Fire Behavior of Thermally Thin Materials in Cone Calorimeter
Abstract
:1. Introduction
2. Materials and Methods
- -
- Ten cotton woven fabrics with an area density ranging from 80 to 270 g/m2. The first eight fabrics (called T1 to T8) are not flame retarded. The other fabrics (called F1 and F3) are derived from T8 sample. T8 is flame retarded using a flame retardant (FR) system based on ammonium polyphosphate (APP) (10–12 µm; Phosphorus and nitrogen contents are respectively 31–32 wt% and 14–15 wt%) provided by Focus Química (São Paulo, Brazil) and sodium montmorillonite (Na-Mt) under the trade name Cloisite® Na+ (Southern Clay Products, Gonzales, TX, USA).
- -
- Two flax fabrics (area density of 200 g/m2), respectively unmodified and flame retarded with a phosphorus additive (vinyl phosphonic acid) grafted using irradiation [24].
- -
- Three jute fabrics of plain-woven material (area density of 180 g/m2) unmodified and flame retarded with Rochelle salt (Potassium sodium tartrate) and borax (sodium borate), respectively.
- -
- -
- Four flax knitted fabrics with the same structures.
- -
- Five PP knitted fabrics with the same structures (studied in a previous work [13]).
- -
- Four PP sheets with a thickness ranging from 1 to 6 mm (studied in a previous work [13]).
- -
- Two aramid-based fabrics (Twill). The exact composition of these two fabrics is unknown. Note that for these two fabrics, the external heat fluxes ranged from 20 to 50 kW/m2.
- -
- One thin (1 mm-thick) sheet of low-density wood, namely balsa (density < 150 g/m3). Note that no grid was used in this case.
3. Results
3.1. Thermal Penetration
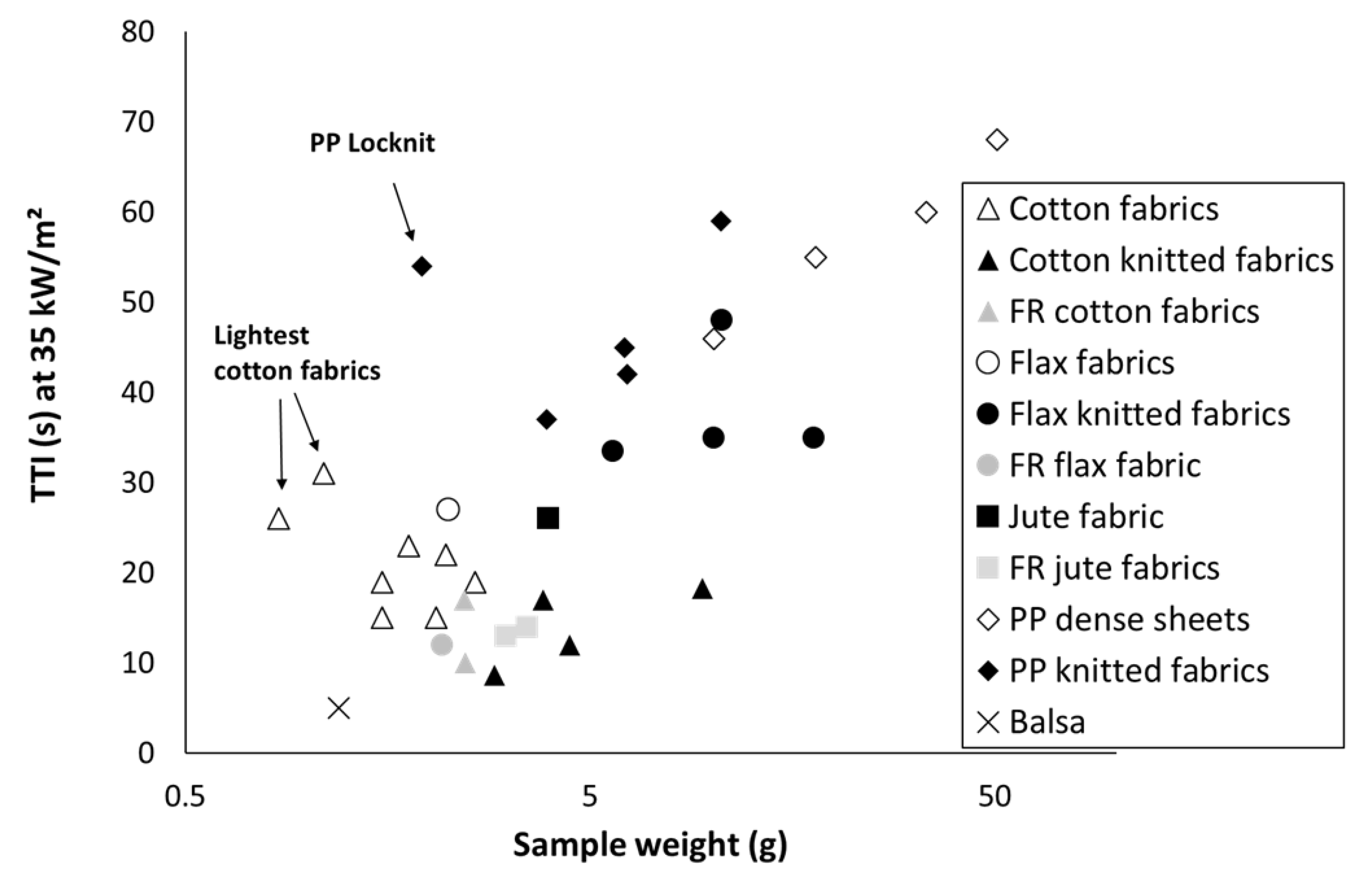
3.2. Time-to-Ignition
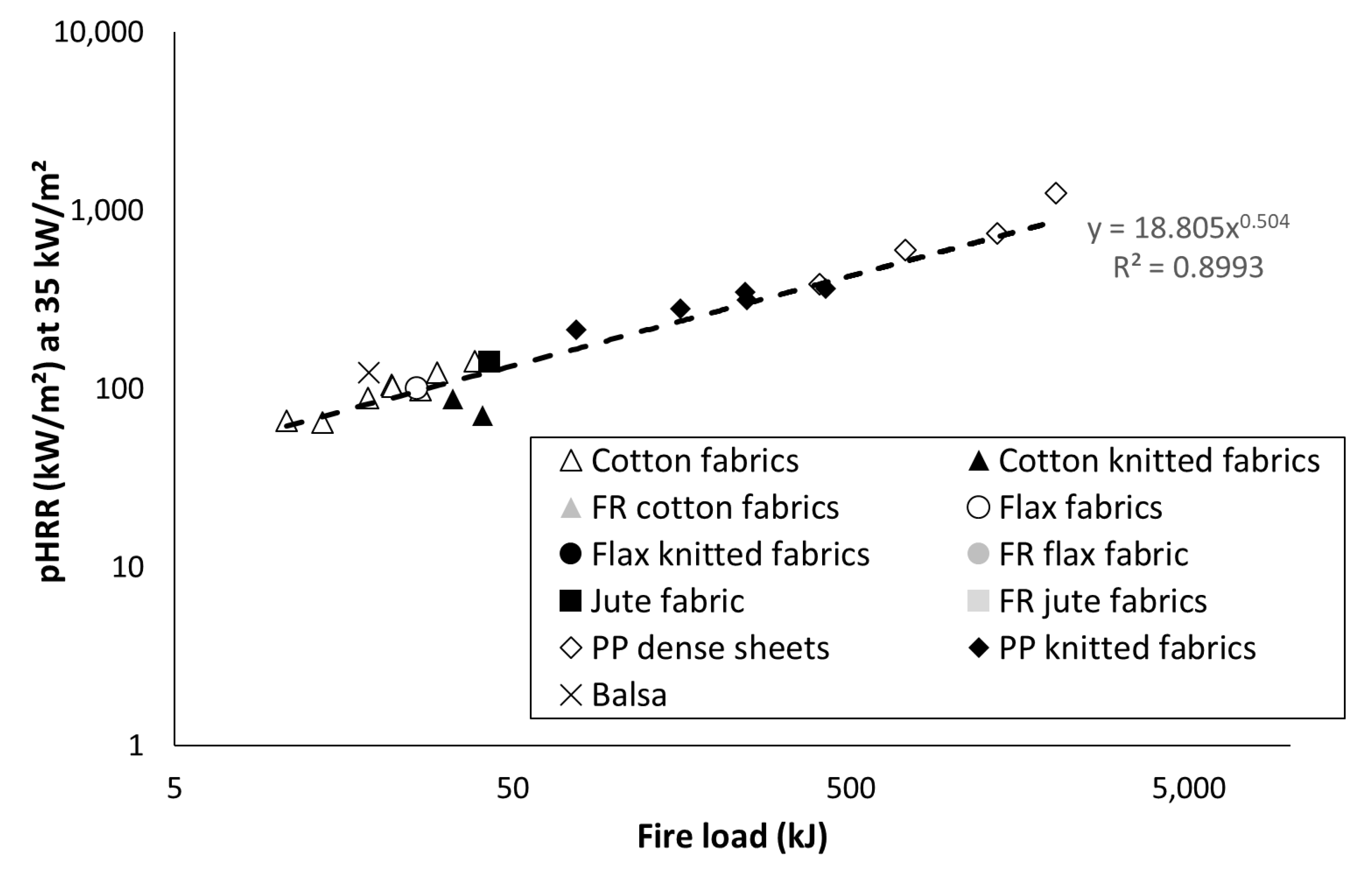
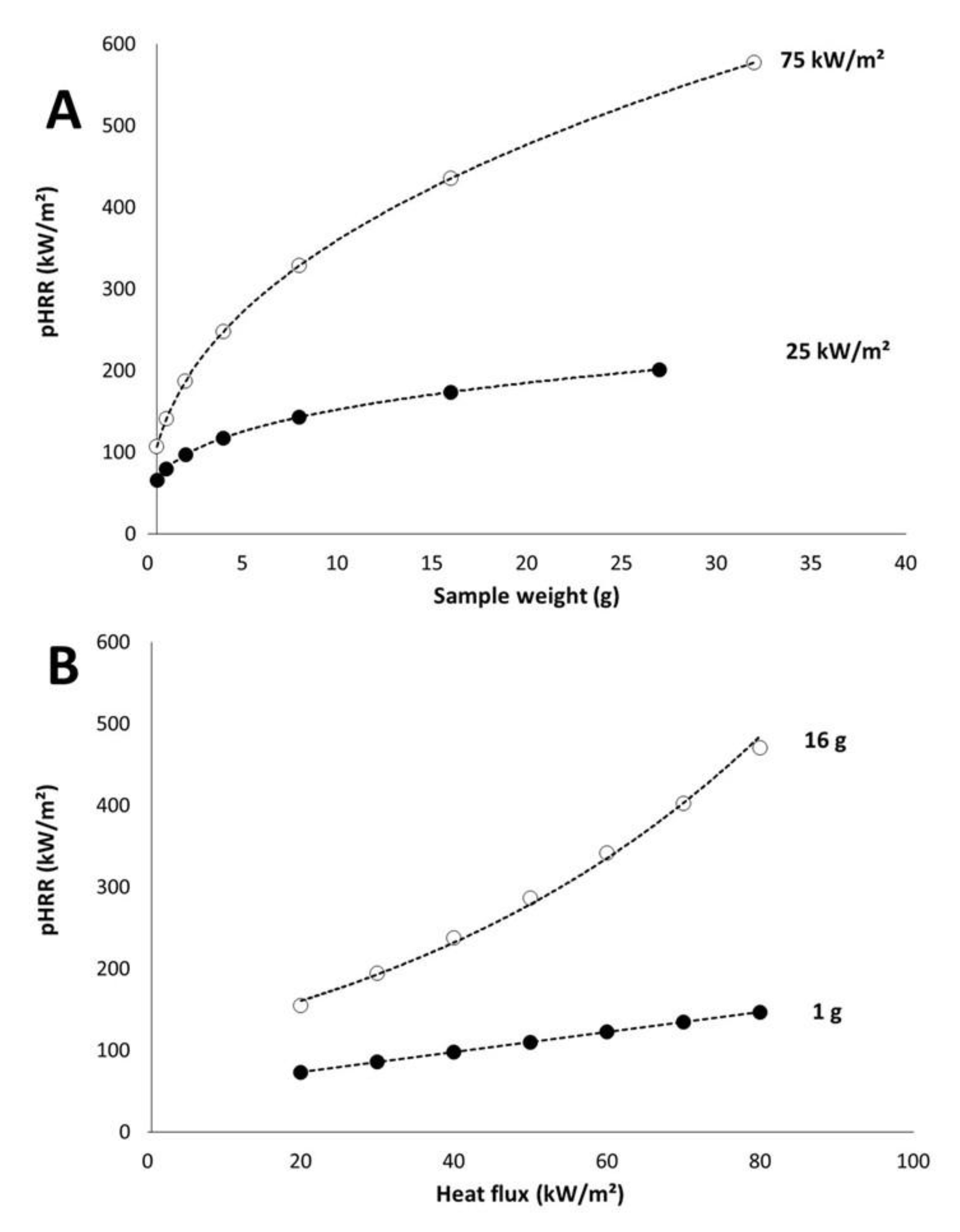
3.3. Peak of Heat Release Rate
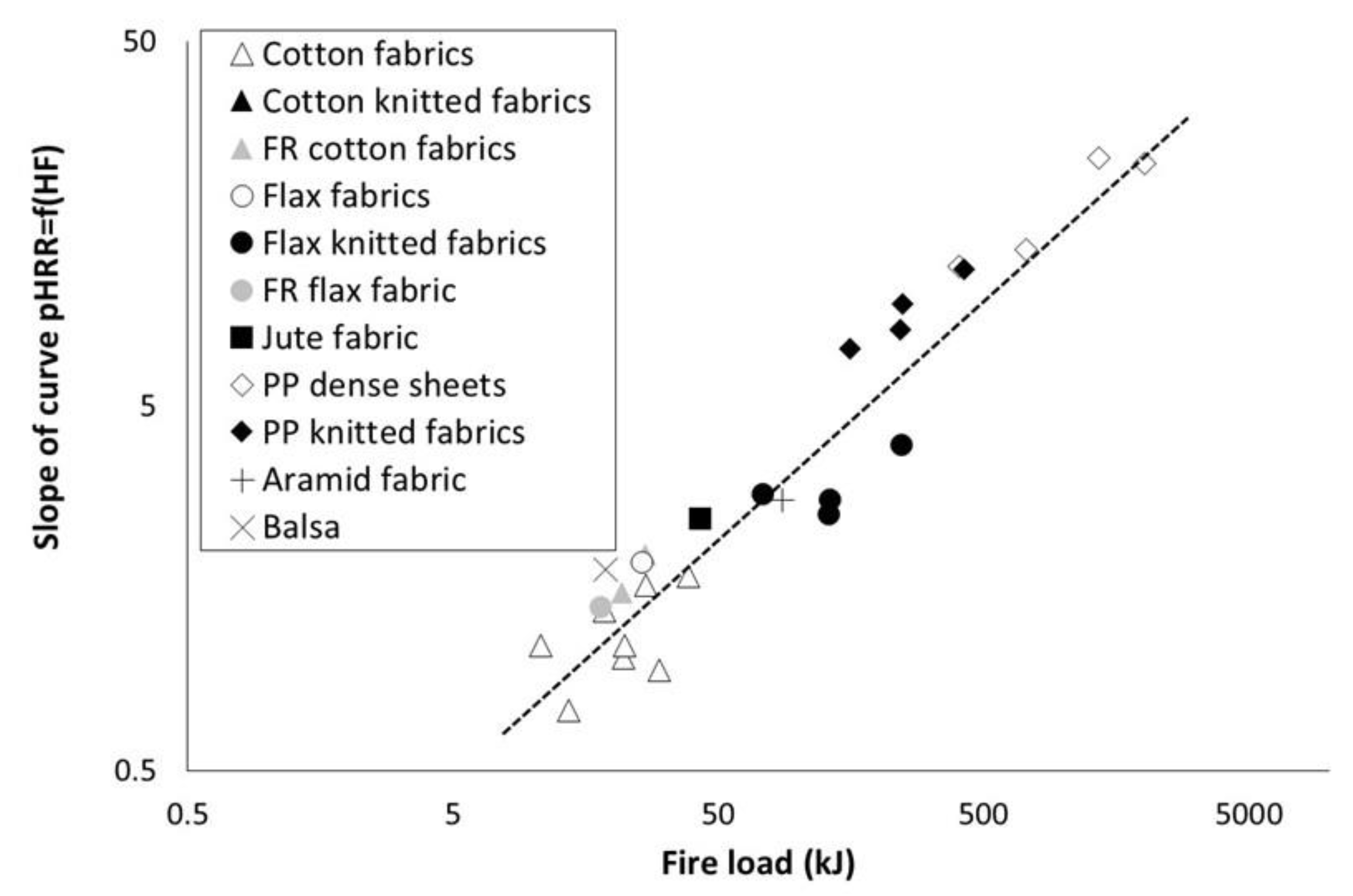
3.4. Phenomenological Modelling
3.5. Some Comments about the Flame Retardancy of Thermally Thin Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cleary, T.; Ohlemiller, T.; Villa, K. The Influence of Ignition Source on the Flaming Fire Hazard or Upholstered Furniture. Fire Saf. J. 1994, 23, 79–102. [Google Scholar] [CrossRef]
- Thompson, S.; Apostolakis, G. A Response Surface Approximation for the Bench-Scale Peak Heat Release Rate from Upholstered Furniture Exposed to a Radiant Heat Source. Fire Saf. J. 1994, 22, 1–22. [Google Scholar] [CrossRef]
- Horrocks, A.; Nazaré, S.; Kandola, B. The particular flammability hazards of nightwear. Fire Saf. J. 2004, 39, 259–276. [Google Scholar] [CrossRef]
- Silva-Santos, M.; Oliveira, M.; Giacomin, A.; Laktim, M.; Baruque-Ramos, J. Flammability on textile of business uniforms: Use of natural fibers. Proc. Eng. 2017, 200, 148–154. [Google Scholar] [CrossRef]
- Horrocks, A. Textile flammability research since 1980 e Personal challenges and partial solutions. Polym. Degrad. Stab. 2013, 98, 2813–2824. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some comments on the use of cone calorimeter data. Polym. Degrad. Stab. 2005, 88, 540–547. [Google Scholar] [CrossRef]
- Babrauskas, V.; Parker, W. Ignitability measurements with the cone calorimeter. Fire Mater. 1987, 11, 31–43. [Google Scholar] [CrossRef]
- Tata, J.; Alongi, J.; Carosio, F.; Frache, A. Optimization of the procedure to burn textile fabrics by cone calorimeter: Part I. Combustion behavior of polyester. Fire Mater. 2011, 35, 397–409. [Google Scholar] [CrossRef]
- Basak, S.; Samanta, K. Thermal behaviour and the cone calorimetric analysis of the jute fabric treated in different pH condition. J. Therm. Anal. Calorim. 2018. [Google Scholar] [CrossRef]
- Nazaré, S.; Kandola, B.; Horrocks, R. Use of cone calorimetry to quantify the burning hazard of apparel fabrics. Fire Mater. 2002, 26, 191–199. [Google Scholar] [CrossRef]
- Alongi, J.; Cuttica, F.; Carosio, F.; Bourbigot, S. How much the fabric grammage may affect cotton combustion? Cellulose 2015, 22, 3477–3489. [Google Scholar] [CrossRef]
- Hernandez, N.; Sonnier, R.; Giraud, S. Influence of grammage on heat release rate of polypropylene fabrics. J. Fire Sci. 2018, 36, 30–46. [Google Scholar] [CrossRef]
- Quintiere, J. Approximate solutions for the ignition of a solid as a function of the Biot number. Fire Mater. 2019, 43, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Delichatsios, M. Ignition Times for Thermally Thick and Intermediate Conditions in Flat and Cylindrical Geometries. In Proceedings of the 6th International Symposium—Fire Safety Science, Poitiers, France, 5–9 July 1999; pp. 233–244. [Google Scholar]
- Haseli, Y.; van Oijen, J.A.; de Goey, L.P.H. Analytical solutions for prediction of the ignition time of wood particles based on a time and space integral method. Thermochim. Acta 2012, 548, 65–75. [Google Scholar] [CrossRef]
- Zhai, C.; Zhang, S.; Yao, S.; Zhan, Q.; Zhang, S.; Wang, Y. Analytical study on ignition time of PMMA exposed to time-decreasing thermal radiation using critical mass flux. Sci. Rep. 2019, 9, 11958–11969. [Google Scholar] [CrossRef]
- Gong, J.; Zhai, C.; Cao, J.; Li, J.; Yang, L.; Zhou, Y.; Wang, Z. Auto-ignition of thermally thick PMMA exposed to linearly decreasing thermal radiation. Combust. Flame 2020, 216, 232–244. [Google Scholar] [CrossRef]
- Hopkins, D.; Quintiere, J. Material fire properties and predictions for thermoplastics. Fire Saf. J. 1996, 26, 241–268. [Google Scholar] [CrossRef]
- Rhodes, B.; Quintiere, J. Burning Rate and Flame Heat Flux for PMMA in a Cone Calorimeter. Fire Saf. J. 1996, 26, 221–240. [Google Scholar] [CrossRef]
- Mikkola, E.; Wichman, I. On the Thermal Ignition of Combustible Materials. Fire Mater. 1989, 14, 87–96. [Google Scholar] [CrossRef]
- Stoliarov, S.; Crowley, S.; Walters, R.; Lyon, R. Prediction of the burning rates of charring polymers. Combust. Flame 2010, 157, 2024–2034. [Google Scholar] [CrossRef]
- Huggett, C. Estimation of rate of heat release by means of oxygen consumption measurements. Fire Mater. 1980, 4, 61–65. [Google Scholar] [CrossRef]
- Hajj, R.; El Hage, R.; Sonnier, R.; Otazaghine, B.; Gallard, B.; Rouif, S.; Nakhl, M.; Lopez-Cuesta, J.M. Grafting of phosphorus flame retardants on flax fabrics: Comparison between two routes. Polym. Degrada. Stab. J. 2018, 147, 25–34. [Google Scholar] [CrossRef]
- Babrauskas, V. Comparative Rates of Heat Release from Five Different Types of Test Apparatuses. J. Fire Sci. 1986, 4, 148–159. [Google Scholar] [CrossRef]
- Ostman, B.; Nussbaum, R. Correlation between Small-Scale Rate of Heat Release and Full-Scale Room Flashover for Surface linings. In Proceedings of the 2nd International Symposium—Fire Safety Science, Tokyo, Japan, 13–17 June 1988; pp. 823–832. [Google Scholar]
- Lyon, R. Polymer Flammability. DOT/FAA/AR-05/14 2005. Available online: https://www.fire.tc.faa.gov/pdf/05-14.pdf (accessed on 12 May 2005).
- Lyons, R.; Walters, R.; Gandhi, S. Combustibility of cyanate ester resins. Fire Mater. 2006, 30, 89–106. [Google Scholar] [CrossRef]
- Lyon, R.; Quintiere, J. Criteria for piloted ignition of combustible solids. Combust. Flame 2007, 151, 551–559. [Google Scholar] [CrossRef]
- Walters, R.; Safronava, N.; Lyon, R. A microscale combustion calorimeter study of gas phase combustion of polymers. Combust. Flame 2015, 162, 855–863. [Google Scholar] [CrossRef]
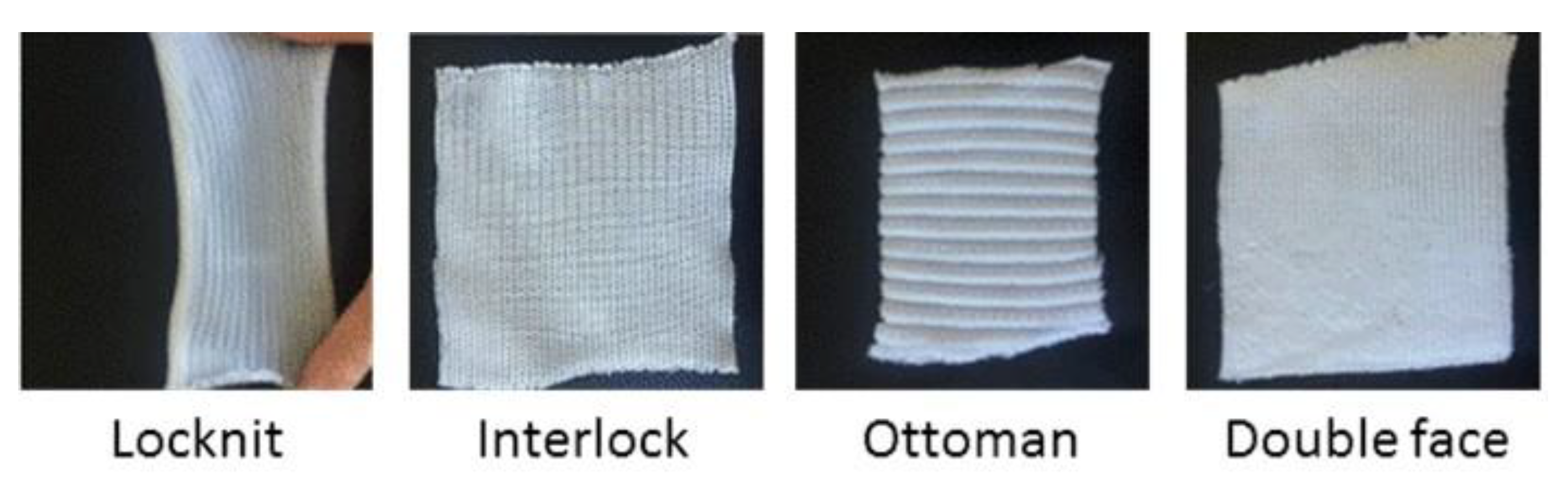




| Sample | Area Density (g/m2) | Sample Weight (g) | Thickness (mm) | Textile Structure |
|---|---|---|---|---|
| Cotton Fabric (T1) | 270 | 2.6 | 0.4 | Plain Weave |
| Cotton Fabric (T2) | 210 | 2.2 | 0.5 | Plain Weave |
| Cotton Fabric (T3) | 150 | 1.53 | 0.6 | Plain Weave |
| Cotton Fabric (T4) | 180 | 1.78 | 0.6 | Plain Weave |
| Cotton Fabric (T5) | 110 | 1.1 | 0.4 | Plain Weave |
| Cotton Fabric (T6) | 170 | 1.53 | 0.6 | Plain Weave |
| Cotton Fabric (T7) | 80 | 0.85 | 0.7 | Plain Weave |
| Cotton Fabric (T8) | 210 | 2.08 | 1.3 | Plain Weave |
| FR T8 Cotton Fabric (F1) | 250 | 2.46 | 0.5 | Plain Weave |
| FR T8 Cotton Fabric (F3) | 250 | 2.45 | 0.5 | Plain Weave |
| Locknit Cotton Knit | 200 | 2.9 | 1.2 | Locknit |
| Interlock Cotton Knit | 400 | 3.84 | 2.8 | Interlock |
| Double Face Cotton Knit | 500 | 4.46 | 2.5 | Double Face |
| Ottoman Cotton Knit | 900 | 9.5 | 4.8 | Ottoman |
| Flax Fabric | 200 | 2.24 | 0.4 | Plain Weave |
| FR Flax Fabric | 200 | 2.16 | 0.42 | Plain Weave |
| Locknit Flax Knit | 700 | 5.7 | 3.1 | Locknit |
| Interlock Flax Knit | 1100 | 10.6 | 3.2 | Interlock |
| Double Face Flax Knit | 1100 | 10.1 | 4.1 | Double Face |
| Ottoman Flax Knit | 1850 | 17.9 | 5 | Ottoman |
| Jute Knit | 380 | 3.95 | 2.1 | Plain Weave |
| Jute Knit (Rochelle Salt) | / | 3.5 | 2.1 | Plain Weave |
| Jute Knit (Borax) | / | 3.1 | 2.1 | Plain Weave |
| Locknit PP Knit | 218 | 1.9 | 1.3 | Locknit |
| Interlock PP Knit | 442 | 3.9 | 2.6 | Interlock |
| Double Face PP Knit | 690 | 6.1 | 3.3 | Double Face |
| Interlock 2 Yarns PP Knit | 698 | 6.2 | 3 | Interlock |
| Ottoman PP Knit | 1195 | 10.6 | 5.4 | Ottoman |
| 1 mm-Thick PP Sheet | 1145 | 10.1 | 1 | Sheet |
| 2 mm-Thick PP Sheet | 2048 | 18.1 | 2 | Sheet |
| 4 mm-Thick PP Sheet | 3831 | 33.9 | 4 | Sheet |
| 6 mm-Thick PP Sheet | 5729 | 50.6 | 6 | Sheet |
| Balsa | / | 1.2 | 1 | Sheet |
| Thin Aramid Fabric | / | 2 | / | Twill |
| Thick Aramid Fabric | / | 6.3 | / | Twill |
| Thickness (mm) | Heat Flux (kW/m2) | |||
|---|---|---|---|---|
| 25 | 35 | 50 | 75 | |
| 1 | 2.4 | 2.0 | 1.6 | 1.3 |
| 2 | 2.8 | 2.2 | 1.8 | 1.4 |
| 4 | 3.0 | 2.3 | 1.9 | 1.5 |
| 6 | 3.4 | 2.5 | 1.9 | 1.4 |
| Sample | Area Density (g/m2) | Sample Weight (g) | 25 kW/m2 | 35 kW/m2 | 50 kW/m2 | 75 kW/m2 | Slope (m2/(kW.s)) | R2 | CHF (kW/m2) |
|---|---|---|---|---|---|---|---|---|---|
| Cotton Fabric (T1) | 270 | 2.6 | 56.5 | 19 | 19 | 10 | 0.0015 | 0.91 | 8.6 |
| Cotton Fabric (T2) | 210 | 2.2 | 48.5 | 22 | 8 | 5 | 0.0037 | 0.98 | 20 |
| Cotton Fabric (T3) | 150 | 1.53 | 57 | 19 | 8 | 6 | 0.003 | 0.95 | 16.5 |
| Cotton Fabric (T4) | 180 | 1.78 | 71 | 23 | 10 | 5 | 0.0038 | 1 | 22.3 |
| Cotton Fabric (T5) | 110 | 1.1 | 65 | 31 | 7 | 4 | 0.005 | 0.97 | 23.8 |
| Cotton Fabric (T6) | 170 | 1.53 | 44 | 15 | 11 | 4 | 0.0044 | 0.95 | 22.2 |
| Cotton Fabric (T7) | 80 | 0.85 | 61 | 26 | 4 | 4 | 0.0052 | 0.76 | 19.4 |
| Cotton Fabric (T8) | 210 | 2.08 | 69 | 15 | 7 | 5 | 0.0037 | 0.96 | 17.4 |
| FR T8 Cotton Fabric (F1) | 250 | 2.46 | 28 | 10 | 2 | 2 | 0.0101 | 0.77 | 18.3 |
| FR T8 Cotton Fabric (F3) | 250 | 2.45 | 39 | 17 | 7 | 3.5 | 0.0053 | 0.99 | 22.2 |
| Locknit Cotton Knit | 200 | 2.9 | 23 | 8.7 | 6 | 3 | 0.0056 | 0.99 | 17.1 |
| Interlock Cotton Knit | 400 | 3.84 | 26.5 | 17 | 9 | 2.5 | 0.0074 | 0.91 | 25.5 |
| Double Face Cotton Knit | 500 | 4.46 | 35 | 12 | 7 | 3 | 0.006 | 0.98 | 22.1 |
| Ottoman Cotton Knit | 900 | 9.5 | 41 | 18.3 | 5.5 | 4 | 0.0047 | 0.94 | 19.6 |
| Flax Fabric | 200 | 2.24 | 78 | 27 | 11 | 4 | 0.0056 | 0.96 | 26.6 |
| FR Flax Fabric | 200 | 2.16 | 38 | 12 | 7.5 | 3 | 0.0061 | 0.97 | 22.2 |
| Locknit Flax Knit | 700 | 5.7 | 63 | 33.5 | 19 | 7.5 | 0.0024 | 0.96 | 21.5 |
| Interlock Flax Knit | 1100 | 10.6 | 92 | 48 | 21 | 11 | 0.0016 | 0.99 | 20.8 |
| Double Face Flax Knit | 1100 | 10.1 | 81 | 35 | 19 | 10 | 0.0019 | 0.99 | 19 |
| Ottoman Flax Knit | 1850 | 17.9 | 88 | 35 | 24 | 10 | 0.0017 | 0.97 | 20.7 |
| Jute Knit | 380 | 3.95 | 44 | 26 | 13 | 5 | 0.0036 | 0.96 | 22.9 |
| Jute Knit (Rochelle Salt) | / | 3.5 | / | 14 | / | / | / | / | / |
| Jute Knit (Borax) | / | 3.1 | / | 13 | / | / | / | / | / |
| Locknit PP Knit | 218 | 1.9 | 109 | 54 | 26 | 13 | 0.0014 | 0.99 | 19.8 |
| Interlock PP Knit | 442 | 3.9 | 71 | 37 | 28 | 16 | 0.0009 | 0.99 | 9.3 |
| Double Face PP Knit | 690 | 6.1 | 72 | 45 | 29 | 19 | 0.0008 | 0.99 | 6.3 |
| Interlock 2 Yarns PP Knit | 698 | 6.2 | 80 | 42 | 26 | 16 | 0.001 | 0.99 | 11.6 |
| Ottoman PP Knit | 1195 | 10.6 | 100 | 59 | 37 | 16 | 0.0011 | 0.97 | 17.8 |
| 1 mm-Thick PP Sheet | 1145 | 10.1 | 66 | 46 | 30 | 19 | 0.0008 | 0.99 | 5.3 |
| 2 mm-Thick PP Sheet | 2048 | 18.1 | 86 | 55 | 38 | 21 | 0.0007 | 0.99 | 10.3 |
| 4 mm-Thick PP Sheet | 3831 | 33.9 | 101 | 60 | 40 | 24 | 0.0006 | 0.99 | 9.7 |
| 6 mm-Thick PP Sheet | 5729 | 50.6 | 131 | 68 | 39 | 23 | 0.0007 | 0.99 | 14.7 |
| Balsa | / | 1.2 | 10 | 5 | 3 | 2 | 0.0079 | 0.99 | 10.6 |
| Sample | Area Density (g/m2) | Sample Weight (g) | 20 kW/m2 | 30 kW/m2 | 40 kW/m2 | 50 kW/m2 | Slope (m2/(kW.s)) | R2 | CHF (kW/m2) |
| Thin Aramid Fabric | / | 2 | / | 7 | 5 | / | 0.0057 | / | 5 |
| Thick Aramid Fabric | / | 6.3 | 35 | 20 | 16 | 11.5 | 0.0019 | 0.99 | 4.6 |
| Sample | Area Density (g/m2) | Sample Weight (g) | 25 kW/m2 | 35 kW/m2 | 50 kW/m2 | 75 kW/m2 | Slope pHRR | EHC (kJ/g) | Fire Load (kJ) |
|---|---|---|---|---|---|---|---|---|---|
| Cotton Fabric (T1) | 270 | 2.6 | 121 | 143 | 169 | 207 | 1.7 | 14.8 | 38.48 |
| Cotton Fabric (T2) | 210 | 2.2 | 109 | 123 | 140 | 157 | 0.94 | 13.6 | 29.92 |
| Cotton Fabric (T3) | 150 | 1.53 | 91 | 106 | 116 | 144 | 1.02 | 14.3 | 21.879 |
| Cotton Fabric (T4) | 180 | 1.78 | 97 | 103 | 124 | 150 | 1.1 | 12.4 | 22.072 |
| Cotton Fabric (T5) | 110 | 1.1 | 63 | 65 | 76 | 98 | 0.73 | 12.4 | 13.64 |
| Cotton Fabric (T6) | 170 | 1.53 | 72 | 89 | 111 | 141 | 1.37 | 12.2 | 18.666 |
| Cotton Fabric (T7) | 80 | 0.85 | 65 | 66 | 85 | 117 | 1.1 | 12.6 | 10.71 |
| Cotton Fabric (T8) | 210 | 2.08 | 92 | 99 | 123 | 170 | 1.61 | 12.8 | 26.624 |
| FR T8 Cotton Fabric (F1) | 250 | 2.46 | 85 | 113 | 135 | 186 | 1.96 | 10.8 | 26.568 |
| FR T8 Cotton Fabric (F3) | 250 | 2.45 | 49 | 103 | 103 | 139 | 1.54 | 8.8 | 21.56 |
| Locknit Cotton Knit | 200 | 2.9 | 67 | 91 | 88 | 156 | 1.67 | 12.2 | 35.38 |
| Interlock Cotton Knit | 400 | 3.84 | 85 | 100 | 125 | 181 | 1.93 | 11.3 | 43.392 |
| Double Face Cotton Knit | 500 | 4.46 | 98 | 110 | 128 | 195 | 1.95 | 11.8 | 52.628 |
| Ottoman Cotton Knit | 900 | 9.5 | 102 | 120 | 163 | 209 | 2.19 | 11.1 | 105.45 |
| Flax Fabric | 200 | 2.24 | 88 | 101 | 127 | 180 | 1.86 | 11.6 | 25.984 |
| FR Flax Fabric | 200 | 2.16 | 61 | 80 | 111 | 131 | 1.4 | 8.3 | 17.928 |
| Locknit Flax Knit | 700 | 5.7 | 153 | 178 | 196 | 299 | 2.87 | 12.9 | 73.53 |
| Interlock Flax Knit | 1100 | 10.6 | 158 | 198 | 227 | 301 | 2.76 | 12.4 | 131.44 |
| Double Face Flax Knit | 1100 | 10.1 | 164 | 186 | 210 | 291 | 2.52 | 12.9 | 130.29 |
| Ottoman Flax Knit | 1850 | 17.9 | 136 | 176 | 237 | 332 | 3.92 | 13.7 | 245.23 |
| Jute Knit | 380 | 3.95 | 126 | 141 | 189 | 245 | 2.46 | 10.8 | 42.66 |
| Jute Knit (Rochelle Salt) | / | 3.5 | / | 88 | / | / | / | 9.5 | 33.25 |
| Jute Knit (Borax) | / | 3.1 | / | 71 | / | / | / | 13.1 | 40.61 |
| Locknit PP Knit | 218 | 1.9 | 119 | 214 | 238 | 278 | / | 40 | 77.0499 |
| Interlock PP Knit | 442 | 3.9 | 215 | 281 | 369 | 576 | 7.2 | 40 | 156.22 |
| Double Face PP Knit | 690 | 6.1 | 320 | 348 | 480 | 710 | 8.1 | 40 | 243.874 |
| Interlock 2 Yarns PP Knit | 698 | 6.2 | 276 | 316 | 422 | 743 | 9.53 | 40 | 246.701 |
| Ottoman PP Knit | 1195 | 10.6 | 286 | 364 | 509 | 870 | 11.81 | 40 | 422.361 |
| 1 mm-Thick PP Sheet | 1145 | 10.1 | 306 | 386 | 725 | 877 | 12.04 | 40 | 404.689 |
| 2 mm-Thick PP Sheet | 2048 | 18.1 | 399 | 598 | 743 | 1093 | 13.4 | 40 | 723.845 |
| 4 mm-Thick PP Sheet | 3831 | 33.9 | 607 | 746 | 1235 | 1764 | 24 | 40 | 1354.03 |
| 6 mm-Thick PP Sheet | 5729 | 50.6 | 913 | 1252 | 1551 | 2100 | 23.1 | 40 | 2024.86 |
| Balsa | / | 1.2 | 110 | 124 | 136 | 200 | 1.78 | 15.6 | 18.72 |
| Sample | Area density (g/m2) | Sample weight (g) | 20 kW/m2 | 30 kW/m2 | 40 kW/m2 | 50 kW/m2 | Slope pHRR | EHC (kJ/g) | Fire load (kJ) |
| Thin aramid fabric | / | 2 | / | 78 | 114 | / | / | 13.9 | 27.8 |
| Thick aramid fabric | / | 6.3 | 99 | 131 | 148 | 185 | 2.75 | 13.8 | 86.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Gazi, M.; Sonnier, R.; Giraud, S.; Batistella, M.; Basak, S.; Dumazert, L.; Hajj, R.; El Hage, R. Fire Behavior of Thermally Thin Materials in Cone Calorimeter. Polymers 2021, 13, 1297. https://doi.org/10.3390/polym13081297
El Gazi M, Sonnier R, Giraud S, Batistella M, Basak S, Dumazert L, Hajj R, El Hage R. Fire Behavior of Thermally Thin Materials in Cone Calorimeter. Polymers. 2021; 13(8):1297. https://doi.org/10.3390/polym13081297
Chicago/Turabian StyleEl Gazi, Marouane, Rodolphe Sonnier, Stéphane Giraud, Marcos Batistella, Shantanu Basak, Loïc Dumazert, Raymond Hajj, and Roland El Hage. 2021. "Fire Behavior of Thermally Thin Materials in Cone Calorimeter" Polymers 13, no. 8: 1297. https://doi.org/10.3390/polym13081297








