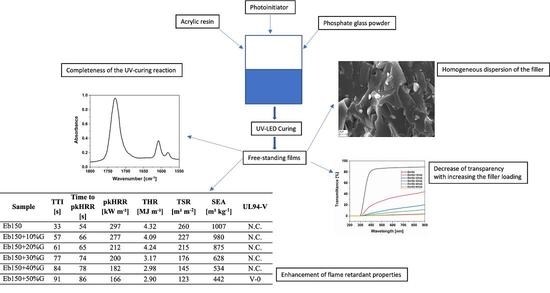
UV-LED Curable Acrylic Films Containing Phosphate Glass Powder: Effect of the Filler Loading on the Thermal, Optical, Mechanical and Flame Retardant Properties
Abstract
:1. Introduction
2. Materials and Methods
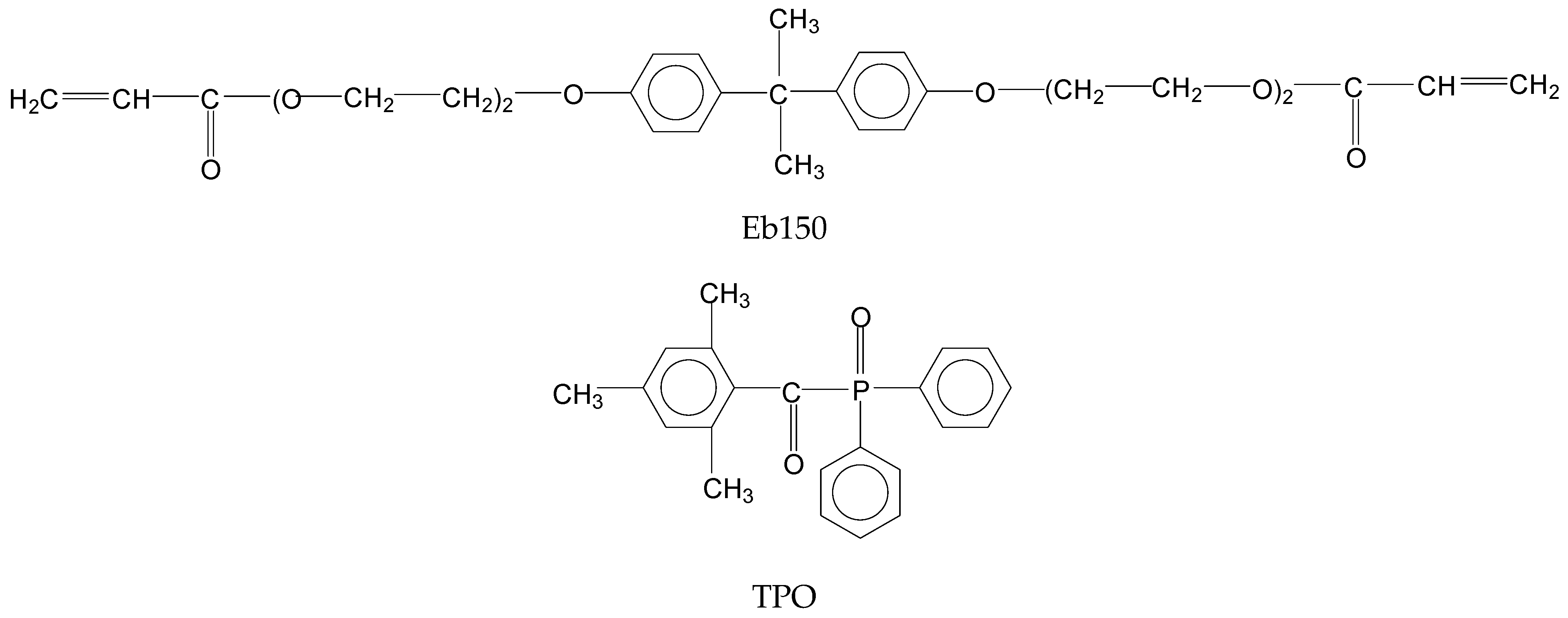
2.1. Materials
2.2. Preparation of the UV-LED Cured Films
2.3. Characterization Techniques
- -
- heating from 0 to 160 °C at 10 °C min−1;
- -
- cooling down to 0 °C at 10 °C min−1;
- -
- final heating from 0 to 160 °C at 10 °C min−1.
3. Results and Discussion
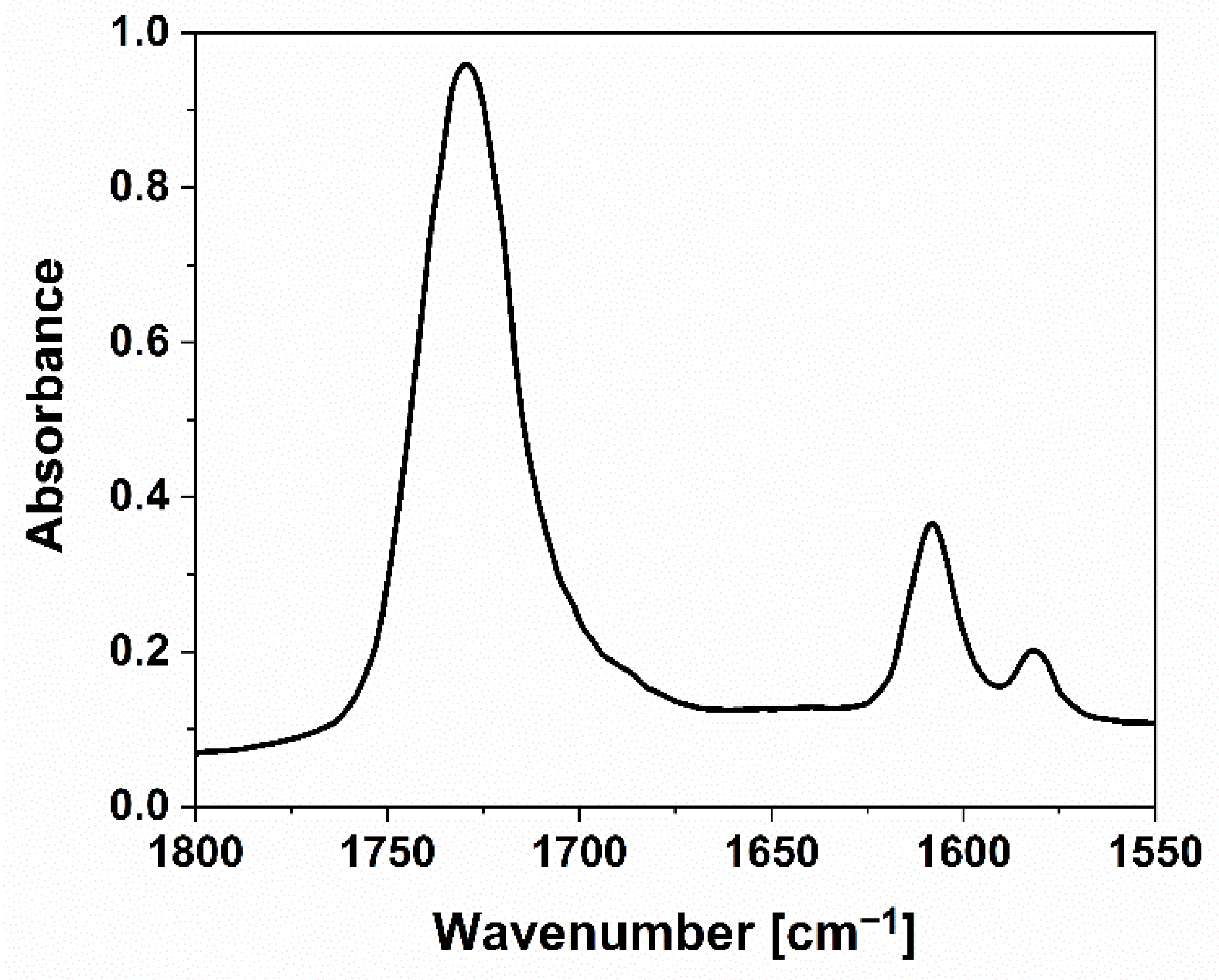
3.1. FTIR-ATR Analyses
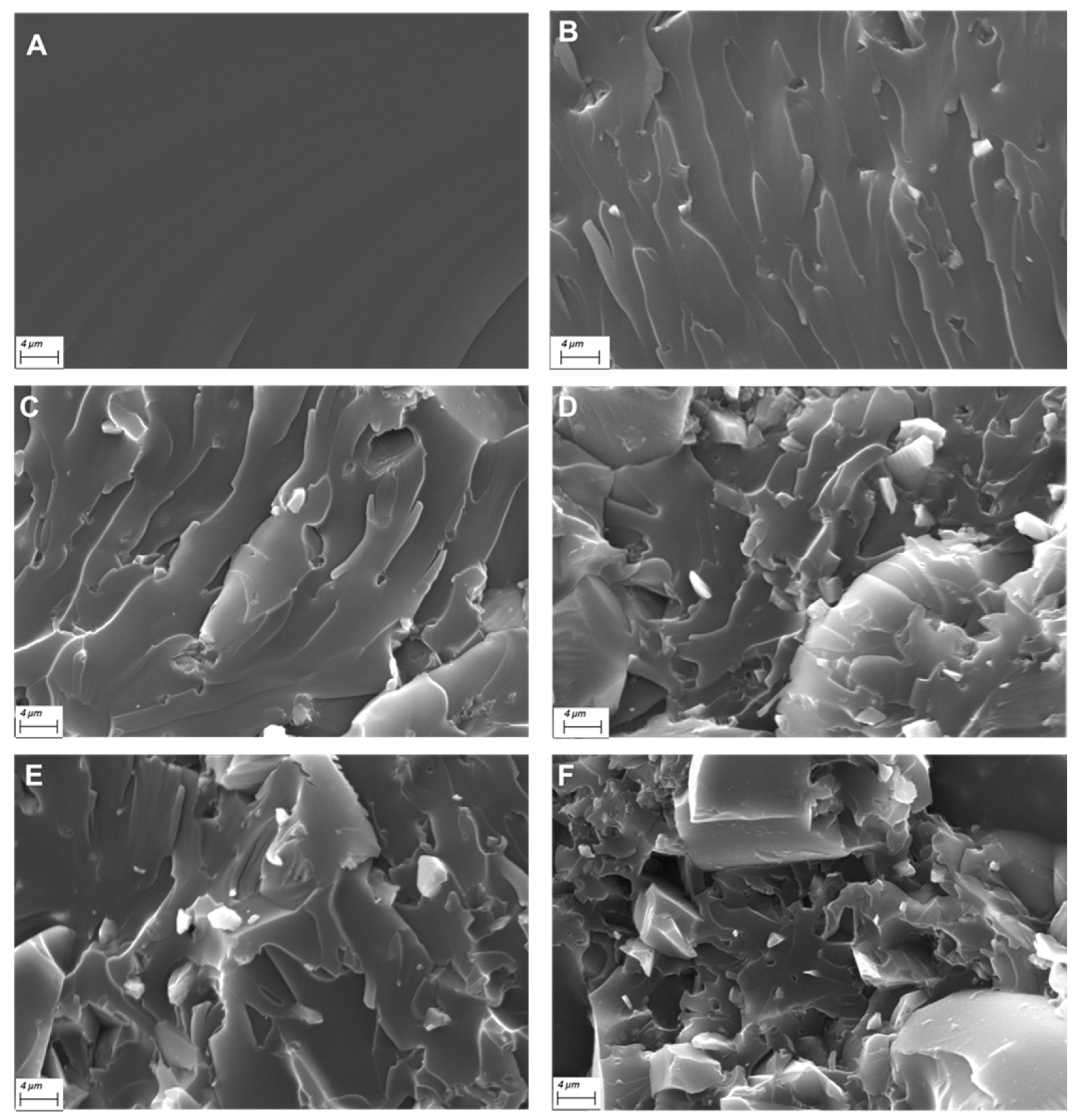
3.2. Morphology of the UV-LED Cured Films
3.3. Thermal Behavior
3.4. Fire Behavior
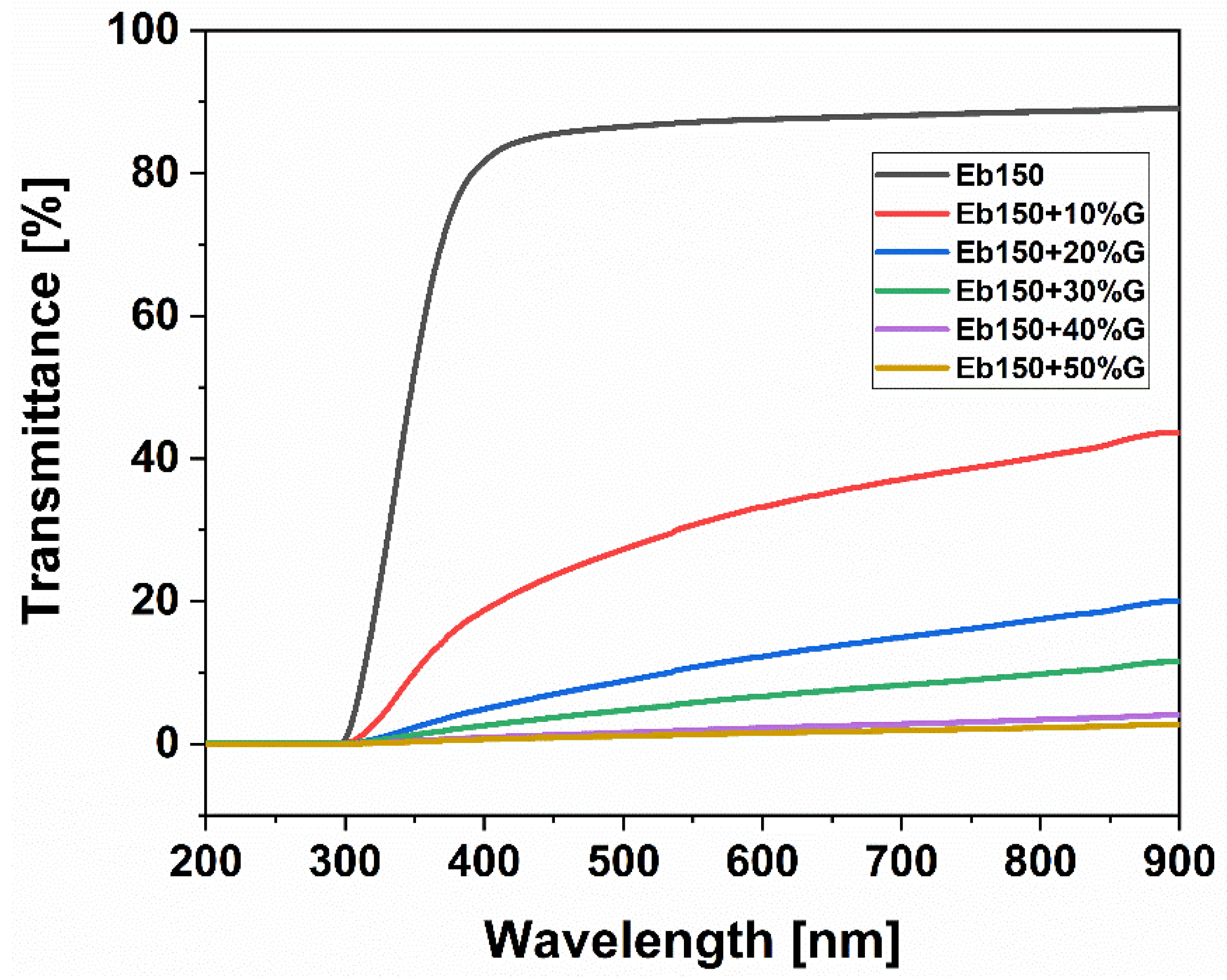
3.5. Optical Properties
3.6. Mechanical Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-art and future challenges of UV curable polymer-based smart materials for printing technologies. Adv. Mater. Technol. 2019, 4, 1800618. [Google Scholar] [CrossRef] [Green Version]
- Ligon-Auer, S.C.; Schwentenwein, M.; Gorsche, C.; Stampfl, J.; Liska, R. Toughening of photo-curable polymer networks: A review. Polym. Chem. 2016, 7, 257–286. [Google Scholar] [CrossRef]
- Decker, C. Photoinitiated crosslinking polymerisation. Prog. Polym. Sci. 1996, 21, 593–650. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Landry, V.; Blanchet, P.; Boivin, G.; Bouffard, J.-F.; Vlad, M. UV-LED curing efficiency of wood coatings. Coatings 2015, 5, 1019–1033. [Google Scholar] [CrossRef] [Green Version]
- Chu, Z.; Feng, Y.; Xie, B.; Yang, Y.; Hu, Y.; Zhou, X.; Yuan, T.; Yang, Z. Bio-based polyfunctional reactive diluent derived from tung oil by thiol-ene click reaction for high bio-content UV-LED curable coatings. Ind. Crops Prod. 2021, 160, 113117. [Google Scholar] [CrossRef]
- Strongone, V.; Bartoli, M.; Jagdale, P.; Arrigo, R.; Tagliaferro, A.; Malucelli, G. Preparation and characterization of UV-LED curable acrylic films containing biochar and/or multiwalled carbon nanotubes: Effect of the filler loading on the rheological, thermal and optical properties. Polymers 2020, 12, 796. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, R.; Bartoli, M.; Torsello, D.; Ghigo, G.; Malucelli, G. Thermal, dynamic-mechanical and electrical properties of UV-LED curable coatings containing porcupine-like carbon structures. Mater. Today Commun. 2021, 28, 102630. [Google Scholar] [CrossRef]
- Javadi, A.; Mehr, H.S.; Sobani, M.; Soucek, M.D. Cure-on-command technology: A review of the current state of the art. Prog. Org. Coat. 2016, 100, 2–31. [Google Scholar] [CrossRef]
- Seipel, S.; Yu, J.; Periyasamy, A.P.; Viková, M.; Vik, M.; Nierstrasz, V.A. Inkjet printing and UV-LED curing of photochromic dyes for functional and smart textile applications. RSC Adv. 2018, 8, 28395–28404. [Google Scholar] [CrossRef] [Green Version]
- Ghazali, S.K.; Adrus, N.; Majid, R.A.; Ali, F.; Jamaluddin, J. UV-LED as a new emerging tool for curable polyurethane acrylate hydrophobic coating. Polymers 2021, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, G. Synthesis and characterization of UV-LED curable nanocomposite coatings. Curr. Org. Chem. 2017, 21, 2314–2321. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Zhang, L.; Protsak, I.; Jamali, R.; Shu, Y.; Pal, P.; Wang, Z.; Yang, J.; Zhang, D. Fast-cured UV-LED polymer materials filled with high mineral contents as wear-resistant, antibacterial coatings. Chem. Eng. J. 2020, 382, 122927. [Google Scholar] [CrossRef]
- Schartel, B.; Schmaucks, G. Flame retardancy synergism in polymers through different inert fillers’ geometry. Polym. Eng. Sci. 2017, 57, 1099–1109. [Google Scholar] [CrossRef]
- Choudhary, S. Characterization of amorphous silica nanofiller effect on the structural, morphological, optical, thermal, dielectric and electrical properties of PVA–PVP blend based polymer nanocomposites for their flexible nanodielectric applications. J. Mater. Sci. Mater. Electron. 2018, 29, 10517–10534. [Google Scholar] [CrossRef]
- Ozcelik Kazancioglu, E.; Batibay, G.S.; Uner, A.; Arsu, N. Thermal and morphological investigation of the effect of POSS-(PEG2000)8 addition to UV curable PEGMEA/PEGDA formulation and simultaneously in situ formed silver nanoparticles. J. Polym. Sci. 2021, 59, 729–737. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A review of the synthesis and applications of polymer–nanoclay composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef] [Green Version]
- Karger-Kocsis, J.; Lendvai, L. Polymer/boehmite nanocomposites: A review. J. Appl. Polym. Sci. 2018, 135, 45573. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, B.; Gao, X.; Xu, D. A review of the interfacial characteristics of polymer nanocomposites containing carbon nanotubes. RSC Adv. 2018, 8, 28048–28085. [Google Scholar] [CrossRef] [Green Version]
- Tsebriienko, T.; Popov, A.I. Effect of poly(titanium oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and nanocellulose in polymer composite materials: A review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Maniadi, A.; Koudoumas, E.; Kenanakis, G.; Romanitan, C.; Tutunaru, O.; Suchea, M.; Kechagias, J. The mechanical and physical properties of 3D-printed materials composed of ABS-ZnO nanocomposites and ABS-ZnO microcomposites. Micromachines 2020, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Sepet, H.; Aydemir, B.; Tarakcioglu, N. Evaluation of mechanical and thermal properties and creep behavior of micro- and nano-CaCO3 particle-filled HDPE nano- and microcomposites produced in large scale. Polym. Bull. 2020, 77, 3677–3695. [Google Scholar] [CrossRef]
- Lee, Y.W.; Sinha, S.; Digonnet, M.J.F.; Byer, R.L.; Jiang, S. 20 W single-mode Yb3+-doped phosphate fiber laser. Opt. Lett. 2006, 31, 3255–3257. [Google Scholar] [CrossRef] [Green Version]
- Pugliese, D.; Boetti, N.G.; Lousteau, J.; Ceci-Ginistrelli, E.; Bertone, E.; Geobaldo, F.; Milanese, D. Concentration quenching in an Er-doped phosphate glass for compact optical lasers and amplifiers. J. Alloys Compd. 2016, 657, 678–683. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.H.; Suratwala, T.I. Nd-doped phosphate glasses for high-energy/high-peak-power lasers. J. Non-Cryst. Solids 2000, 263–264, 318–341. [Google Scholar] [CrossRef]
- Campbell, J.H.; Hayden, J.S.; Marker, A. High-power solid-state lasers: A laser glass perspective. Int. J. Appl. Glass Sci. 2011, 2, 3–29. [Google Scholar] [CrossRef]
- Ceci-Ginistrelli, E.; Pugliese, D.; Boetti, N.G.; Novajra, G.; Ambrosone, A.; Lousteau, J.; Vitale-Brovarone, C.; Abrate, S.; Milanese, D. Novel biocompatible and resorbable UV-transparent phosphate glass based optical fiber. Opt. Mater. Express 2016, 6, 2040–2051. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, W.; Zong, J.; Nguyen, D.; Norwood, R.A.; Chavez-Pirson, A.; Peyghambarian, N. 976 nm single-frequency distributed Bragg reflector fiber laser. Opt. Lett. 2012, 37, 4167–4169. [Google Scholar] [CrossRef]
- Yu, D.; Kleemeier, M.; Wu, G.M.; Schartel, B.; Liu, W.Q.; Hartwig, A. A low melting organic-inorganic glass and its effect on flame retardancy of clay/epoxy composites. Polymer 2011, 52, 2120–2131. [Google Scholar] [CrossRef]
- Yu, D.; Kleemeier, M.; Wu, G.M.; Schartel, B.; Liu, W.Q.; Hartwig, A. Phosphorus and silicon containing low-melting organic–inorganic glasses improve flame retardancy of epoxy/clay composites. Macromol. Mater. Eng. 2011, 296, 952–964. [Google Scholar] [CrossRef]
- Liu, W.; Pan, Y.-T.; Zhang, J.; Zhang, L.; Moya, J.S.; Cabal, B.; Wang, D.-Y. Low-melting phosphate glasses as flame-retardant synergists to epoxy: Barrier effects vs flame retardancy. Polym. Degrad. Stab. 2021, 185, 109495. [Google Scholar] [CrossRef]
- Signore, M.A.; De Pascali, C.; Duraccio, D.; Malucelli, G.; Fioravanti, A.; Melissano, E.; Martucci, M.C.; Masieri, M.; Siciliano, P.; Francioso, L. Synthesis and characterization of UV-curable nanocellulose/ZnO/AlN acrylic flexible films: Thermal, dynamic mechanical and piezoelectric response. J. Appl. Polym. Sci. 2021, 138, 49731. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Dickens, S.H. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent. Mater. 2001, 17, 71–79. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: New York, NY, USA, 2004; pp. 1–364. [Google Scholar]
- Nielsen, L.E.; Landel, R.F. Mechanical Properties of Polymers and Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 1–580. [Google Scholar]
- Ross, P.; Escobar, G.; Sevilla, G.; Quagliano, J. Micro and nanocomposites of polybutadiene-based polyurethane liners with mineral fillers and nanoclay: Thermal and mechanical properties. Open Chem. 2017, 15, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Yew, M.C.; Ramli Sulong, N.H.; Yew, M.K.; Amalina, M.A.; Johan, M.R. The formulation and study of the thermal stability and mechanical properties of an acrylic coating using chicken eggshell as a novel bio-filler. Prog. Org. Coat. 2013, 76, 1549–1555. [Google Scholar] [CrossRef]
- Kleps, T.; Jaroszynska, D.; Piaskiewicz, M. Influence of fillers on the thermal stability of elastomers. Thermogravimetric study. J. Therm. Anal. 1990, 36, 2257–2260. [Google Scholar] [CrossRef]
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Unal, H. Morphology and mechanical properties of composites based on polyamide 6 and mineral additives. Mater. Des. 2004, 25, 483–487. [Google Scholar] [CrossRef]
- Duraccio, D.; Strongone, V.; Malucelli, G.; Auriemma, F.; De Rosa, C.; Mussano, F.D.; Genova, T.; Faga, M.G. The role of alumina-zirconia loading on the mechanical and biological properties of UHMWPE for biomedical applications. Compos. Part B 2019, 164, 800–808. [Google Scholar] [CrossRef]
- Keaney, E.; Shearer, J.; Panwar, A.; Mead, J. Refractive index matching for high light transmission composite systems. J. Compos. Mater. 2018, 52, 3299–3307. [Google Scholar] [CrossRef]
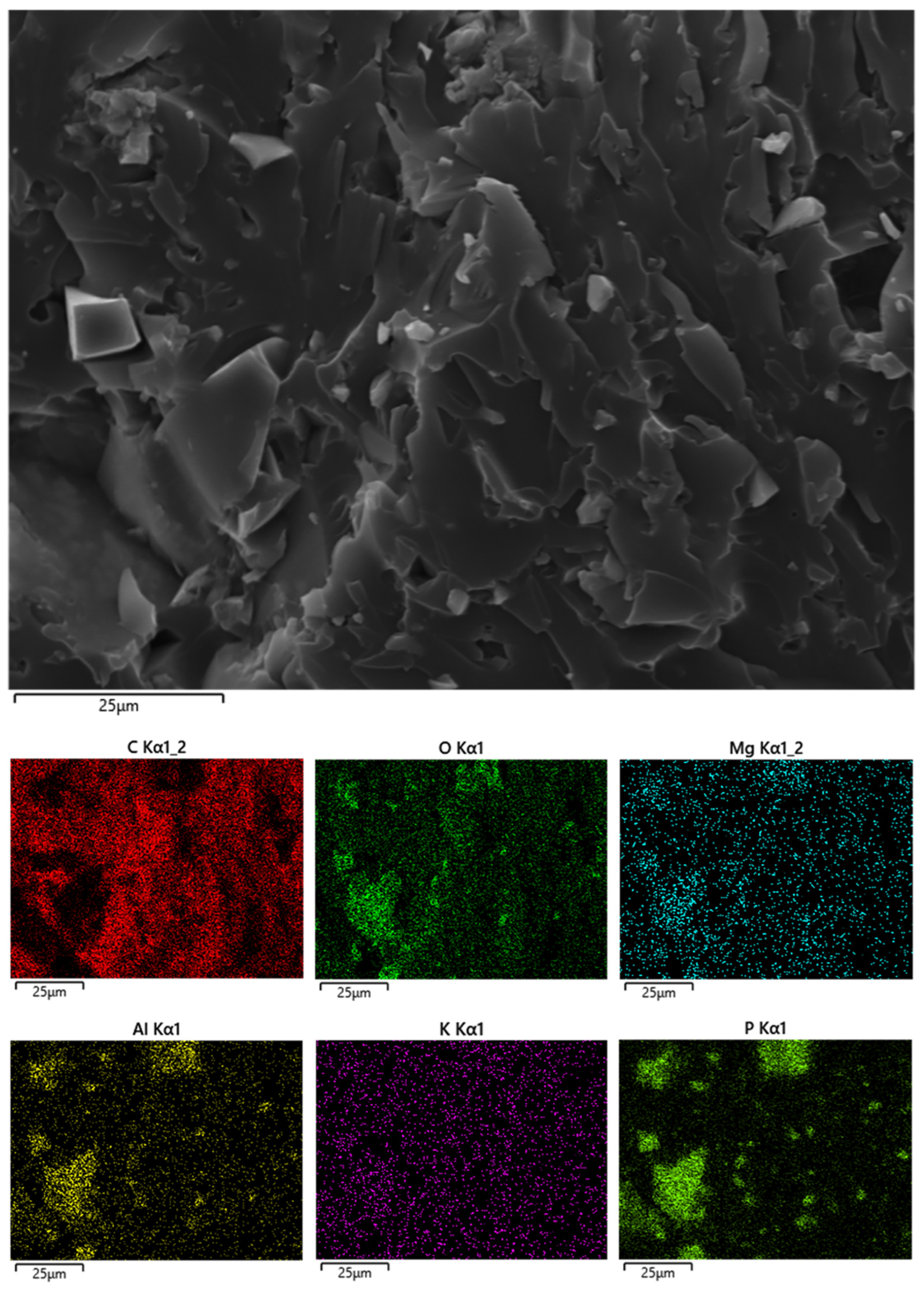


| Sample | Tg [°C] |
|---|---|
| Eb150 | 58.5 |
| Eb150 + 10%G | 59.3 |
| Eb150 + 20%G | 59.5 |
| Eb150 + 30%G | 61.0 |
| Eb150 + 40%G | 62.1 |
| Eb150 + 50%G | 65.1 |
| Atmosphere: Nitrogen | ||||||
|---|---|---|---|---|---|---|
| Sample | T5% [°C] | Tmax 1 * [°C] | Residue @ Tmax 1 [%] | Tmax 2 * [°C] | Residue @ Tmax 2 [%] | Residue @ 700 °C [%] |
| Eb150 | 299 | 441 | 40.0 | - | - | 4.1 |
| Eb150 + 10%G | 312 | 439 | 48.8 | - | - | 14.3 |
| Eb150 + 20%G | 319 | 440 | 53.6 | - | - | 24.4 |
| Eb150 + 30%G | 331 | 439 | 60.1 | - | - | 34.9 |
| Eb150 + 40%G | 369 | 435 | 67.3 | - | - | 44.2 |
| Eb150 + 50%G | 377 | 433 | 73.1 | - | - | 53.8 |
| Atmosphere: Air | ||||||
| Sample | T5% [°C] | Tmax 1* [°C] | Residue @ Tmax 1 [%] | Tmax 2* [°C] | Residue @ Tmax 2 [%] | Residue @ 700 °C [%] |
| Eb150 | 295 | 430 | 48.3 | 559 | 8.1 | 0.0 |
| Eb150 + 10%G | 301 | 431 | 50.4 | 553 | 27.2 | 14.0 |
| Eb150 + 20%G | 308 | 430 | 52.3 | 549 | 34.4 | 23.8 |
| Eb150 + 30%G | 326 | 433 | 54.1 | 556 | 40.1 | 34.2 |
| Eb150 + 40%G | 330 | 431 | 64.2 | 547 | 47.8 | 43.3 |
| Eb150 + 50%G | 345 | 431 | 74.6 | 547 | 56.4 | 53.7 |
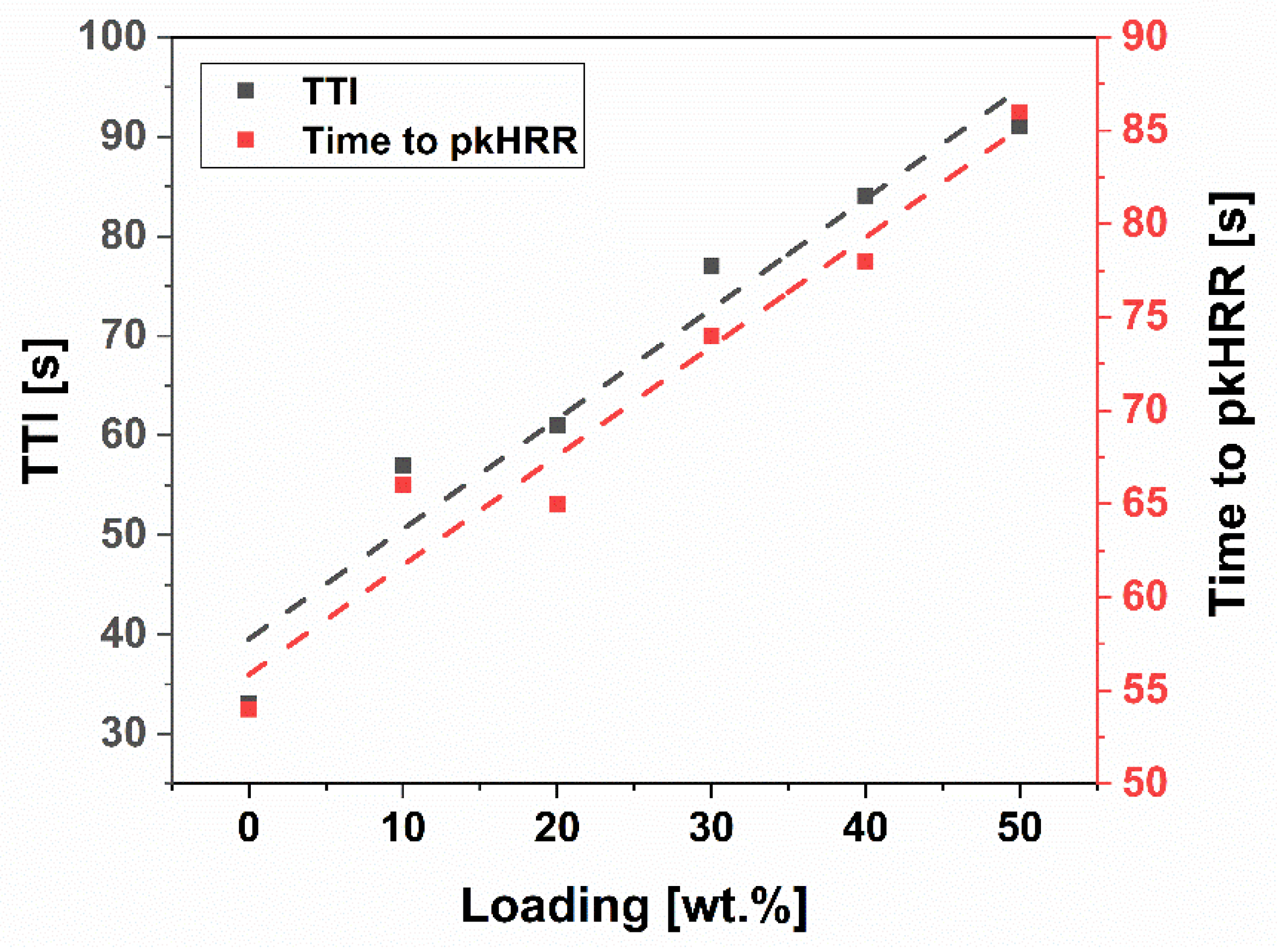
| Sample | TTI [s] | Time to pkHRR [s] | pkHRR [kW m−2] | THR [MJ m−2] | TSR [m2 m−2] | SEA [m2 kg−1] | CO/CO2 | Residue Mass [%] |
|---|---|---|---|---|---|---|---|---|
| Eb150 | 33 | 54 | 297 | 4.32 | 260 | 1007 | 0.40 | 1.7 |
| Eb150 + 10%G | 57 | 66 | 277 | 4.09 | 227 | 980 | 0.48 | 16.2 |
| Eb150 + 20%G | 61 | 65 | 212 | 4.24 | 215 | 875 | 0.46 | 27.4 |
| Eb150 + 30%G | 77 | 74 | 200 | 3.17 | 176 | 628 | 0.46 | 36.8 |
| Eb150 + 40%G | 84 | 78 | 182 | 2.98 | 145 | 534 | 0.47 | 47.7 |
| Eb150 + 50%G | 91 | 86 | 166 | 2.90 | 123 | 442 | 0.47 | 59.3 |
| Sample | n @ 633 nm ± 0.001 | n @ 825 nm ± 0.001 |
|---|---|---|
| Eb150 | 1.565 | 1.556 |
| Eb150 + 10%G | 1.568 | 1.559 |
| Eb150 + 20%G | 1.566 | 1.554 |
| Eb150 + 30%G | 1.563 | 1.556 |
| Eb150 + 40%G | 1.564 | 1.554 |
| Eb150 + 50%G | 1.563 | 1.554 |
| Sample | Young’s Modulus [MPa] | Elongation @break [%] | Pencil Hardness |
|---|---|---|---|
| Eb150 | 2280 ± 65 | 13.0 ± 0.6 | 2B |
| Eb150 + 10%G | 2455 ± 88 | 8.4 ± 0.5 | B |
| Eb150 + 20%G | 2870 ± 84 | 7.1 ± 0.7 | HB |
| Eb150 + 30%G | 3005 ± 96 | 5.3 ± 1.0 | H |
| Eb150 + 40%G | 3280 ± 112 | 3.4 ± 0.9 | 2H |
| Eb150 + 50%G | 3450 ± 127 | 2.8 ± 0.9 | 3H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, D.; Malucelli, G. UV-LED Curable Acrylic Films Containing Phosphate Glass Powder: Effect of the Filler Loading on the Thermal, Optical, Mechanical and Flame Retardant Properties. Polymers 2022, 14, 1899. https://doi.org/10.3390/polym14091899
Pugliese D, Malucelli G. UV-LED Curable Acrylic Films Containing Phosphate Glass Powder: Effect of the Filler Loading on the Thermal, Optical, Mechanical and Flame Retardant Properties. Polymers. 2022; 14(9):1899. https://doi.org/10.3390/polym14091899
Chicago/Turabian StylePugliese, Diego, and Giulio Malucelli. 2022. "UV-LED Curable Acrylic Films Containing Phosphate Glass Powder: Effect of the Filler Loading on the Thermal, Optical, Mechanical and Flame Retardant Properties" Polymers 14, no. 9: 1899. https://doi.org/10.3390/polym14091899