Customizable Collagen Vitrigel Membranes and Preliminary Results in Corneal Engineering
Abstract
:1. Introduction
2. Methods
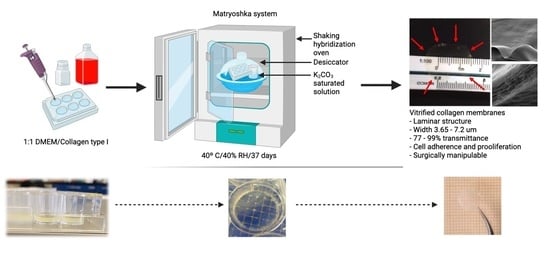
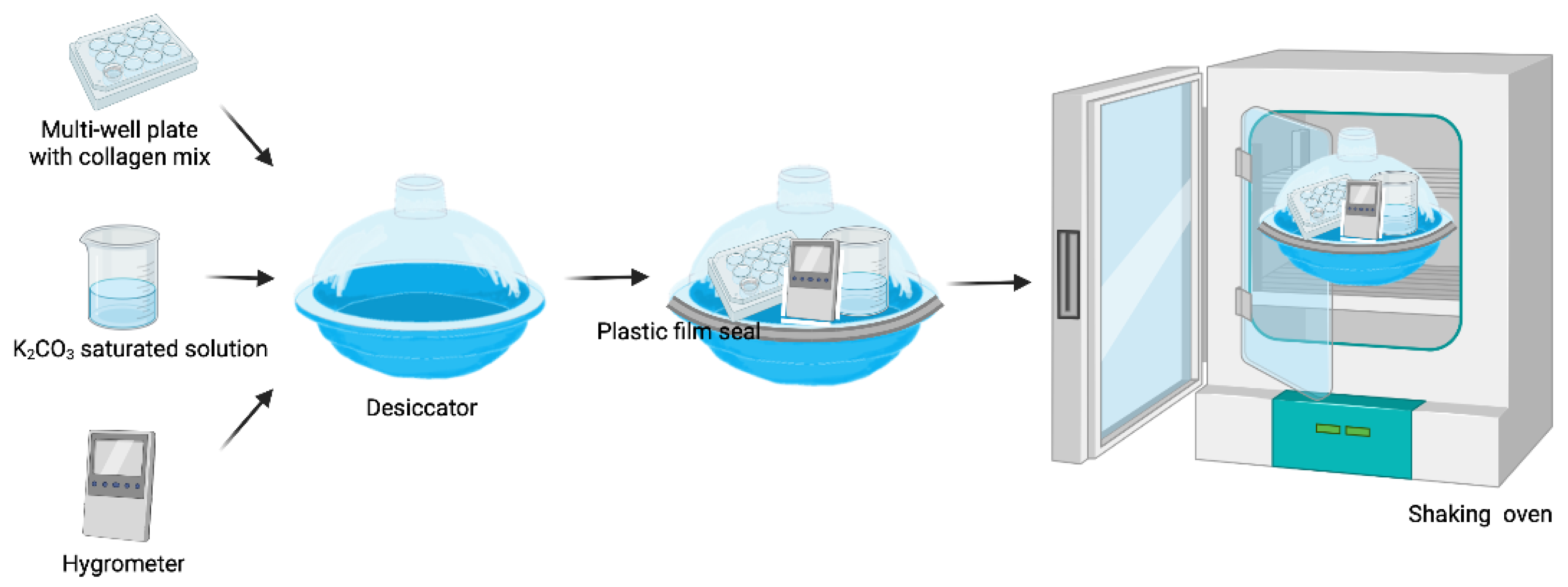
2.1. Matryoshka System Assembly for the Production of Collagen Membranes
2.2. Collagen-Based Membrane Production
2.2.1. Collagen Gel Preparation
2.2.2. Desiccation
2.2.3. Membrane Re Hydration
2.3. Collagen-Based Membrane Characterization
2.3.1. Optical Microscopy
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. D Confocal Microscopy
2.3.4. In Vitro Cell Adherence, Viability, and Cytotoxicity
2.3.5. Fourier-Transformed Infrared Spectra (FTIR)
2.3.6. Transmittance Analysis
2.3.7. X-ray Diffraction Analysis (XRD)
2.3.8. Ex Vivo Surgical Manipulation Test
2.4. Engineered Corneal Endothelium Assembling
2.5. Pilot Study of the Transplantation of Engineered Corneal Endothelium in an Animal Model
Statistical Analysis
3. Results
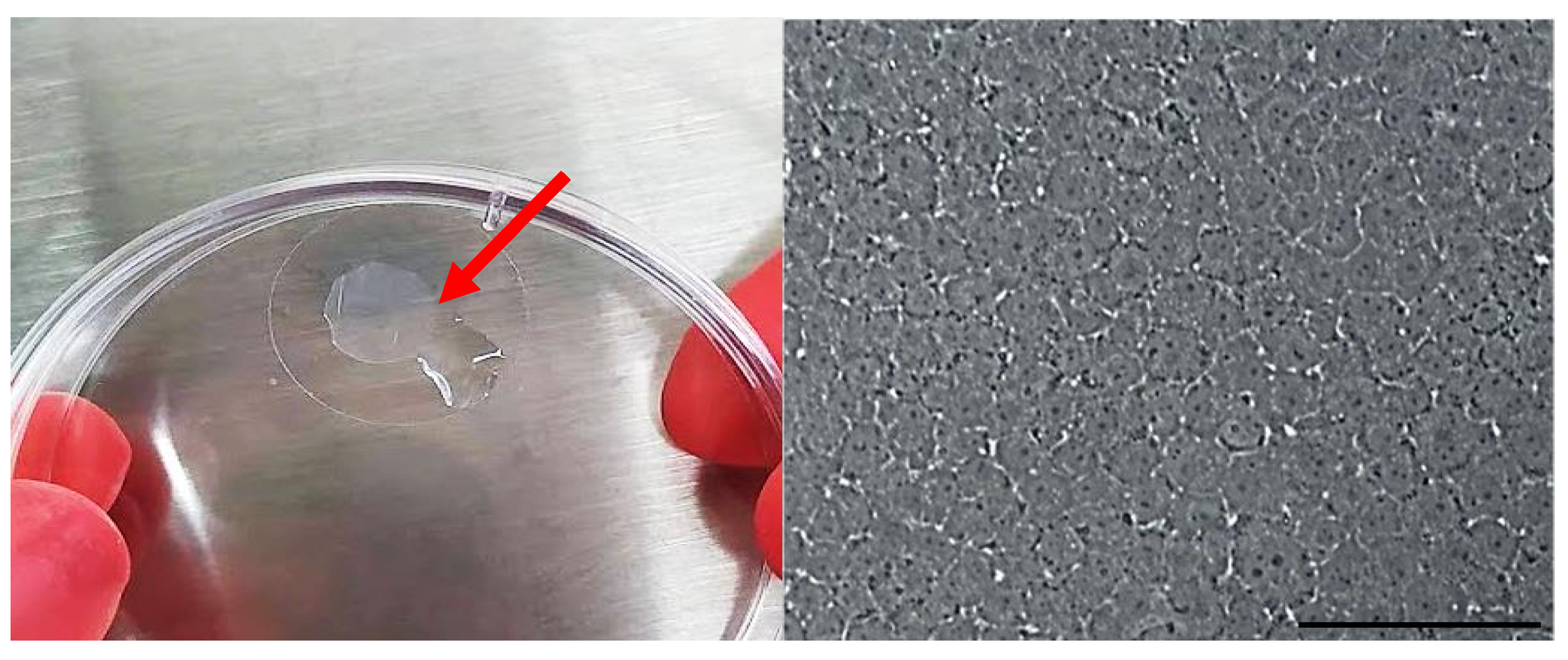
3.1. Matryoshka System Assembling and Stabilization
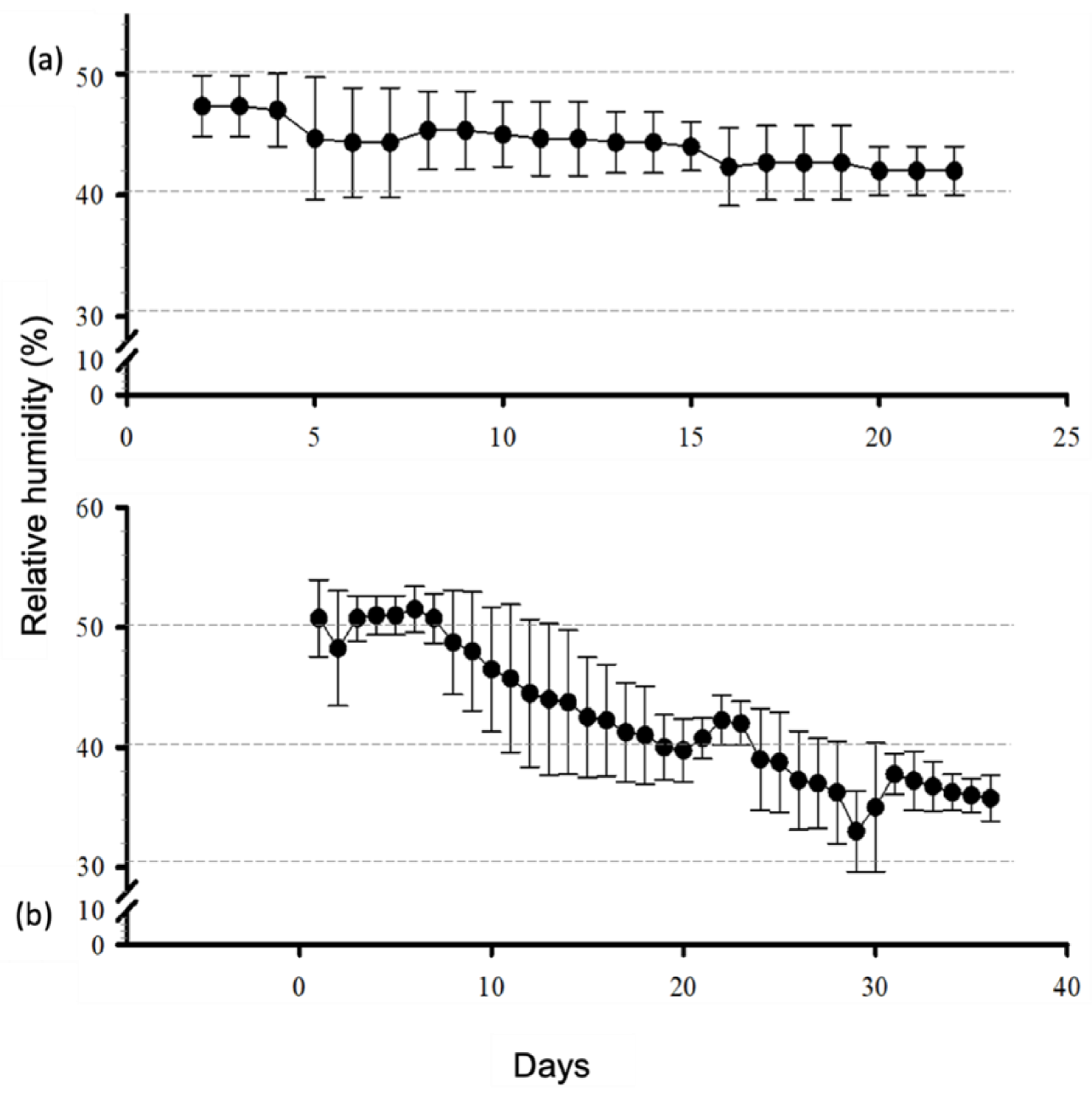
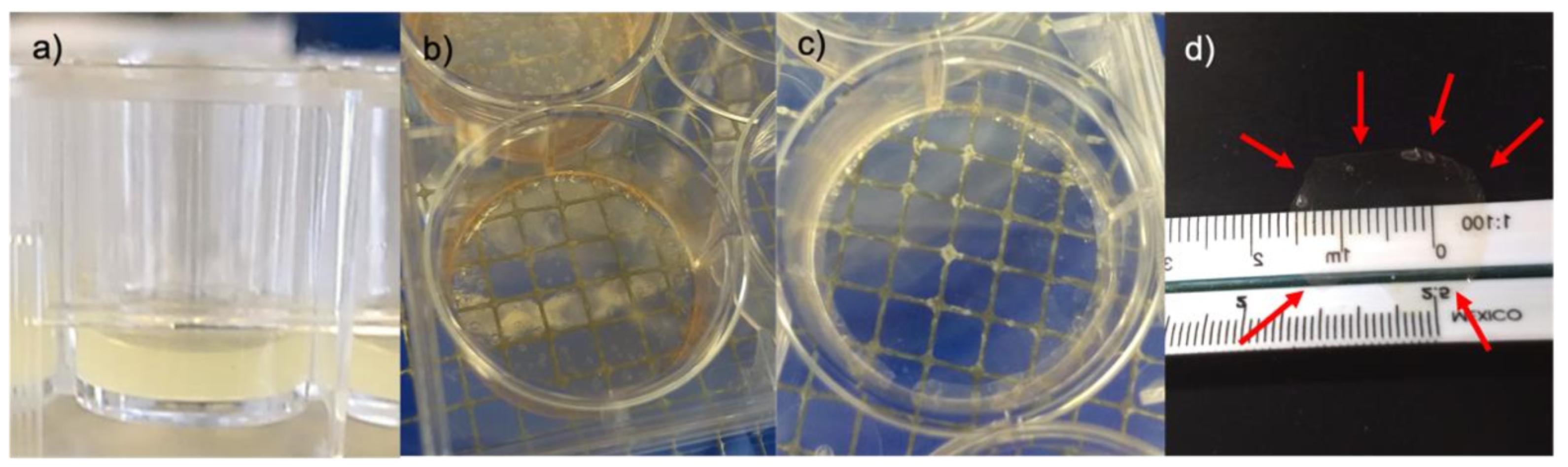
3.2. Membrane Desiccation and Rehydration
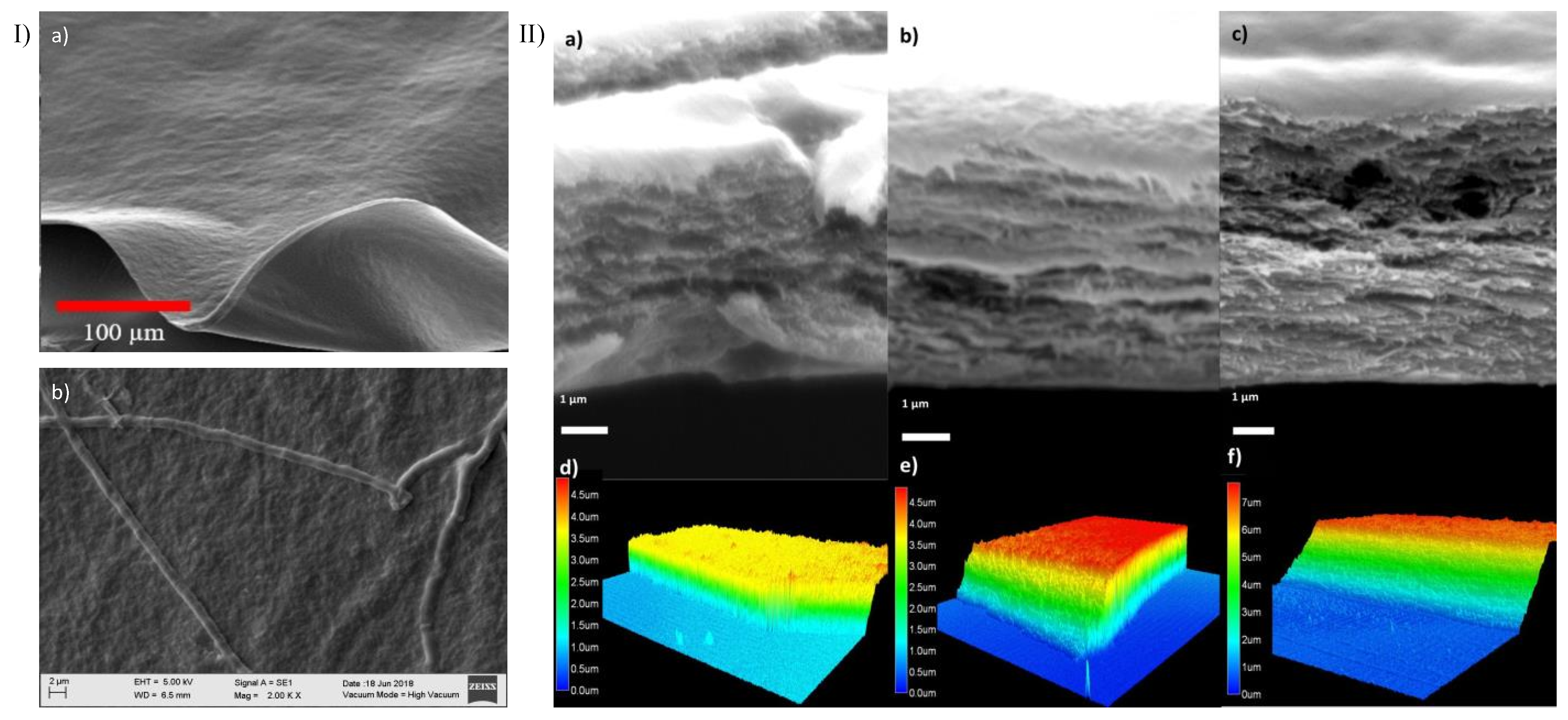
3.3. Confocal, Optical, and SEM Characterization
3.4. Membrane Characterization: Cell Viability, IR Spectra, Transmittance, and X-ray Diffraction
3.5. Wet Lab with Ex Vivo Model for Surgical Manipulation Test
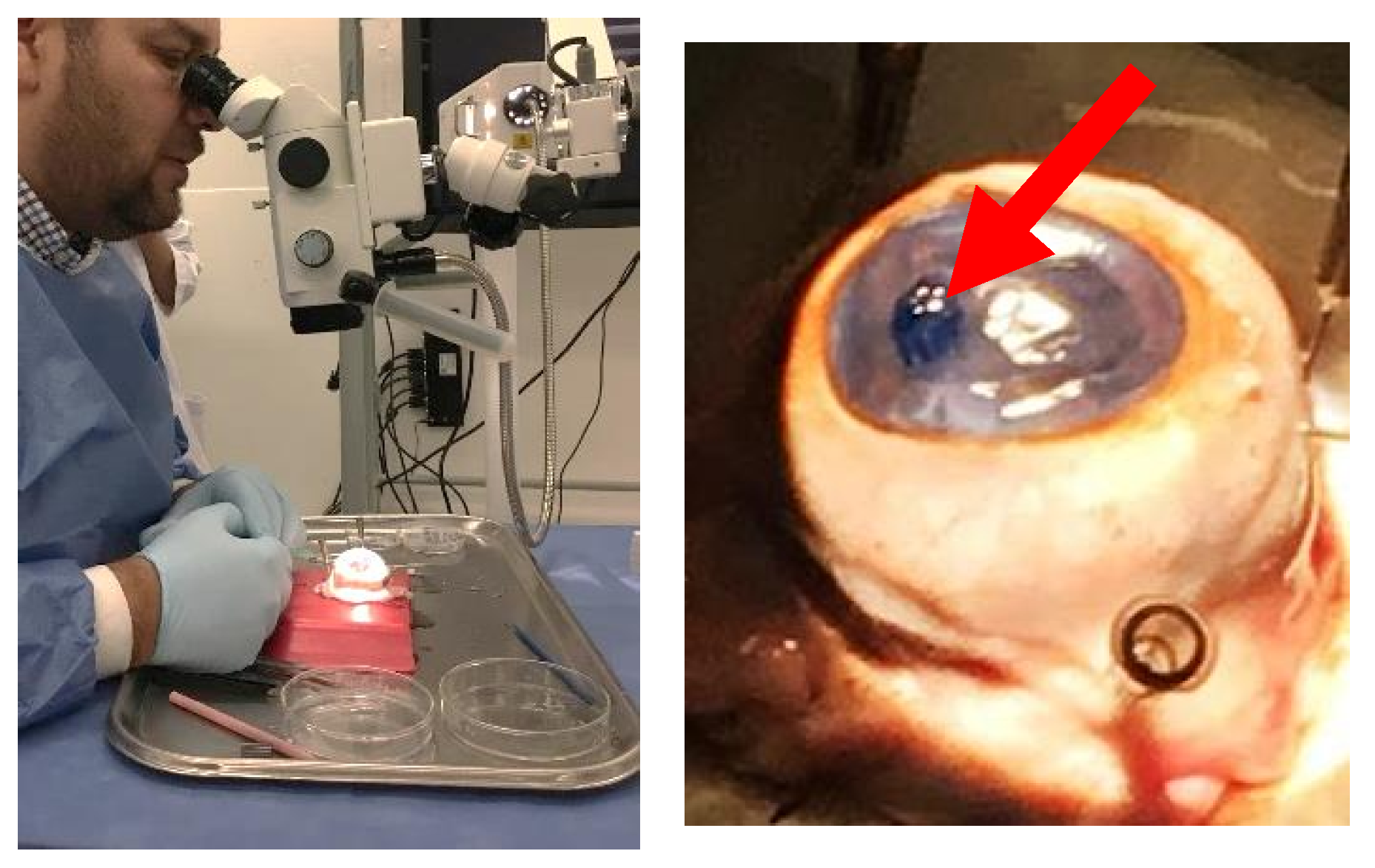
3.6. Engineered Corneal Endothelium
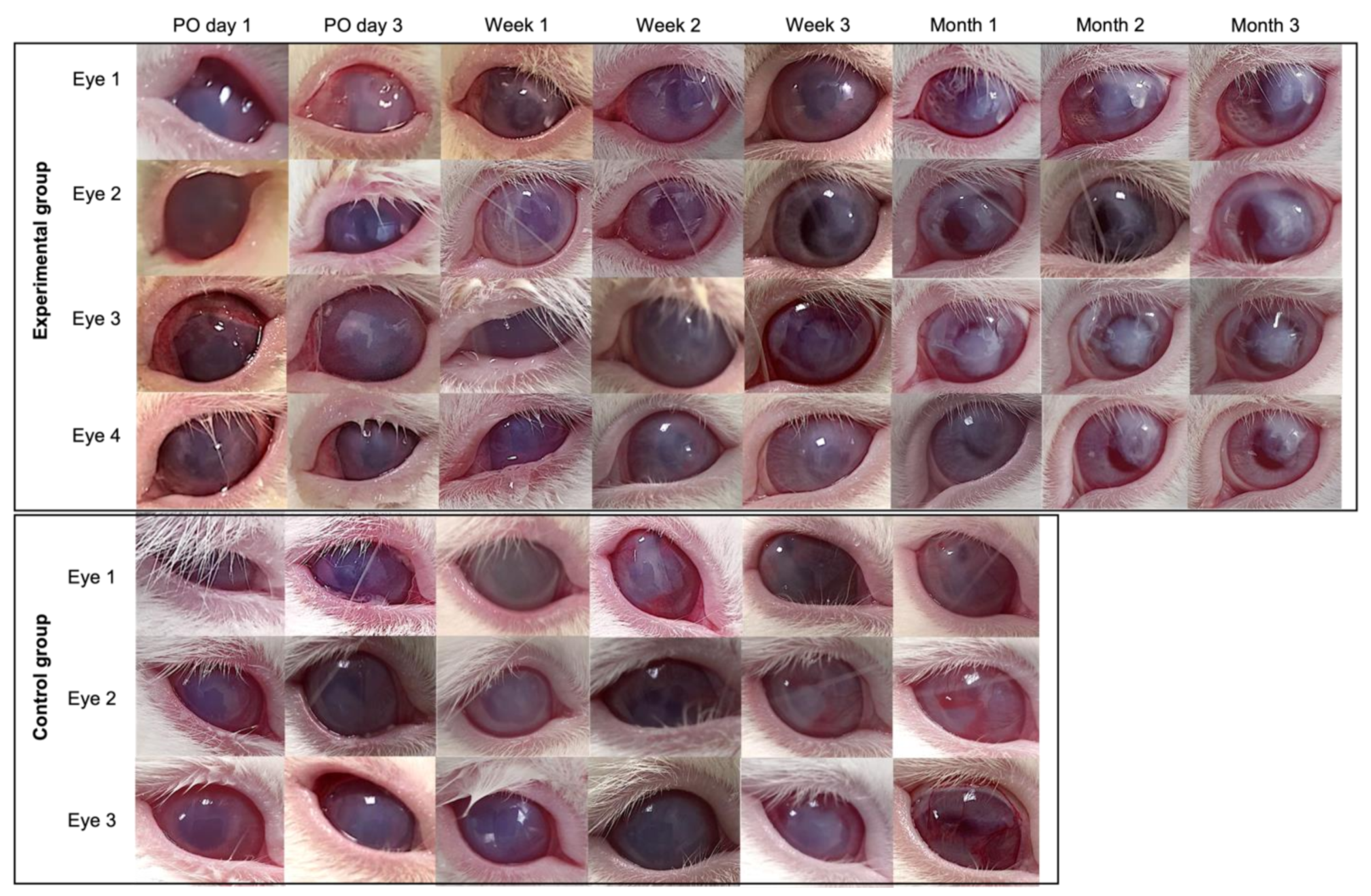
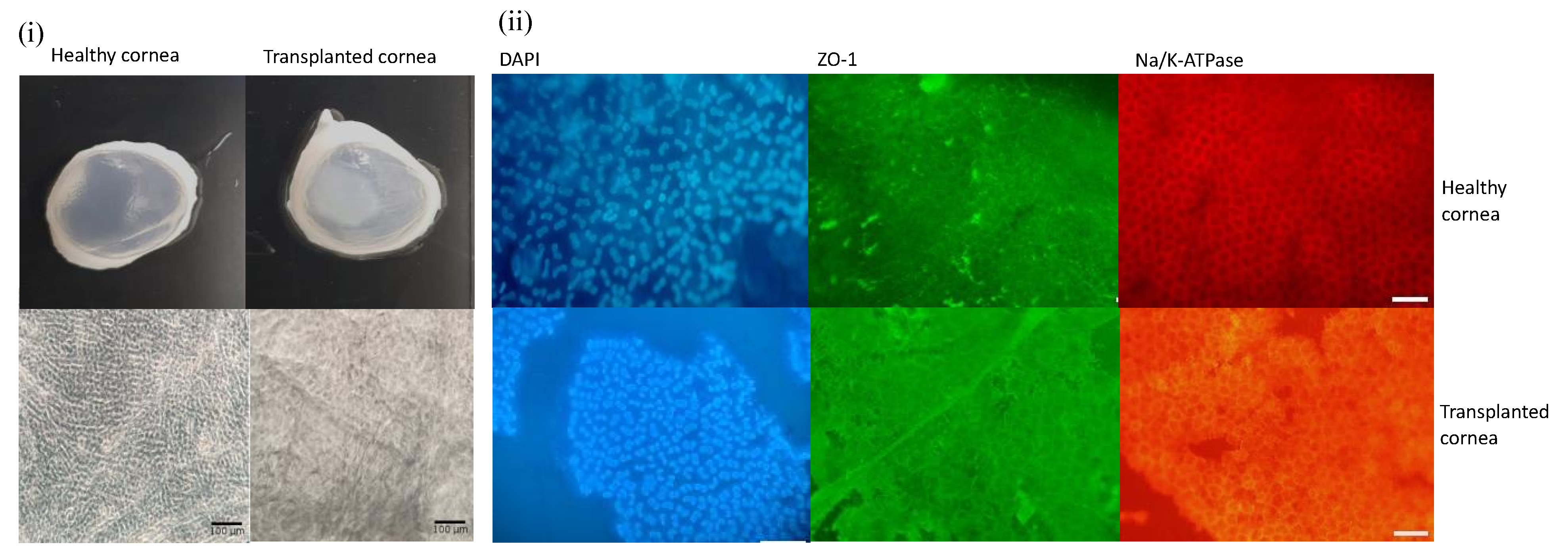
3.7. Pilot Transplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wong, Y.L.; Walkden, A. Current Perspectives on Corneal Transplantation. Clin. Ophthalmol. 2022, 16, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.A.; Steinmetz, J.D.; Saylan, M.; Mersha, A.M.; Weldemariam, A.H.; Wondmeneh, T.G.; Sreeramareddy, C.T.; Pinheiro, M.; Yaseri, M.; Yu, C.; et al. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet. Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Li, Z.; Wang, T.; Liu, P. Prevalence and Causes of Corneal Blindness. Clin. Exp. Ophthalmol. 2014, 42, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Zavala, J.; López Jaime, G.R.; Rodríguez Barrientos, C.A.; Valdez-Garcia, J. Corneal Endothelium: Developmental Strategies for Regeneration. Eye 2013, 27, 579–588. [Google Scholar] [CrossRef]
- Ong, H.S.; Ang, M.; Mehta, J. Evolution of Therapies for the Corneal Endothelium: Past, Present and Future Approaches. Br. J. Ophthalmol. 2021, 105, 454. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Usui, T.; Tanaka, K.; Hattori, S.; Irie, S.; Miyata, K.; Araie, M.; Amano, S. Cultured Human Corneal Endothelial Cell Transplantation with a Collagen Sheet in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2992–2997. [Google Scholar] [CrossRef]
- Levis, H.J.; Peh, G.S.L.; Toh, K.P.; Poh, R.; Shortt, A.J.; Drake, R.A.L.; Mehta, J.S.; Daniels, J.T. Plastic Compressed Collagen as a Novel Carrier for Expanded Human Corneal Endothelial Cells for Transplantation. PLoS ONE 2012, 7, 11. [Google Scholar] [CrossRef]
- Duncan, T.J.; Tanaka, Y.; Shi, D.; Kubota, A.; Quantock, A.J.; Nishida, K. Flow-Manipulated, Crosslinked Collagen Gels for Use as Corneal Equivalents. Biomaterials 2010, 31, 8996–9005. [Google Scholar] [CrossRef]
- Walckling, M.; Waterstradt, R.; Baltrusch, S. Collagen Remodeling Plays a Pivotal Role in Endothelial Corneal Dystrophies. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef] [PubMed]
- Nocera, A.D.; Comín, R.; Salvatierra, N.A.; Cid, M.P. Development of 3D Printed Fibrillar Collagen Scaffold for Tissue Engineering. Biomed. Microdevices 2018, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.M.; Ianchis, R.; Alexa, R.L.; Gifu, I.C.; Kaya, M.G.A.; Savu, D.I.; Popescu, R.C.; Alexandrescu, E.; Ninciuleanu, C.M.; Preda, S.; et al. Development of New Collagen/Clay Composite Biomaterials. Int. J. Mol. Sci. 2022, 23, 401. [Google Scholar] [CrossRef]
- Dems, D.; Rodrigues Da Silva, J.; Hélary, C.; Wien, F.; Marchand, M.; Debons, N.; Muller, L.; Chen, Y.; Schanne-Klein, M.C.; Laberty-Robert, C.; et al. Native Collagen: Electrospinning of Pure, Cross-Linker-Free, Self-Supported Membrane. ACS Appl. Bio Mater. 2020, 3, 2948–2957. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Tunable Collagen I Hydrogels for Engineered Physiological Tissue Micro-Environments. PLoS ONE 2015, 10, e0122500. [Google Scholar] [CrossRef]
- Takezawa, T.; Ozaki, K.; Nitani, A.; Takabayashi, C.; Shimo-Oka, T. Collagen Vitrigel: A Novel Scaffold That Can Facilitate a Three-Dimensional Culture for Reconstructing Organoids. Cell Transpl. 2004, 13, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Colón, X.; Xia, Z.; Breidenich, J.L.; Mulreany, D.G.; Guo, Q.; Uy, O.M.; Tiffany, J.E.; Freund, D.E.; McCally, R.L.; Schein, O.D.; et al. Structure and Properties of Collagen Vitrigel Membranes for Ocular Repair and Regeneration Applications. Biomaterials 2012, 33, 8286–8295. [Google Scholar] [CrossRef]
- Ambrose, W.M.I.; Salahuddin, A.; So, S.; Ng, S.; Márquez, S.P.; Takezawa, T.; Schein, O.; Elisseeff, J. Collagen Vitrigel Membranes for the In Vitro Reconstruction of Separate Corneal Epithelial, Stromal, and Endothelial Cell Layers. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 818–831. [Google Scholar] [CrossRef]
- Ni, M.; Tong, W.H.; Choudhury, D.; Rahim, N.A.A.; Iliescu, C.; Yu, H. Cell Culture on MEMS Platforms: A Review. Int. J. Mol. Sci. 2009, 10, 5411. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev. 2014, 20, 683. [Google Scholar] [CrossRef] [Green Version]
- Hollister, S.J. Scaffold Engineering: A Bridge to Where? Biofabrication 2009, 1, 012001. [Google Scholar] [CrossRef] [PubMed]
- Girlanda, R. Deceased Organ Donation for Transplantation: Challenges and Opportunities. World J. Transplant. 2016, 6, 451. [Google Scholar] [CrossRef] [PubMed]
- PAHO/WHO|Pan American Health Organization. Organ Donation and Transplants. Available online: https://www.paho.org/en/topics/organ-donation-and-transplants (accessed on 25 May 2022).
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Li, X.; Xiao, Z.; Yao, Y.; Chu, Y.; Farkas, B.; Romano, I.; Brandi, F.; Dai, J. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv. Healthc. Mater. 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Composite Calcium Phosphate and Collagen Scaffolds for Bone Regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Sensini, A.; Gualandi, C.; Zucchelli, A.; Boyle, L.A.; Kao, A.P.; Reilly, G.C.; Tozzi, G.; Cristofolini, L.; Focarete, M.L. Tendon Fascicle-Inspired Nanofibrous Scaffold of Polylactic Acid/Collagen with Enhanced 3D-Structure and Biomechanical Properties. Sci. Rep. 2018, 8, 17167. [Google Scholar] [CrossRef]
- Bertram, U.; Steiner, D.; Poppitz, B.; Dippold, D.; Köhn, K.; Beier, J.P.; Detsch, R.; Boccaccini, A.R.; Schubert, D.W.; Horch, R.E.; et al. Vascular Tissue Engineering: Effects of Integrating Collagen into a PCL Based Nanofiber Material. Biomed. Res. Int. 2017, 2017, 9616939. [Google Scholar] [CrossRef]
- Do Amaral, R.J.F.C.; Zayed, N.M.A.; Pascu, E.I.; Cavanagh, B.; Hobbs, C.; Santarella, F.; Simpson, C.R.; Murphy, C.M.; Sridharan, R.; González-Vázquez, A.; et al. Functionalising Collagen-Based Scaffolds With Platelet-Rich Plasma for Enhanced Skin Wound Healing Potential. Front. Bioeng. Biotechnol. 2019, 7, 371. [Google Scholar] [CrossRef]
- Montalvo-Parra, M.D.; Vidal-Paredes, I.A.; Calzada-Rodríguez, C.E.; Cárdenas-Rodríguez, I.T.; Torres-Guerrero, G.F.; Gómez-Elizondo, D.; López-Martínez, M.; Zavala, J.; Valdez-García, J.E. Experimental Design of a Culture Approach for Corneal Endothelial Cells of New Zealand White Rabbit. Heliyon 2020, 6, 10. [Google Scholar] [CrossRef]
- Rodríguez-Barrientos, C.-A.; Trevino, V.; Zavala, J.; Montalvo-Parra, M.-D.; Guerrero-Ramírez, G.-I.; Aguirre-Gamboa, R.; Valdez-García, J.-E. Arresting Proliferation Improves the Cell Identity of Corneal Endothelial Cells in the New Zealand Rabbit. Mol. Vis. 2019, 25, 745–755. [Google Scholar]
- Valdez-García, J.E.; Mendoza, G.; Zavala, J.; Zavala-Pompa, A.; Brito, G.; Cortés-Ramírez, J.A.; Elisseeff, J. In Vivo Biocompatibility of Chitosan and Collagen–vitrigel Membranes for Corneal Scaffolding: A Comparative Analysis. Curr. Tissue Eng. 2015, 5, 123–129. [Google Scholar] [CrossRef]
- Valdez-Garcia, J.E.; Lozano-Ramirez, J.F.; Zavala, J. Adult White New Zealand Rabbit as Suitable Model for Corneal Endothelial Engineering. BMC Res. Notes 2015, 8, 28. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Baba, K.; Duncan, T.J.; Kubota, A.; Asahi, T.; Quantock, A.J.; Yamato, M.; Okano, T.; Nishida, K. Transparent, Tough Collagen Laminates Prepared by Oriented Flow Casting, Multi-Cyclic Vitrification and Chemical Cross-Linking. Biomaterials 2011, 32, 3358–3366. [Google Scholar] [CrossRef]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen Fibril Formation. Biochem. J. 1996, 316 Pt 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Long, K.; Liu, Y.; Li, W.; Wang, L.; Liu, S.; Wang, Y.; Wang, Z.; Ren, L. Improving the Mechanical Properties of Collagen-Based Membranes Using Silk Fibroin for Corneal Tissue Engineering. J. Biomed. Mater. Res. A 2015, 103, 1159–1168. [Google Scholar] [CrossRef]
- Chae, J.J.; McIntosh Ambrose, W.; Espinoza, F.A.; Mulreany, D.G.; Ng, S.; Takezawa, T.; Trexler, M.M.; Schein, O.D.; Chuck, R.S.; Elisseeff, J.H. Regeneration of Corneal Epithelium Utilizing a Collagen Vitrigel Membrane in Rabbit Models for Corneal Stromal Wound and Limbal Stem Cell Deficiency. Acta Ophthalmol. 2015, 93, e57–e66. [Google Scholar] [CrossRef]
- Huibertus van Essen, T.; Lin, C.C.; Hussain, A.K.; Maas, S.; Lai, H.J.; Linnartz, H.; van den Berg, T.J.T.P.; Salvatori, D.C.F.; Luyten, G.P.M.; Jager, M.J. A Fish Scale-Derived Collagen Matrix as Artificial Cornea in Rats: Properties and Potential. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3224–3233. [Google Scholar] [CrossRef]
- Vázquez, N.; Chacón, M.; Rodríguez-Barrientos, C.A.; Merayo-Lloves, J.; Naveiras, M.; Baamonde, B.; Alfonso, J.F.; Zambrano-Andazol, I.; Riestra, A.C.; Meana, Á. Human Bone Derived Collagen for the Development of an Artificial Corneal Endothelial Graft. In Vivo Results in a Rabbit Model. PLoS ONE 2016, 11, e0167578. [Google Scholar] [CrossRef]
- Achilli, M.; Mantovani, D. Tailoring Mechanical Properties of Collagen-Based Scaffolds for Vascular Tissue Engineering: The Effects of PH, Temperature and Ionic Strength on Gelation. Polymers 2010, 2, 664–680. [Google Scholar] [CrossRef]
- Offeddu, G.S.; Ashworth, J.C.; Cameron, R.E.; Oyen, M.L. Multi-Scale Mechanical Response of Freeze-Dried Collagen Scaffolds for Tissue Engineering Applications. J. Mech. Behav. Biomed. Mater. 2015, 42, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.; Li, W.; Lewis, R.V.; Segre, C.U.; Wang, R. E-Spun Composite Fibers of Collagen and Dragline Silk Protein: Fiber Mechanics, Biocompatibility, and Application in Stem Cell Differentiation. Biomacromolecules 2015, 16, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, L.; Wang, Y. Crosslinked Collagen-Gelatin-Hyaluronic Acid Biomimetic Film for Cornea Tissue Engineering Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.F.; Capaldi, M.J.; Chapman, J.A. Reconstitution of Collagen Fibrils in Vitro; the Assembly Process Depends on the Initiating Procedure. Int. J. Biol. Macromol. 1986, 8, 161–166. [Google Scholar] [CrossRef]
- Christiansen, D.L.; Huang, E.K.; Silver, F.H. Assembly of Type I Collagen: Fusion of Fibril Subunits and the Influence of Fibril Diameter on Mechanical Properties. Matrix Biol. 2000, 19, 409–420. [Google Scholar] [CrossRef]
- Nair, M.; Calahorra, Y.; Kar-Narayan, S.; Best, S.M.; Cameron, R.E. Self-Assembly of Collagen Bundles and Enhanced Piezoelectricity Induced by Chemical Crosslinking†. Nanoscale 2019, 11, 15120. [Google Scholar] [CrossRef]
- Beems, E.M.; van Best, J.A. Light Transmission of the Cornea in Whole Human Eyes. Exp. Eye Res. 1990, 50, 393–395. [Google Scholar] [CrossRef]
- Park, S.N.; Park, J.C.; Kim, H.O.; Song, M.J.; Suh, H. Characterization of Porous Collagen/Hyaluronic Acid Scaffold Modified by 1-Ethyl-3-(3-Dimethylaminopropyl)Carbodiimide Cross-Linking. Biomaterials 2002, 23, 1205–1212. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, E.Y.; Kim, M.J.; Giegengack, M.; Khan, F.A.; Khang, G.; Soker, S. In Vitro Evaluation of the Interactions between Human Corneal Endothelial Cells and Extracellular Matrix Proteins. Biomed. Mater. 2013, 8, 014108. [Google Scholar] [CrossRef]
- Spinozzi, D.; Miron, A.; Lie, J.T.; Rafat, M.; Lagali, N.; Melles, G.R.J.; Dhubhghaill, S.N.; Dapena, I.; Oellerich, S. In Vitro Evaluation and Transplantation of Human Corneal Endothelial Cells Cultured on Biocompatible Carriers. Cell Transpl. 2020, 29, 963689720923577. [Google Scholar] [CrossRef]
- Srirampur, A.; Mansoori, T. A Simplified Ex Vivo Model to Learn the Correct Orientation of Descemet Membrane Endothelial Graft. Indian J. Ophthalmol. 2021, 69, 151–152. [Google Scholar] [CrossRef]
- Yoshida, J.; Yokoo, S.; Oshikata-Miyazaki, A.; Amano, S.; Takezawa, T.; Yamagami, S. Transplantation of Human Corneal Endothelial Cells Cultured on Bio-Engineered Collagen Vitrigel in a Rabbit Model of Corneal Endothelial Dysfunction. Curr. Eye Res. 2017, 42, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing Scaffolds for Corneal Regeneration. Adv. Funct. Mater. 2020, 30, 1908996. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalvo-Parra, M.D.; Ortega-Lara, W.; Loya-García, D.; Bustamante-Arias, A.; Guerrero-Ramírez, G.-I.; Calzada-Rodríguez, C.E.; Torres-Guerrero, G.F.; Hernández-Sedas, B.; Cárdenas-Rodríguez, I.T.; Guevara-Quintanilla, S.E.; et al. Customizable Collagen Vitrigel Membranes and Preliminary Results in Corneal Engineering. Polymers 2022, 14, 3556. https://doi.org/10.3390/polym14173556
Montalvo-Parra MD, Ortega-Lara W, Loya-García D, Bustamante-Arias A, Guerrero-Ramírez G-I, Calzada-Rodríguez CE, Torres-Guerrero GF, Hernández-Sedas B, Cárdenas-Rodríguez IT, Guevara-Quintanilla SE, et al. Customizable Collagen Vitrigel Membranes and Preliminary Results in Corneal Engineering. Polymers. 2022; 14(17):3556. https://doi.org/10.3390/polym14173556
Chicago/Turabian StyleMontalvo-Parra, María Dolores, Wendy Ortega-Lara, Denise Loya-García, Andrés Bustamante-Arias, Guillermo-Isaac Guerrero-Ramírez, Cesar E. Calzada-Rodríguez, Guiomar Farid Torres-Guerrero, Betsabé Hernández-Sedas, Italia Tatnaí Cárdenas-Rodríguez, Sergio E. Guevara-Quintanilla, and et al. 2022. "Customizable Collagen Vitrigel Membranes and Preliminary Results in Corneal Engineering" Polymers 14, no. 17: 3556. https://doi.org/10.3390/polym14173556