Facile Synthesis of Microporous Ferrocenyl Polymers Photocatalyst for Degradation of Cationic Dye
Abstract
:1. Introduction
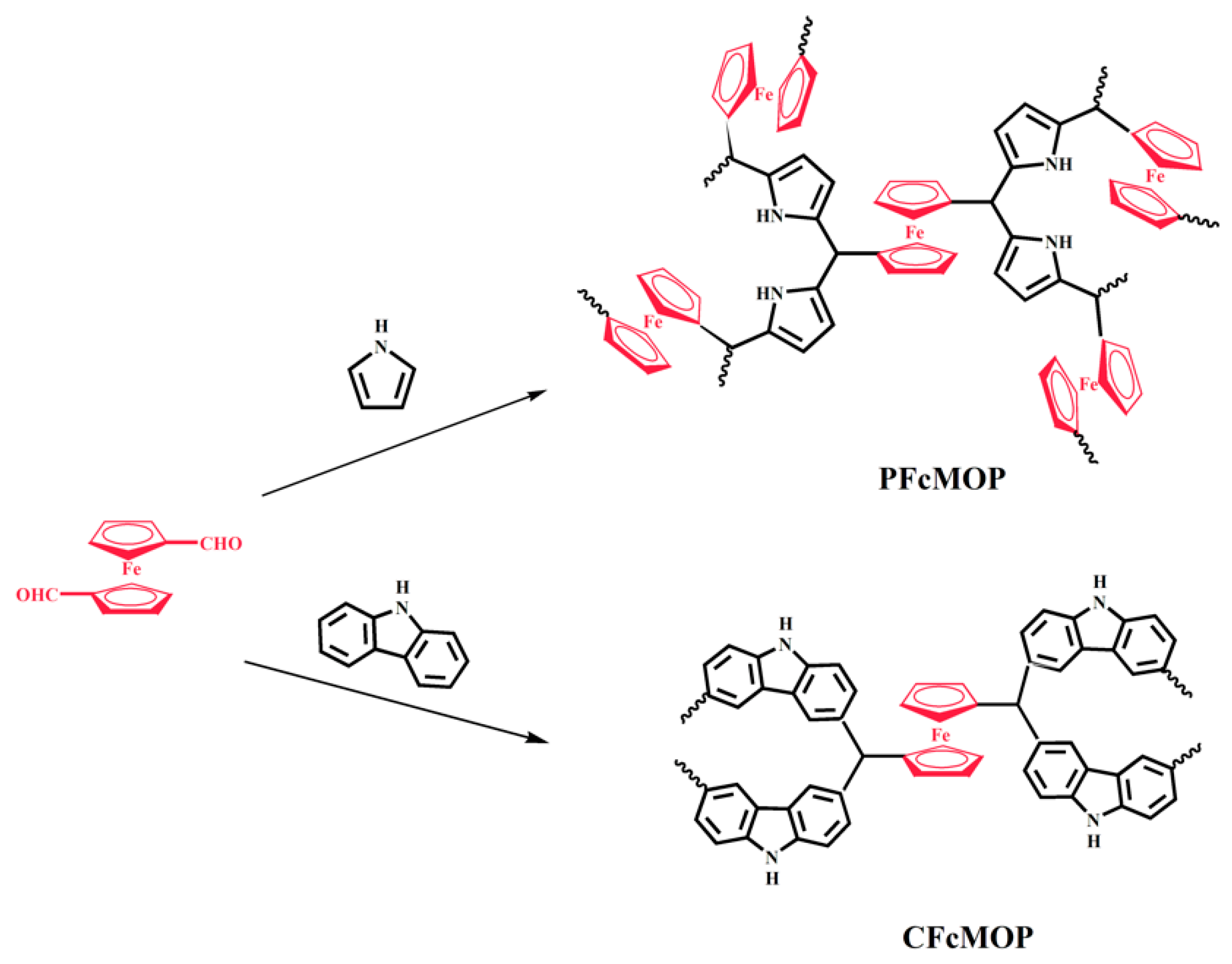
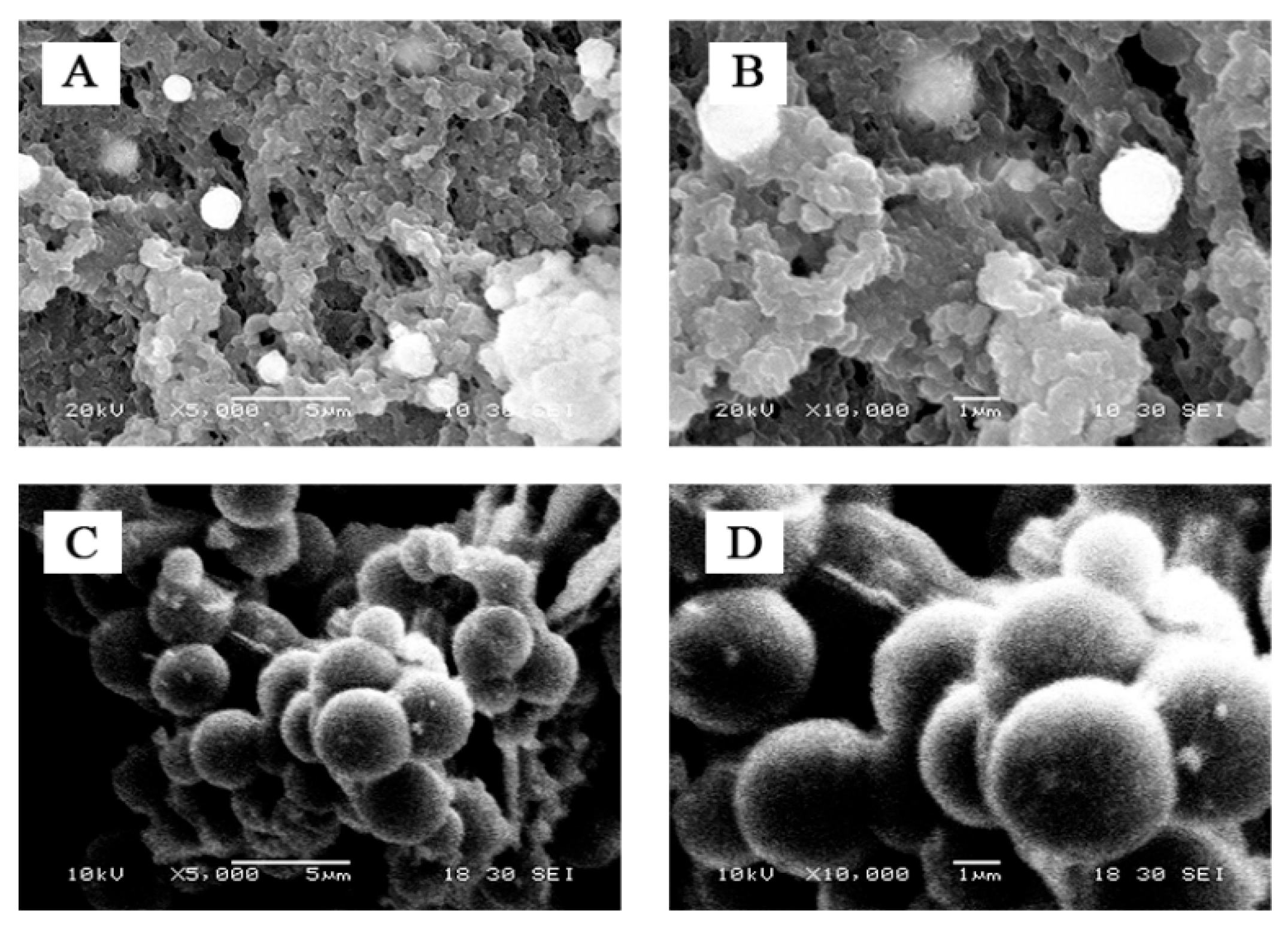
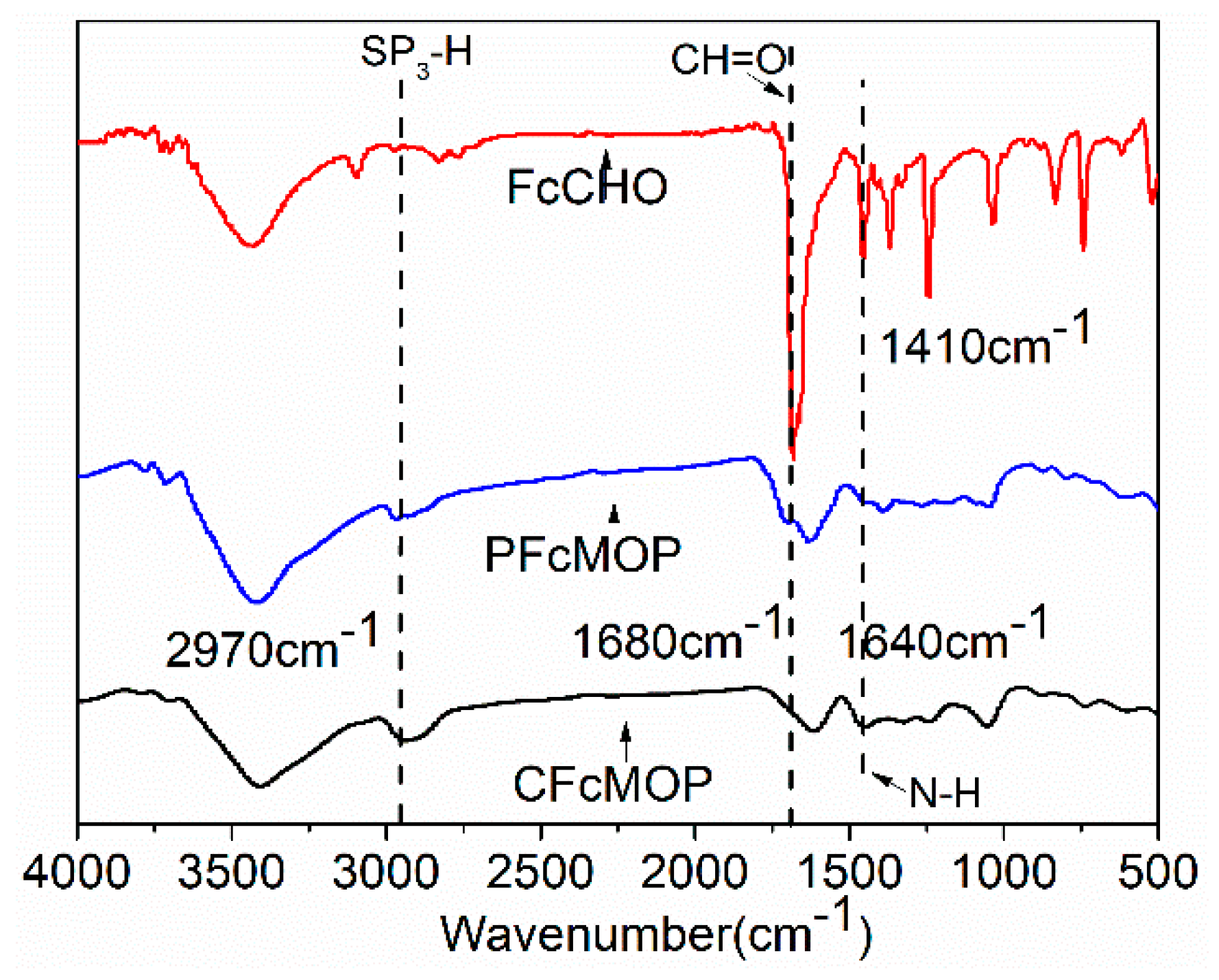
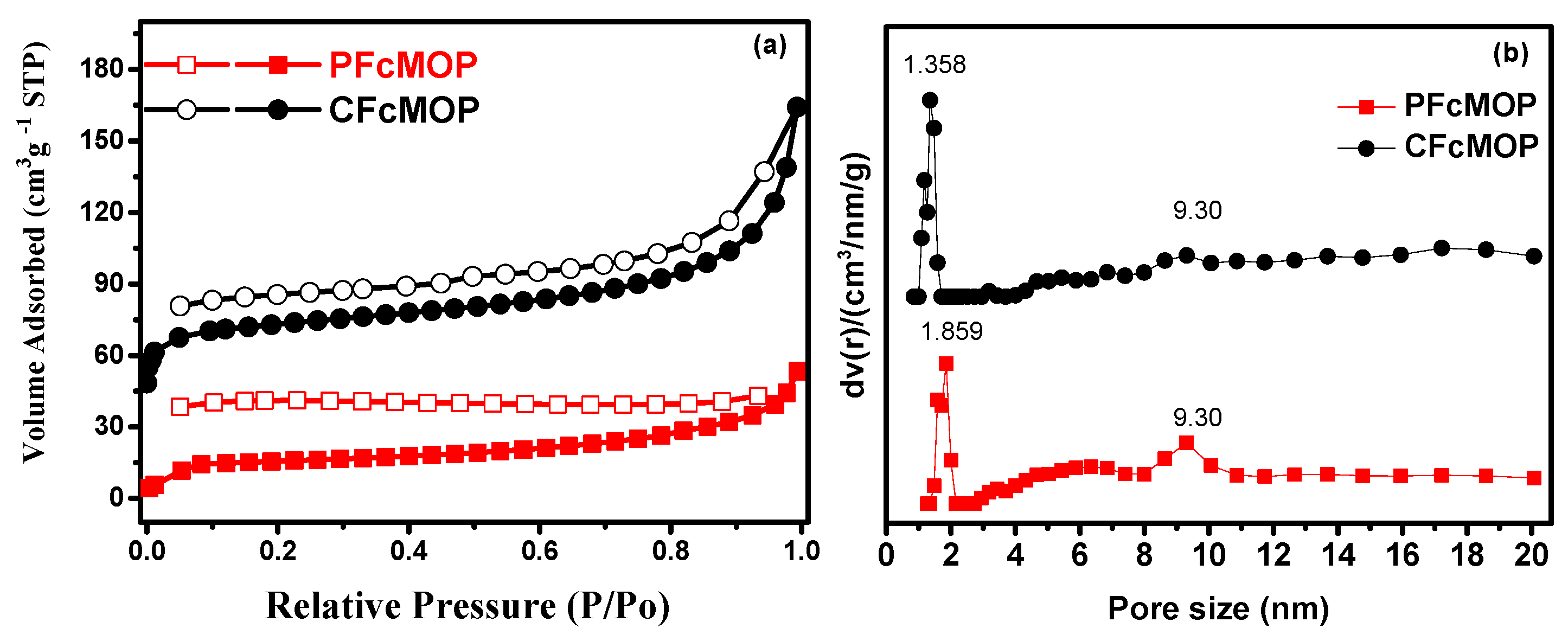
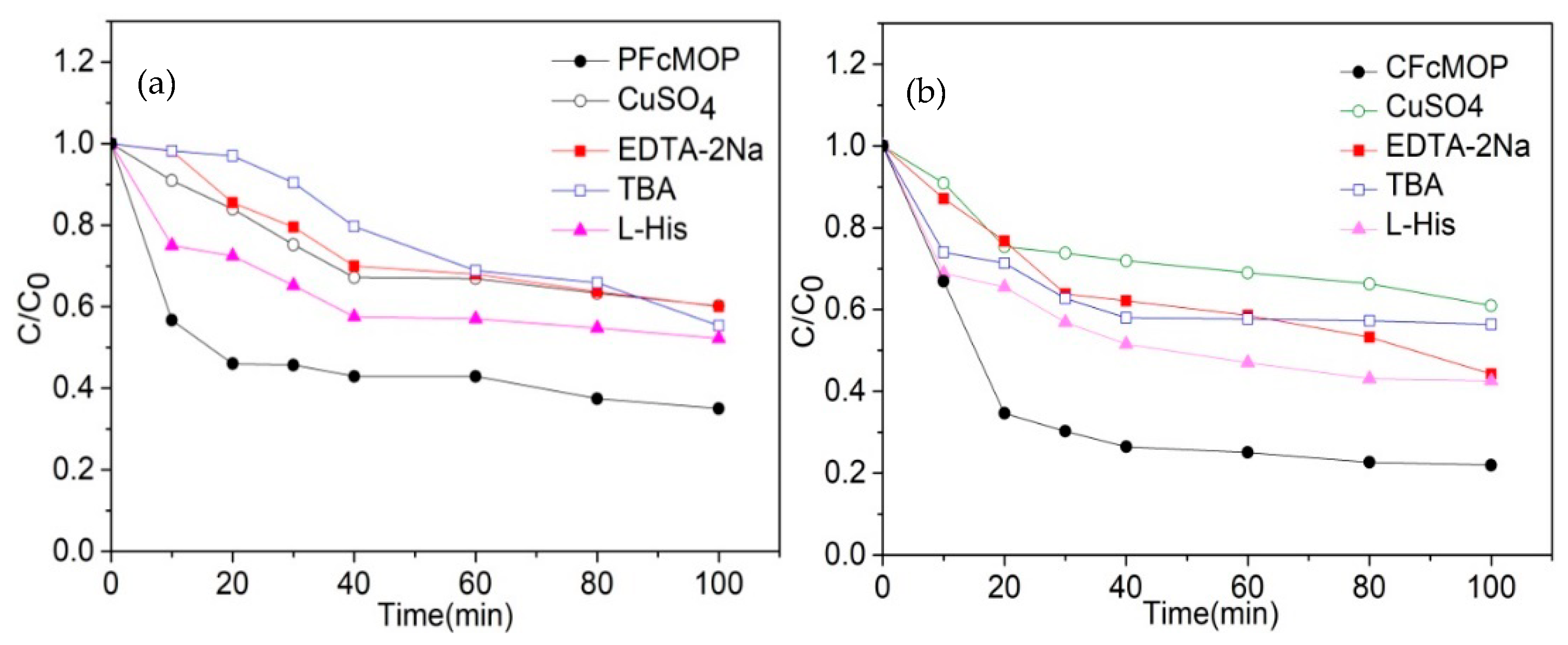
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, T.-T.; Liu, X.-H.; Zou, K.; Deng, W.-Q. Capture and conversion of CO2 at ambient conditions by a conjugated microporous polymer. Nat. Commun. 2013, 4, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Peng, Q.; Yang, H.; Fang, Z.; Deng, J.; Yu, G.; Liao, Y.; Liao, S.; Liu, Q. Modulating Carrier Transfer over Carbazolic Conjugated Microporous Polymers via Donor Structural Design for Functionalization of Thiophenols. ACS Appl. Mater. Interfaces 2021, 13, 60072–60083. [Google Scholar] [CrossRef] [PubMed]
- Novotney, J.L.; Dichtel, W.R. Conjugated porous polymers for TNT vapor detection. ACS Macro Lett. 2013, 2, 423–426. [Google Scholar] [CrossRef]
- Kaleeswaran, D.; Vishnoi, P.; Murugavel, R. Imine and β-ketoenamine tethered fluorescent covalent-organic frameworks for CO2 uptake and nitroaromatic sensing. J. Mater. Chem. C. 2015, 3, 7159–7171. [Google Scholar] [CrossRef]
- Meng, S.; Ma, H.; Jiang, L.; Ren, H.; Zhu, G. A facile approach to prepare porphyrinic porous aromatic frameworks for small hydrocarbon separation. J. Mater. Chem. A 2014, 2, 14536–14541. [Google Scholar] [CrossRef]
- Huang, J.; Tan, Z.-Q.; Su, H.-M.; Guo, Y.-W.; Liu, H.; Liao, B.; Liu, Q.-Q. Ferrocenyl building block constructing porous organic polymer for gas capture and methyl violet adsorption. J. Central South Univ. 2020, 27, 1247–1261. [Google Scholar] [CrossRef]
- Suresh, V.M.; Bonakala, S.; Roy, S.; Balasubramanian, S.; Maji, T.K. Synthesis, characterization, and modeling of a functional conjugated microporous polymer: CO2 storage and light harvesting. J. Phys. Chem. C. 2014, 118, 24369–24376. [Google Scholar] [CrossRef]
- Vyas, V.; Vishwakarma, M.; Moudrakovski, I.; Haase, F.; Savasci, G.; Ochsenfeld, C.; Spatz, J.P.; Lotsch, B.V. Exploiting Noncovalent Interactions in an Imine-Based Covalent Organic Framework for Quercetin Delivery. Adv. Mater. 2016, 28, 8749–8754. [Google Scholar] [CrossRef]
- Luo, Y.; Li, B.; Wang, W.; Wu, K.; Tan, B. Hypercrosslinked Aromatic Heterocyclic Microporous Polymers: A New Class of Highly Selective CO2Capturing Materials. Adv. Mater. 2012, 24, 5703–5707. [Google Scholar] [CrossRef]
- Liu, Q.; Xia, B.; Huang, J.; Liao, B.; Liu, H.; Ou, B.; Chen, L.; Zhou, Z. Hypercrosslinked polystyrene microspheres with ultrahigh surface area and their application in gas storage. Mater. Chem. Phys. 2017, 199, 616–622. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Z.; Ou, B.; Liu, L.; Zhou, Z.; Shen, S.; Duan, Y. Design, preparation, and application of ordered porous polymer materials. Mater. Chem. Phys. 2014, 144, 213–225. [Google Scholar] [CrossRef]
- Cheng, G.; Bonillo, B.; Sprick, R.S.; Adams, D.J.; Hasell, T.; Cooper, A.I. Conjugated Polymers of Intrinsic Microporosity (C-PIMs). Adv. Funct. Mater. 2014, 24, 5219–5224. [Google Scholar] [CrossRef]
- Lu, G.L.; Yang, H.S.; Zhu, Y.L.; Huggins, T.; Jason Ren, Z.Y.; Liu, Z.N.; Zhang, W. Synthesis of a conjugated porous Co (II) porphyrinylene–ethynylene framework through alkyne metathesis and its catalytic activity study. J. Mater. Chem. A. 2015, 3, 4954–4959. [Google Scholar] [CrossRef]
- Xu, Y.H.; Jin, S.B.; Xu, H.; Nagai, A.; Jiang, D.L. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef]
- Díaz, U.; Corma, A. Ordered covalent organic frameworks, COFs and PAFs. From preparation to application. Coord. Chem. Rev. 2016, 311, 85–124. [Google Scholar] [CrossRef]
- Zhan, X.; Chen, Z.; Zhang, Q. Recent progress in two-dimensional COFs for energy-related applications. J. Mater. Chem. A 2017, 5, 14463–14479. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Li, G.; Tang, Z.; Chen, L.J.; Liao, B.; Ou, B.L.; Zhou, Z.H.; Zhou, H. Design and synthesis conjugated microporous polymers with different pore-size distribution and tunable surface area. Mater. Chem. Phys. 2017, 186, 11–18. [Google Scholar] [CrossRef]
- Zhou, G.; Baumgarten, A.M.; Müllen, K. Arylamine-Substituted Oligo(ladder-type pentaphenylene)s: Electronic Communication between Bridged Redox Centers. J. Am. Chem. Soc. 2007, 129, 12211–12221. [Google Scholar] [CrossRef]
- Yu, M.; Wang, X.; Yang, X.; Zhao, Y.; Jiang, J.-X. Conjugated microporous copolymer networks with enhanced gas adsorption. Polym. Chem. 2015, 6, 3217–3223. [Google Scholar] [CrossRef]
- Zhang, K.; Tieke, B.; Vilela, F.; Skabara, P.J. Conjugated microporous networks on the basis of 2,3,5,6-tetraarylated diketopyrrolo [3, 4-c] pyrrole. Macromol. Rapid Commun. 2011, 32, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Zhang, B.; Wang, Z. Highly selective separation of CO2, CH4, and C2–C4 hydrocarbons in ultramicroporous semicycloaliphatic polyimides. ACS Appl. Mater. Interfaces 2018, 10, 26618–26627. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.R.; Stöckel, E.; Adams, D.J.; Cooper, A.I. High Surface Area Networks from Tetrahedral Monomers: Metal-Catalyzed Coupling, Thermal Polymerization, and “Click” Chemistry. Macromolecules 2010, 43, 8531–8538. [Google Scholar] [CrossRef]
- Farha, O.K.; Spokoyny, A.M.; Hauser, B.G.; Bae, Y.S.; Brown, S.E.; Snurr, R.Q.; Mirkin, C.A.; Hupp, J.T. Synthesis, properties, and gas separation studies of a robust diimide-based microporous organic polymer. Chem. Mater. 2009, 21, 3033–3035. [Google Scholar] [CrossRef]
- Katsoulidis, A.P.; Kanatzidis, M.G. Phloroglucinol based microporous polymeric organic frameworks with −OH functional groups and high CO2 capture capacity. Chem. Mater. 2011, 23, 1818–1824. [Google Scholar] [CrossRef]
- Rao, K.V.; Haldar, R.; Kulkarni, C.; Maji, T.K.; George, S.J. Perylene based porous polyimides: Tunable, high surface area with tetrahedral and pyramidal monomers. Chem. Mater. 2012, 24, 969–971. [Google Scholar] [CrossRef]
- Rabbani, M.G.; El-Kaderi, H.M. Template-free synthesis of a highly porous benzimidazole-linked polymer for CO2 capture and H2 storage. Chem. Mater. 2011, 23, 1650–1653. [Google Scholar] [CrossRef]
- Zhang, B.F.; Wang, Z.G. Microporous thermosetting film constructed from hyperbranched polyarylate precursors containing rigid tetrahedral core: Synthesis, characterization, and properties. Chem. Mater. 2010, 22, 2780–2789. [Google Scholar] [CrossRef]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, Y.G.; Krishnakumar, B.; Malik, M.A.; Alhayyani, S. Design and Preparation of Biomass-Derived Activated Carbon Loaded TiO2 Photocatalyst for Photocatalytic Degradation of Reactive Red 120 and Ofloxacin. Polymers 2022, 14, 880. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Ye, J. Efficient Photocatalytic Decomposition of Organic Contaminants over CaBi2O4 under Visible-Light Irradiation. Angew. Chem. Int. Ed. 2004, 43, 4463–4466. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Chen, W. Synthesis of novel CaCO3/Ag2CO3/AgI/Ag plasmonic photocatalyst with enhanced visible light photocatalytic activity. Sci. China Technol. Sci. 2015, 58, 1864–1870. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, R.; Wang, Y.; Chen, W. Plasmonic Ag@AgCl nanotubes fabricated from copper nanowires as high-performance visible light photocatalyst. ACS Appl. Mater. Interfaces 2014, 6, 14819–14826. [Google Scholar] [CrossRef]
- Du, C.; He, S.; Gao, X.; Chen, W. Hierarchical Cu@MnO2 core-shell nanowires: A nonprecious-metal catalyst with an excellent catalytic activity toward the reduction of 4-nitrophenol. Chem. Cat. Chem. 2016, 8, 2885–2889. [Google Scholar] [CrossRef]
- Stegbauer, L.; Schwinghammer, K.; Lotsch, B.V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014, 5, 2789–2793. [Google Scholar] [CrossRef] [Green Version]
- Weder, C. Hole Control in Microporous Polymers. Angew. Chem. Int. Ed. 2008, 47, 448–450. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Tang, X.; Tang, J.; Pan, C.; Yu, G. Phenothiazine core promoted charge transfer in conjugated microporous polymers for photocatalytic Ugi-type reaction and aerobic selenation of indoles. Appl. Catal. B Environ. 2020, 272, 118982. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, J.; Yu, W.; Huang, Q.; Fu, Y.; Kuang, G.-C.; Pan, C.; Yu, G. Visible Light-Driven C-3 Functionalization of Indoles over Conjugated Microporous Polymers. ACS Catal. 2018, 8, 8084–8091. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Zhang, Y.-B.; Su, J.; Furukawa, H.; Yun, Y.; Gándara, F.; Duong, A.; Zou, X.; Yaghi, O.M. Single-Crystal Structure of a Covalent Organic Framework. J. Am. Chem. Soc. 2013, 135, 16336–16339. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, B.; Yan, J.; Wang, Z. The directing effect of linking units on building microporous architecture in tetraphenyladmantane-based poly(Schiff base) networks. Chem. Commun. 2014, 50, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhou, Y.; Xin, X.; Zhang, Q.; Zhang, L.; Wang, R.; Sun, D. Iron(III) Porphyrin-Based Porous Material as Photocatalyst for Highly Efficient and Selective Degradation of Congo Red. Macromol. Chem. Phys. 2016, 217, 599–604. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Tan, Z.; Zhang, Y.; Liu, Q.; Li, Q.; Li, G. Facile Synthesis of Microporous Ferrocenyl Polymers Photocatalyst for Degradation of Cationic Dye. Polymers 2022, 14, 1900. https://doi.org/10.3390/polym14091900
Zhang B, Tan Z, Zhang Y, Liu Q, Li Q, Li G. Facile Synthesis of Microporous Ferrocenyl Polymers Photocatalyst for Degradation of Cationic Dye. Polymers. 2022; 14(9):1900. https://doi.org/10.3390/polym14091900
Chicago/Turabian StyleZhang, Bing, Zhiqiang Tan, Yinhu Zhang, Qingquan Liu, Qianxia Li, and Gen Li. 2022. "Facile Synthesis of Microporous Ferrocenyl Polymers Photocatalyst for Degradation of Cationic Dye" Polymers 14, no. 9: 1900. https://doi.org/10.3390/polym14091900






