351. [Google Scholar] [CrossRef]
- Nabar, Y.; Raquez, J.M.; Dubois, P.; Narayan, R. Production of Starch Foams by Twin-Screw Extrusion: Effect of Maleated Poly(butylene adipate-co-terephthalate) as a Compatibilizer. Biomacromolecules 2005, 6, 807–817. [Google Scholar] [CrossRef]
- Prasong, W.; Ishigami, A.; Thumsorn, S.; Kurose, T.; Ito, H. Improvement of Interlayer Adhesion and Heat Resistance of Biodegradable Ternary Blend Composite 3D Printing. Polymers 2021, 13, 740. [Google Scholar] [CrossRef]
- Sagnelli, D.; Hebelstrup, K.H.; Leroy, E.; Rolland-Sabate, A.; Guilois, S.; Kirkensgaard, J.J.K.; Mortensen, K.; Lourdin, D.; Blennow, A. Plant-crafted starches for bioplastics production. Carbohydr. Polym. 2016, 152, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Thanpitcha, T.; Kritchayanon, N.; Pentrakoon, D.; Pimpan, V. An Initial Study of Starch-g-polystyrene Foam Prepared by a Steaming Process. J. Met. Mater. Miner. 2003, 12, 1–6. [Google Scholar]
- Wu, F.; Misra, M.; Mohanty, A.K. Tailoring the toughness of sustainable polymer blends from biodegradable plastics via morphology transition observed by atomic force microscopy. Polym. Degrad. Stab. 2020, 173, 109066. [Google Scholar] [CrossRef]
- Hu, D.; Xue, K.; Liu, Z.; Xu, Z.; Zhao, L. The essential role of PBS on PBAT foaming under supercritical CO2 toward green engineering. J. CO2 Util. 2022, 60, 101965. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Nabar, Y.; Narayan, R.; Dubois, P. In situ compatibilization of maleated thermoplastic starch/polyester melt-blends by reactive extrusion. Polym. Eng. Sci. 2008, 48, 1747–1754. [Google Scholar] [CrossRef]
- Stagner, J.A.; Alves, V.D.; Narayan, R. Application and performance of maleated thermoplastic starch-poly(butylene adipate-co-terephthalate) blends for films. J. Appl. Polym. Sci. 2012, 126, E135–E142. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwak, H.W.; Kwon, S.; Jang, H.; Park, S.I. Characterization of PLA/PBSeT Blends Prepared with Various Hexamethylene Diisocyanate Contents. Materials 2021, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmuş, A. Ductility improvements of PLA-based binary and ternary blends with controlled morphology using PBAT, PBSA, and nanoclay. Compos. Part B Eng. 2020, 182, 107661. [Google Scholar] [CrossRef]
- Zhang, J.; Hirschberg, V.; Rodrigue, D. Mechanical fatigue of biodegradable polymers: A study on polylactic acid (PLA), polybutylene succinate (PBS) and polybutylene adipate terephthalate (PBAT). Int. J. Fatigue 2022, 159, 106798. [Google Scholar] [CrossRef]
- Banpean, A.; Takagi, H.; Shimizu, N.; Igarashi, N.; Sakurai, S. Small- and wide-angle X-ray scattering studies on confined crystallization of Poly(ethylene glycol) in Poly(L-lactic acid) spherulite in a PLLA/PEG blend. Polymer 2021, 229, 123971. [Google Scholar] [CrossRef]
- Bijarimi, M.; Ahmad, S.; Rasid, R.; Khushairi, M.A.; Zakir, M. Poly(lactic acid)/Poly(ethylene glycol) blends: Mechanical, thermal and morphological properties. AIP Conf. Proc. 2016, 1096, 020002. [Google Scholar] [CrossRef] [Green Version]
- Hassouna, F.; Raquez, J.-M.; Addiego, F.; Dubois, P.; Toniazzo, V.; Ruch, D. New approach on the development of plasticized polylactide (PLA): Grafting of poly(ethylene glycol) (PEG) via reactive extrusion. Eur. Polym. J. 2011, 47, 2134–2144. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, Y.S.; Topolkaraev, V.; Hiltner, A.; Baer, E. Crystallization and phase separation in blends of high stereoregular poly(lactide) with poly(ethylene glycol). Polymer 2003, 44, 5681–5689. [Google Scholar] [CrossRef]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Park, B.-S.; Song, J.C.; Park, D.H.; Yoon, K.-B. PLA/chain-extended PEG blends with improved ductility. J. Appl. Polym. Sci. 2012, 123, 2360–2367. [Google Scholar] [CrossRef]
- Pillin, I.; Montrelay, N.; Grohens, Y. Thermo-mechanical characterization of plasticized PLA: Is the miscibility the only significant factor? Polymer 2006, 47, 4676–4682. [Google Scholar] [CrossRef]
- Taki, K.; Nitta, K.; Kihara, S.-I.; Ohshima, M. CO2 foaming of poly(ethylene glycol)/polystyrene blends: Relationship of the blend morphology, CO2 mass transfer, and cellular structure. J. Appl. Polym. Sci. 2005, 97, 1899–1906. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, B.; Zhao, H.; Yu, P.; Fu, D.; Wen, J.; Peng, X. Processing and characterization of supercritical CO2 batch foamed poly(lactic acid)/poly(ethylene glycol) scaffold for tissue engineering application. J. Appl. Polym. Sci. 2013, 130, 3066–3073. [Google Scholar] [CrossRef]
- Xu, L.; Qian, S.; Zheng, W.; Bai, Y.; Zhao, Y. Formation Mechanism and Tuning for Bimodal Open-Celled Structure of Cellulose Acetate Foams Prepared by Supercritical CO2 Foaming and Poly(ethylene glycol) Leaching. Ind. Eng. Chem. Res. 2018, 57, 15690–15696. [Google Scholar] [CrossRef]
- Chang, C.J.; Venkatesan, M.; Cho, C.J.; Chung, P.Y.; Chandrasekar, J.; Lee, C.H.; Wang, H.T.; Wong, C.M.; Kuo, C.C. Thermoplastic Starch with Poly(butylene adipate-co-terephthalate) Blends Foamed by Supercritical Carbon Dioxide. Polymers 2022, 14, 1952. [Google Scholar] [CrossRef]
- Dehouche, N.; Idres, C.; Kaci, M.; Zembouai, I.; Bruzaud, S. Effects of various surface treatments on Aloe Vera fibers used as reinforcement in poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) biocomposites. Polym. Degrad. Stab. 2020, 175, 109131. [Google Scholar] [CrossRef]




| Symbols | Blends (by Weight) Ref. |
|---|---|
| [N-1] | 50% TPS/50% PBAT [36] |
| [P-1] | 50% (TPS with 10PHR PEG-10)/50% PBAT |
| [P-2] | 50% (TPS with 10PHR PEG-20)/50% PBAT |
| [P-3] | 50% (TPS with 10PHR PEG-30)/50% PBAT |
| [SP-1] | 50% (TPS with 5PHR SA + 5PHR PEG-10)/50% PBAT |
| [SP-2] | 50% (TPS with 5PHR SA + 5PHR PEG-20)/50% PBAT |
| [SP-3] | 50% (TPS with 5PHR SA + 5PHR PEG-30)/50% PBAT |
| Tensile Strength (kPa) | Elongation (%) Ref. | |
|---|---|---|
| [N-1] (F) | 20 ± 3 [36] | |
| [P-1] * (F) | 42 ± 2 | |
| [P-1] (F) | 45 ± 1 | |
| [P-2] (F) | 43 ± 2 | |
| [P-3] (F) | 45 ± 3 | |
| [SP-1] * (F) | 48 ± 1 | |
| [SP-1] (F) | 45 ± 2 | |
| [SP-2] (F) | 44 ± 1 | |
| [SP-3] (F) | 46 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-J.; Chandrasekar, J.; Cho, C.-J.; Venkatesan, M.; Huang, P.-S.; Yang, C.-W.; Wang, H.-T.; Wong, C.-M.; Kuo, C.-C. Reinforcing a Thermoplastic Starch/Poly(butylene adipate-co-terephthalate) Composite Foam with Polyethylene Glycol under Supercritical Carbon Dioxide. Polymers 2023, 15, 129. https://doi.org/10.3390/polym15010129
Chang C-J, Chandrasekar J, Cho C-J, Venkatesan M, Huang P-S, Yang C-W, Wang H-T, Wong C-M, Kuo C-C. Reinforcing a Thermoplastic Starch/Poly(butylene adipate-co-terephthalate) Composite Foam with Polyethylene Glycol under Supercritical Carbon Dioxide. Polymers. 2023; 15(1):129. https://doi.org/10.3390/polym15010129
Chicago/Turabian StyleChang, Chih-Jen, Jayashree Chandrasekar, Chia-Jung Cho, Manikandan Venkatesan, Pin-Shu Huang, Ching-Wei Yang, Hsin-Ta Wang, Chang-Ming Wong, and Chi-Ching Kuo. 2023. "Reinforcing a Thermoplastic Starch/Poly(butylene adipate-co-terephthalate) Composite Foam with Polyethylene Glycol under Supercritical Carbon Dioxide" Polymers 15, no. 1: 129. https://doi.org/10.3390/polym15010129