Crystallinity and Gas Permeability of Poly (Lactic Acid)/Starch Nanocrystal Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of SNCs
2.3. Preparation of PLA/g-SNC Nanocomposite
2.4. Methods
2.4.1. Differential Scanning Calorimetry (DSC)
2.4.2. Wide-Angle X-ray Diffraction (WAXD)
2.4.3. Polarized Optical Microscopy (POM)
2.4.4. Small Angle X-ray Scattering (SAXS)
2.4.5. Scanning Electron Microscopy (SEM)
2.4.6. Oxygen Permeability (OP)
2.4.7. Water Vapour Permeability (WVP)
3. Results and Discussion
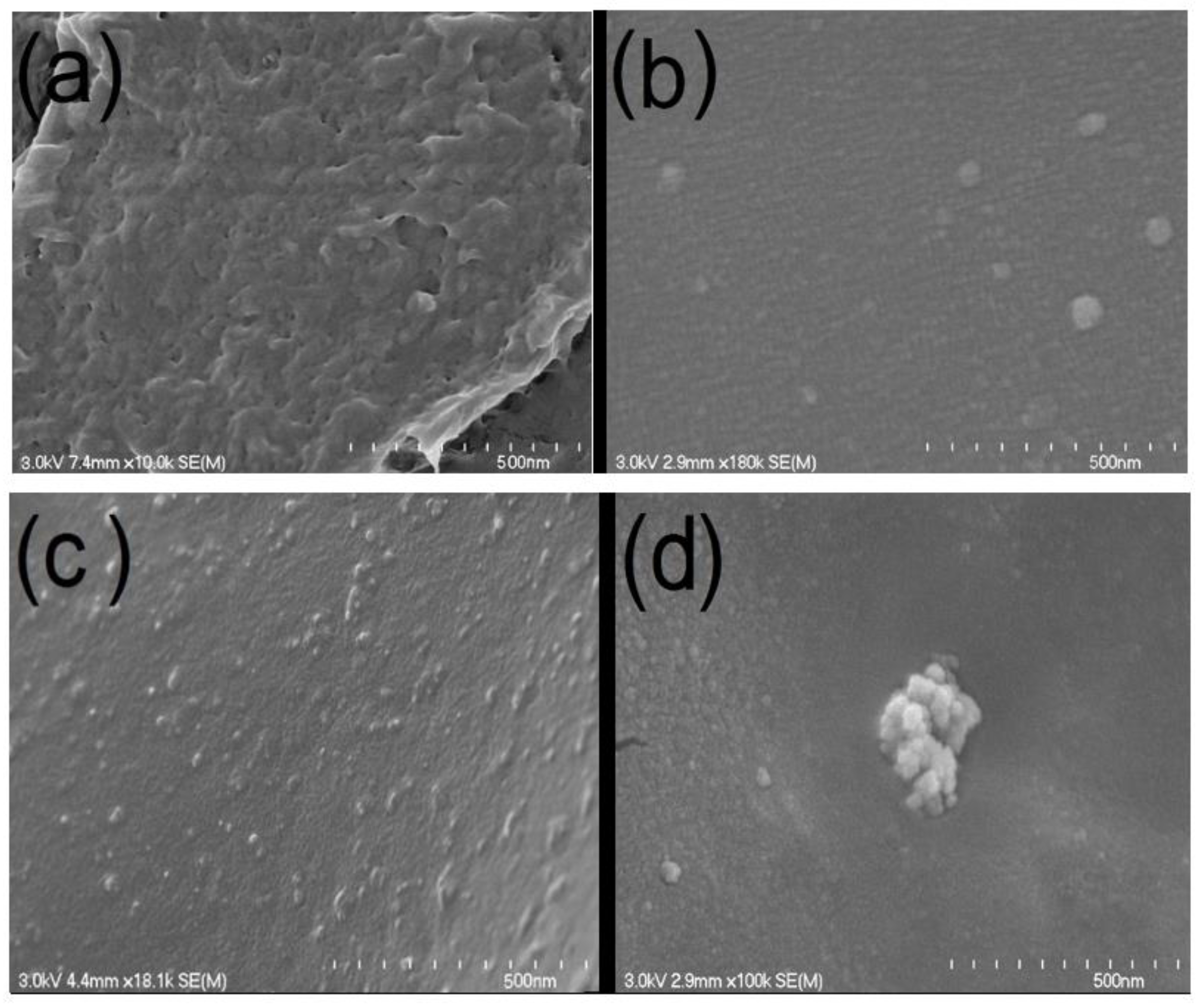
3.1. SEM Analysis
3.2. Melting Behavior of PLA/g-SNC Nanocomposites
3.3. Crystalline Structure of PLA/g-SNC Nanocomposites
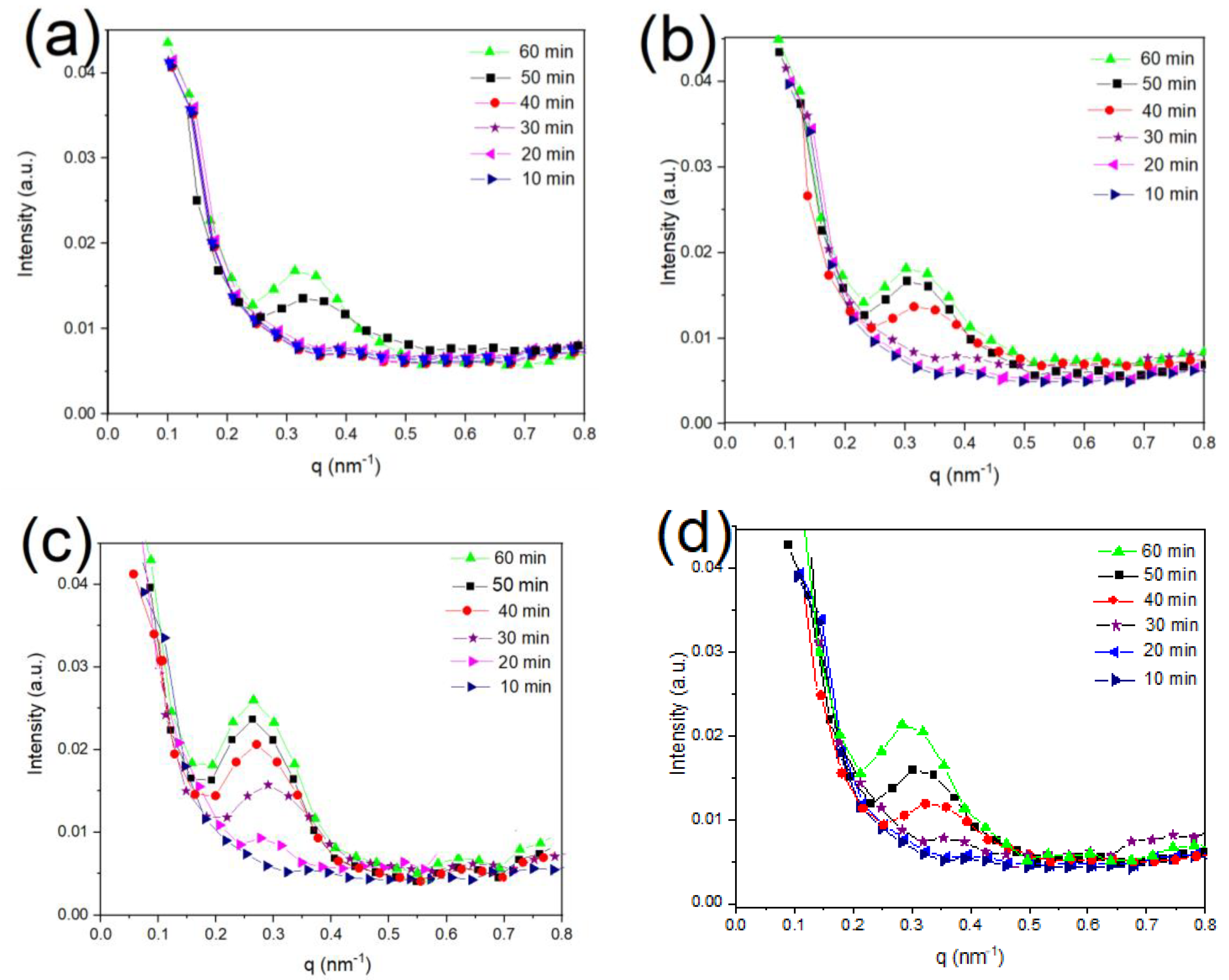
3.4. Long Period (Lac) of PLA/g-SNC Nanocomposites
3.5. Spherulite Morphology of PLA/g-SNC Nanocomposites
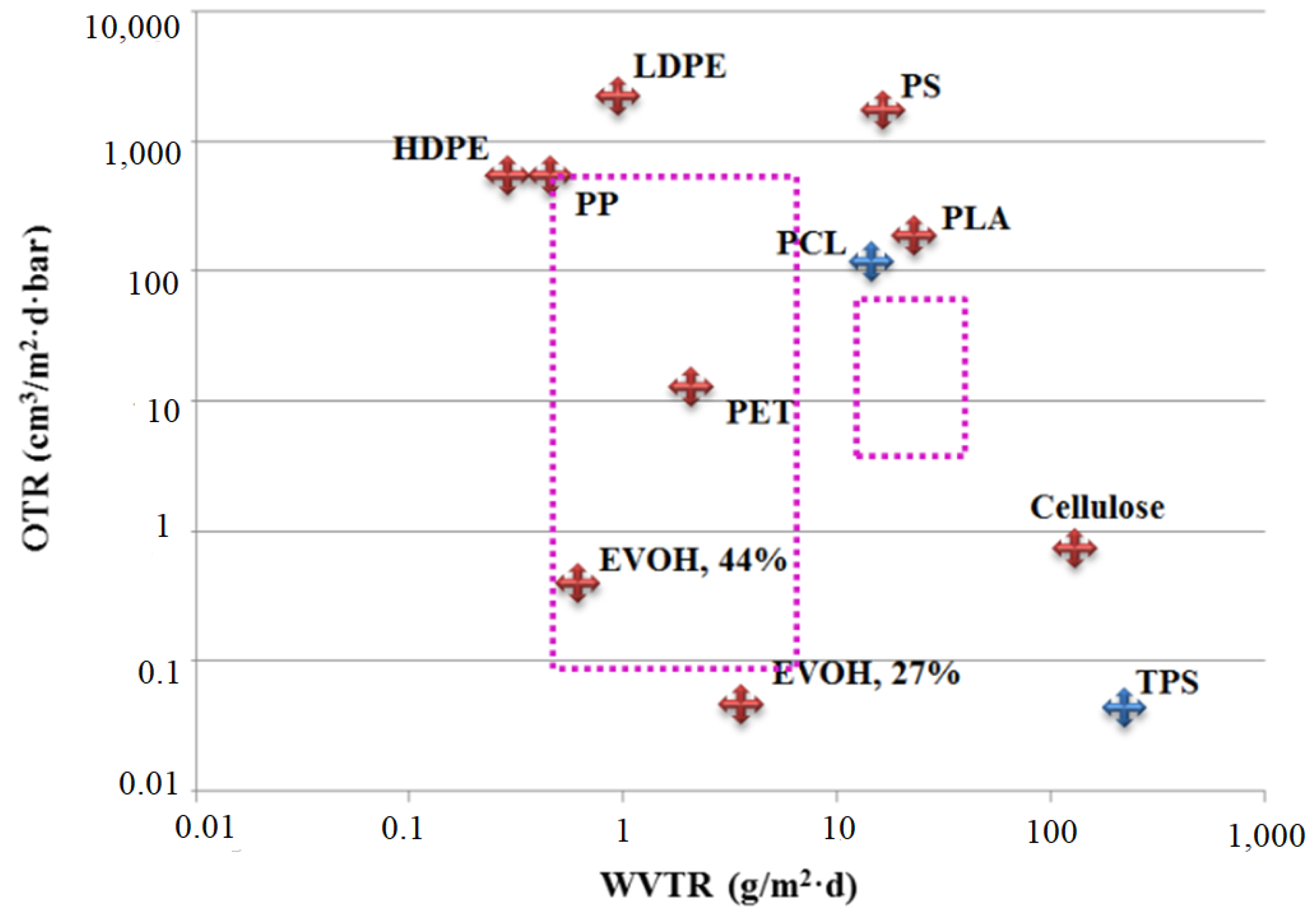
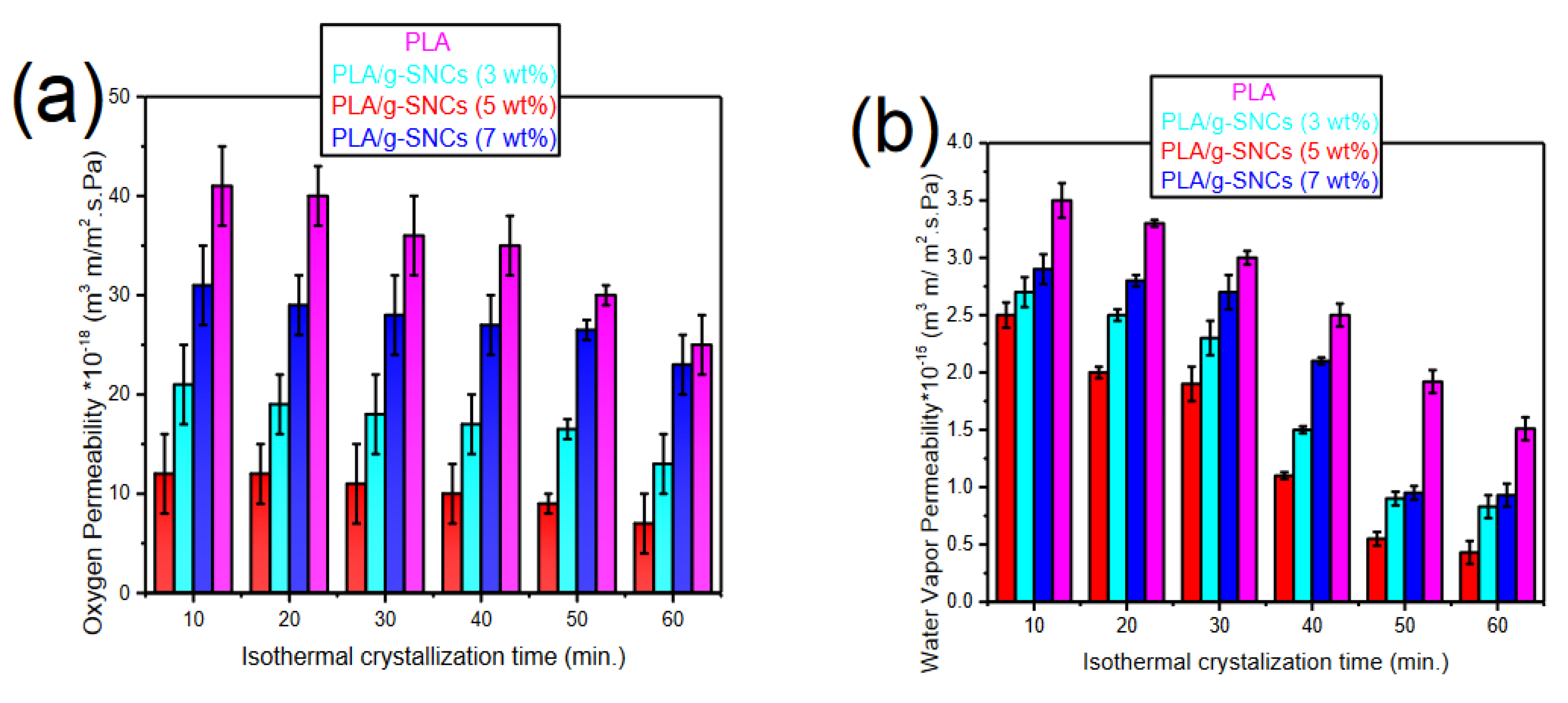
3.6. Gas Transport Properties of PLA/g-SNC Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.S.F.K.S.; Sherwani, S.F.K. Nanocellulose reinforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food packaging applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Mustățea, G.; Ungureanu, E.L.; Belc, N. Polylactic acid (PLA) for food packaging applications-a short overview. Ann. Food Sci. Technol. (N. Y.) 2019, 20, 9–14. [Google Scholar]
- Zheng, Y.; Pan, P. Crystallization of biodegradable and biobased polyesters: Polymorphism, cocrystallization, and structure-property relationship. Prog. Polym. Sci. 2020, 109, 101291. [Google Scholar] [CrossRef]
- Yu, W.; Wang, X.; Ferraris, E.; Zhang, J. Melt crystallization of PLA/Talc in fused filament fabrication. Mater. Des. 2019, 182, 108013. [Google Scholar] [CrossRef]
- Cocca, M.; Di Lorenzo, M.L.; Malinconico, M.; Frezza, V. Influence of crystal polymorphism on mechanical and barrier properties of poly(l-lactic acid). Eur. Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous crystallization and multiple melting behavior of poly (l-lactide): Molecular weight dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Kai, W.; Serizawa, S.; Iji, M.; Inoue, Y. Crystallization behavior and mechanical properties of bio-based green composites based on poly(l-lactide) and kenaf fiber. J. Appl. Polym. Sci. 2007, 105, 1511–1520. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Jiang, S.; Chen, X.; An, L. Crystal structure and morphology influenced by shear effect of poly(l-lactide) and its melting behavior revealed by WAXD, DSC and in-situ POM. Polymer 2011, 52, 3478–3487. [Google Scholar] [CrossRef]
- Kalish, J.P.; Aou, K.; Yang, X.; Hsu, S.L. Spectroscopic and thermal analyses of α′ and α crystalline forms of poly (l-lactic acid). Polymer 2011, 52, 814–821. [Google Scholar] [CrossRef]
- Sun, Y.S.; Woo, E.M. Morphology and crystal structure of cold-crystallized syndiotactic polystyrene. Polymer 2001, 42, 2241–2245. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Sato, H.; Tsuji, H.; Noda, I.; Yan, S.; Ozaki, Y. Crystal Modifications and Thermal Behavior of Poly(l-lactic acid) Revealed by Infrared Spectroscopy. Macromolecules 2005, 38, 10. [Google Scholar] [CrossRef]
- Guinault, A.; Sollogoub, C.; Domenek, S.; Grandmontagne, A.; Ducruet, V. Influence of crystallinity on gas barrier and mechanical properties of pla food packaging films. Int. J. Mater. Form. 2010, 3, 603–606. [Google Scholar] [CrossRef]
- Ruggiero, M.T.; Kölbel, J.; Li, Q.; Zeitler, J.A. Predicting the structures and associated phase transition mechanisms in disordered crystals via a combination of experimental and theoretical methods. Faraday Discuss. 2018, 2011, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.; Liang, Z.; Nakamura, N.; Miyagawa, T.; Inoue, Y. Uracil as Nucleating Agent for Bacterial Poly[(3-Hydroxybutyrate)-co-(3-hydroxyhexanoate)] Copolymers. Macromol. Biosci. 2009, 9, 585–595. [Google Scholar] [CrossRef]
- Lai, W.C. Thermal Behavior and Crystal Structure of Poly (l-lactic acid) with 1,3:2,4-Dibenzylidene-d-sorbitol. J. Phys. Chem. B 2011, 115, 11029–11037. [Google Scholar] [CrossRef]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of whey-protein-coated films and laminates as novel recyclable food packaging materials with excellent barrier properties. Int. J. Polym. Sci. 2012, 2012, 562381. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Toro, R.; Bonilla, J.; Talens, P.; Chiralt, A. Future of starch-based materials in food packaging. In Starch-Based Materials in Food Packaging; Academic Press: Cambridge, MA, USA, 2017; pp. 257–312. [Google Scholar]
- Râpă, M.; Miteluţ, A.C.; Tănase, E.E.; Grosu, E.; Popescu, P.; Popa, M.E.; Rosnes, J.T.; Sivertsvik, M.; Darie-Niţă, R.N.; Vasile, C. Influence of chitosan on mechanical, thermal, barrier and antimicrobial properties of PLA-biocomposites for food packaging. Compos. Part B Eng. 2016, 102, 112–121. [Google Scholar] [CrossRef]
- Chabrat, E.; Abdillahi, H.; Rouilly, A.; Rigal, L. Influence of citric acid and water on thermoplastic wheat flour/poly (lactic acid) blends. I: Thermal, mechanical and morphological properties. Ind. Crops Prod. 2012, 37, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Chen, L.; Jin, Z.; Jiao, A. Research progress of starch-based biodegradable materials: A review. J. Mater. Sci. 2021, 56, 11187–11208. [Google Scholar] [CrossRef]
- Wang, R. Poly (Lactic Acid)(PLA), Poly (ε-Caprolactone)(PCL) and Thermoplastic Starch (TPS) Blends for Compostable Packaging Applications; Rochester Institute of Technology: New York, NY, USA, 2018. [Google Scholar]
- Noshirvani, N.; Ghanbarzadeh, B.; Fasihi, H.; Almasi, H. Starch–PVA Nanocomposite Film Incorporated with Cellulose Nanocrystals and MMT: A Comparative Study. Int. J. Food Eng. 2016, 12, 37–48. [Google Scholar] [CrossRef]
- Mao, J.; Tang, Y.; Wang, Y.; Huang, J.; Dong, X.; Chen, Z.; Lai, Y. Particulate matter capturing via naturally dried ZIF-8/graphene aerogels under harsh conditions. iScience 2019, 16, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Zeng, J.; Wang, C.; Pan, Z. Preparation and properties of cross-linked starch nanocrystals/polylactic acid nanocomposites. Int. J. Polym. Sci. 2015, 10, 1155. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.M.O.; Pires, A.T.N.; Yamashita, F. Characterization of thermoplastic starch/poly(lactic acid) blends obtained by extrusion and thermopressing. J. Braz. Chem. Soc. 2012, 23, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Battegazzore, D.; Alongi, J.; Frache, A. Poly(lactic acid)-Based Composites Containing Natural Fillers: Thermal, Mechanical and Barrier Properties. J. Polym. Environ. 2013, 22, 88–98. [Google Scholar] [CrossRef]
- Zamir, S.S.; Frouzanmehr, M.R.; Nagalakshmaiah, M.; Ajji, A.; Robert, M.; Elkoun, S. Chemical compatibility of lactic acid-grafted starch nanocrystals (SNCs) with polylactic acid (PLA). Polym. Bull. 2018, 76, 3481–3499. [Google Scholar] [CrossRef]
- McCready, M.J.; Schultz, J.M.; Lin, J.S.; Hendricks, R.W. Effect of crystallization time on the properties of melt-crystallized linear polyethylene. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 725–740. [Google Scholar] [CrossRef]
- Strobl, G.R.; Schneider, M. Direct evaluation of the electron density correlation function of partially crystalline polymers. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 1343–1359. [Google Scholar] [CrossRef]
- Buchanan, D.R. Analysis of small-angle X-ray diffraction from polymers. J. Polym. Sci. Part A-2 Polym. Phys. 1971, 9, 645–658. [Google Scholar] [CrossRef]
- Murthy, N.S.; Kotliar, A.M.; Sibilia, J.P.; Sacks, W. Structure and properties of talc-filled polyethylene and nylon 6 films. J. Appl. Polym. Sci. 1986, 31, 2569–2582. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, Y.; Rieger, J.; Enderle, H.F.; Lilge, D.; Roth, S.V.; Gehrke, R.; Heckmann, W.; Men, Y. Two lamellar to fibrillar transitions in the tensile deformation of high-density polyethylene. Macromolecules 2010, 43, 4727–4732. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Fu, L.; Lu, Y.; Men, Y. Lamellar thickness and stretching temperature dependency of cavitation in semicrystalline polymers. PLoS ONE 2014, 9, 97234. [Google Scholar]
- Shogren, R. Water vapor permeability of biodegradable polymers. J. Polym. Environ. 1997, 5, 91–95. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Lagaron, J.M. On the use of plant cellulose nanowhiskers to enhance the barrier properties of polylactic acid. Cellulose 2010, 17, 987–1004. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.M.; Chiralt, A.; Torre, L. Production and characterization of PLA_PBS biodegradable blends reinforced with cellulose nanocrystals extracted from hemp fibres. Ind. Crops Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]
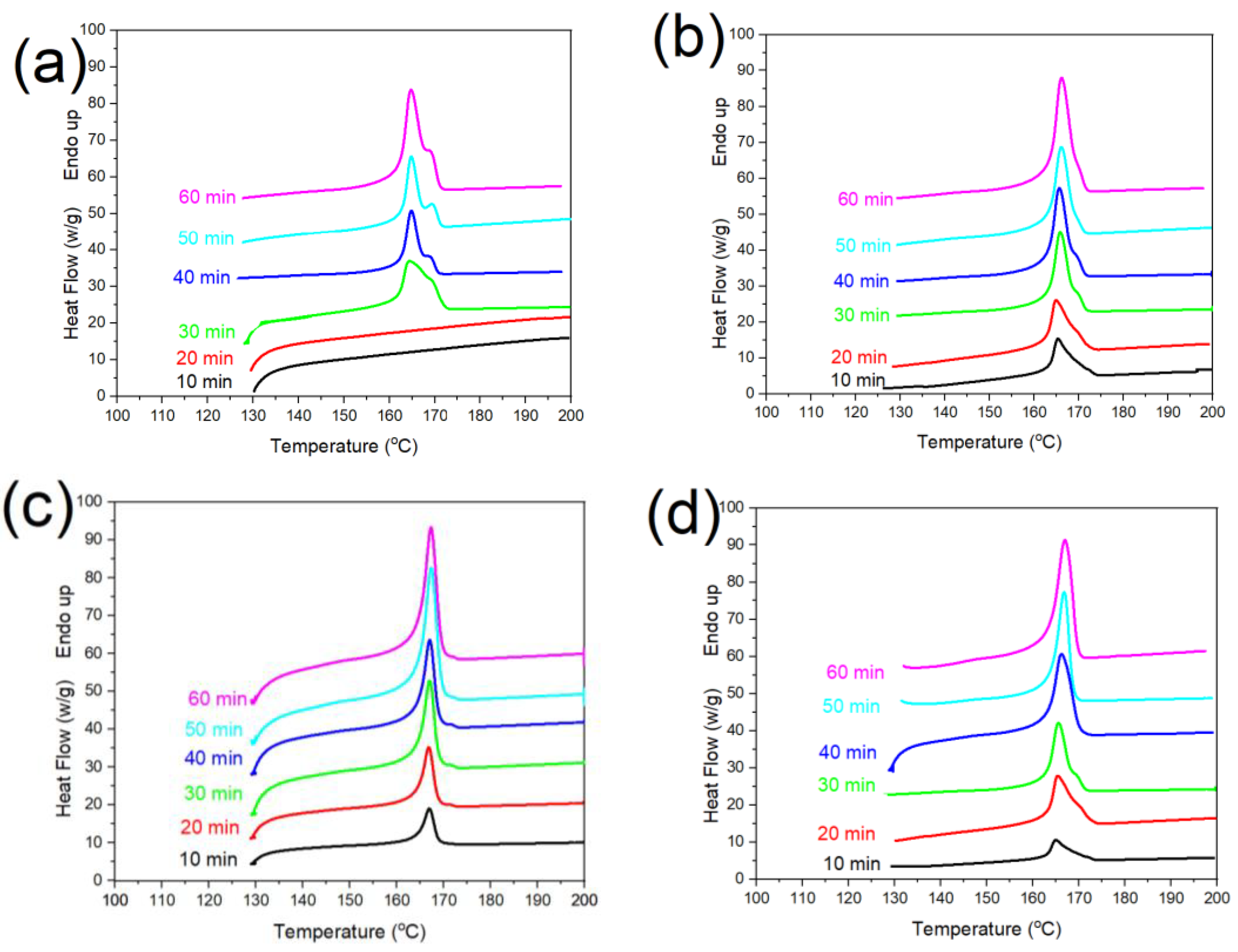
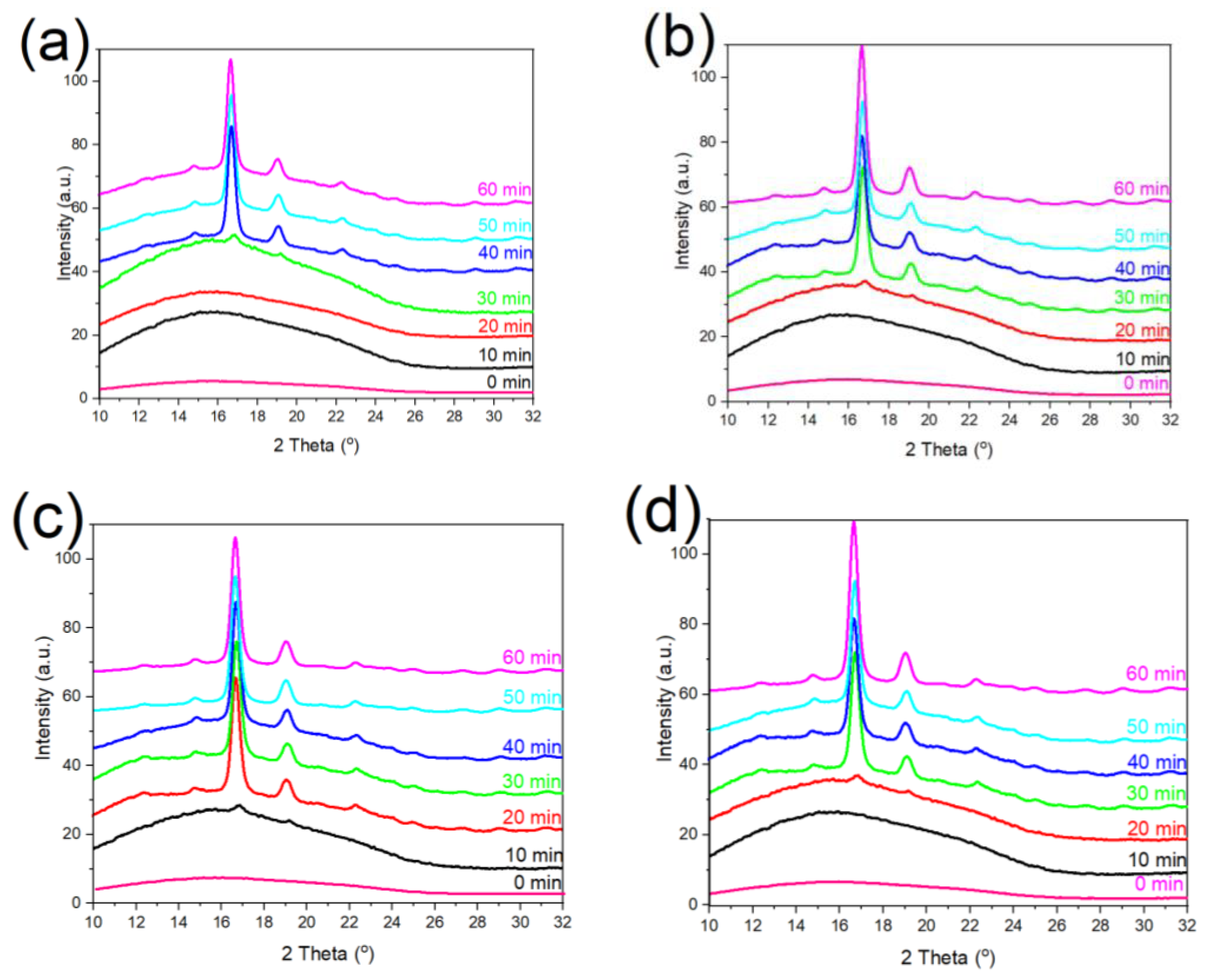

| Sample | PLA | PLA/g-SNC (3 wt%) | PLA/g-SNC (5 wt%) | PLA/g-SNC (7 wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(min) | (°C) | (°C) | (%) | (%) | (°C) | (%) | (%) | (°C) | (%) | (%) | (°C) | (%) | (%) |
| 10 | - | - | - | - | 165.6 | - | 1.00 | 166.1 | - | 4.20 | 166.5 | - | 1.20 |
| 20 | - | - | - | - | 165.8 | - | 10.3 | 167.4 | 24.9 | 23.3 | 167.6 | - | 13.4 |
| 30 | 169.2 | 167.3 | - | 10.7 | 168.7 | 21.9 | 15.0 | 168.9 | 37.5 | 37.0 | 167.7 | 33.9 | 17.0 |
| 40 | 165.5 | 168.3 | - | 12.5 | 168.9 | 22.7 | 25.5 | 169.2 | 38.9 | 38.0 | 168.1 | 34.7 | 27.5 |
| 50 | 164.4 | 167.4 | - | 15.7 | 168.3 | 35.5 | 32.1 | 169.0 | 39.0 | 38.9 | 168.5 | 36.5 | 31.1 |
| 60 | 164.1 | 167.7 | 35.2 | 17.1 | 169.5 | 36.3 | 33.4 | 169.1 | 41.8 | 41.1 | 168.6 | 37.8 | 32.4 |
| PLA | PLA/g-SNC (3 wt%) | PLA/g-SNC (5 wt%) | PLA/g-SNC (7 wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | ||||||||||||
| 20 | - | - | - | - | - | - | 19.0 | 14.0 | 5.0 | - | - | - |
| 30 | - | - | - | - | - | - | 19.9 | 15.1 | 4.8 | - | - | - |
| 40 | - | - | - | 20.9 | 14.9 | 6.0 | 20.9 | 15.4 | 5.5 | 20.9 | 15.0 | 5.9 |
| 50 | 20.9 | 14.8 | 6.1 | 21.7 | 15.5 | 6.2 | 22.4 | 16.4 | 6.0 | 21.7 | 15.4 | 6.3 |
| 60 | 21.7 | 15.6 | 6.1 | 21.9 | 15.9 | 6.0 | 22.4 | 16.5 | 5.9 | 22.1 | 16.1 | 6.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharafi Zamir, S.; Fathi, B.; Ajji, A.; Robert, M.; Elkoun, S. Crystallinity and Gas Permeability of Poly (Lactic Acid)/Starch Nanocrystal Nanocomposite. Polymers 2022, 14, 2802. https://doi.org/10.3390/polym14142802
Sharafi Zamir S, Fathi B, Ajji A, Robert M, Elkoun S. Crystallinity and Gas Permeability of Poly (Lactic Acid)/Starch Nanocrystal Nanocomposite. Polymers. 2022; 14(14):2802. https://doi.org/10.3390/polym14142802
Chicago/Turabian StyleSharafi Zamir, Somayeh, Babak Fathi, Abdellah Ajji, Mathieu Robert, and Said Elkoun. 2022. "Crystallinity and Gas Permeability of Poly (Lactic Acid)/Starch Nanocrystal Nanocomposite" Polymers 14, no. 14: 2802. https://doi.org/10.3390/polym14142802






