Matching Rheology, Conductivity and Joule Effect in PU/CNT Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Measurements
3. Results
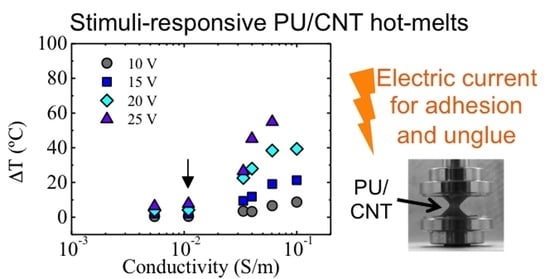
3.1. Thermal Properties
3.2. Rheological Properties
3.3. Electrical Conductivity
3.4. Joule Heating Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breuer, O.; Sundararaj, U. Big returns from small fibers: A review of polymer/carbon nanotube composites. Polym. Compos. 2004, 25, 630–645. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Summerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Progr. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Guragain, S.; Bastakoti, B.P.; Malgras, V.; Nakashima, K.; Yamauchi, Y. Multi-Stimuli-Responsive Polymeric Materials. Chem. Eur. J. 2015, 21, 13164–13174. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Jiang, H.Y.; Junger, O.; Langer, R. Light-induced shape-memory polymers. Nature 2005, 434, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liang, F.; Gou, J. Nanopaper Enabled Shape-Memory Nanocomposite with Vertically Aligned Nickel Nanostrand: Controlled Synthesis and Electrical Actuation. Soft Matter 2011, 7, 7416–7423. [Google Scholar] [CrossRef]
- Lu, H.; Min Huang, W. Synergistic effect of self-assembled carboxylic acid-functionalized carbon nanotubes and carbon fiber for improved electro-activated polymeric shape-memory nanocomposite. Appl. Phys. Lett. 2013, 102, 231910. [Google Scholar] [CrossRef]
- Mohr, R.; Kratz, K.; Weigel, T.; Lucka-Gabor, M.; Moneke, M.; Lendlein, A. Initiation of shape-memory effect by inductive heating of magnetic nanoparticles in thermoplastic polymers. Proc. Natl. Acad. Sci. USA 2006, 103, 3540–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Liu, Y.; Leng, J.; Du, S. Qualitative Separation of the Physical Swelling Effect on the Recovery Behavior of Shape Memory Polymer. Eur. Polym. J. 2010, 46, 1908–1914. [Google Scholar] [CrossRef]
- Fernández, M.; Landa, M.; Muñoz, M.E.; Santamaria, A. Tackiness of an electrically conducting polyurethane-nanotube nanocomposite. Int. J. Adhes. Adhes. 2010, 30, 609–614. [Google Scholar] [CrossRef]
- Fernández, M.; Landa, M.; Muñoz, M.E.; Santamaria, A. Thermal and viscoelastic features of new nanocomposites based on a hot-melt adhesive polyurethane and multi-walled carbon nanotubes. Macromol. Mat. Engin. 2010, 295, 1031–1041. [Google Scholar] [CrossRef]
- Fernández, M.; Landa, M.; Muñoz, M.E.; Santamaria, A. Electrical conductivity of PUR/MWCNT nanocomposites in the molten state, during crystallization and in the solid state. Eur. Polym. J. 2011, 47, 2078–2086. [Google Scholar] [CrossRef]
- El-Tantawy, F. Joule Heating treatments of conductive butyl rubber/ceramic superconductor composites. A new way for improving the stability and reproducibility? Eur. Polym. J. 2001, 37, 565–574. [Google Scholar] [CrossRef]
- Chapartegui, M.; Barcena, J.; Irastorza, X.; ELizetxea, C.; Fernandez, M.; Santamaria, A. Analysis of the conditions to manufacture a MWCNT buckypaper/benzoxazine nanocomposite. Compos. Sci. Technol. 2012, 72, 489–497. [Google Scholar] [CrossRef]
- Chapartegui, M.; Barcena, J.; Irastorza, X.; Elizetxea, C.; Fiamegkou, E.; Kostopoulos, V.; Santamaria, A. Manufacturing, characterization and thermal conductivity of epoxy and benzoxazine multi-walled carbon nanotube buckypaper composites. J. Compos. Mat. 2012, 47, 1705–1715. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Del Rosario, G.; Ureña, A. Coupled thermal-electrical analysis of carbon nanotube/epoxy composites. Polym. Engin. Sci. 2014, 54, 1976–1982. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Moriche, R.; Del Rosario, G.; Jiménez-Suárez, A.; Prolongo, M.G.; Ureña, A. Joule effect self-heating of epoxy composites reinforced with graphitic nanofillers. J. Polym. Res. 2016, 23, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Prolongo, S.G.; Moriche, R.; Jiménez-Suárez, A.; Delgado, A.; Ureña, A. Printable self-heating coatings based on the use of carbon nanoreinforcements. Polym. Compos. 2020, 41, 271–278. [Google Scholar] [CrossRef]
- Jeong, Y.G.; An, J.E. UV-cured epoxy/graphene nanocomposite films: Preparation, structure and electric heating performance. Polym. Int. 2014, 63, 1895–1901. [Google Scholar] [CrossRef]
- Gómez-Barreiro, S.; Gracia-Fernández, C.; Löpez-Beceiro, J.; Artiaga, R. Rheological testing of a curing process controlled by Joule heating. Polym. Test. 2016, 55, 97–100. [Google Scholar] [CrossRef]
- Wu, T.-M.; Chen, E.-C. Isothermal and nonisothermal crystallization kinetics of poly(ε-caprolactone)/multi-walled carbon nanotube composites. Polym. Eng. Sci. 2006, 46, 1309–1317. [Google Scholar] [CrossRef]
- Wu, D.; Wu, L.; Sun, Y.; Zhang, M. Rheological properties and crystallization behavior of multi-walled carbon nanotube/poly(ε-caprolactone) composites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 3137–3147. [Google Scholar] [CrossRef]
- Jeon, K.; Lumata, L.; Tokumoto, T.; Steven, E.; Brooks, J.; Alamo, R.G. Low electrical conductivity threshold and crystalline morphology of single-walled carbon nanotubes – high density polyethylene nanocomposites characterized by SEM, Raman spectroscopy and AFM. Polymer 2007, 48, 4751–4764. [Google Scholar] [CrossRef]
- Trujillo, M.; Arnal, M.L.; Müller, A.J.; Laredo, E.; Bredeau, S.; Bonduel, D.; Dubois, P. Thermal and Morphological Characterization of Nanocomposites Prepared by in-Situ Polymerization of High-Density Polyethylene on Carbon Nanotubes. Macromolecules 2007, 40, 6268–6276. [Google Scholar] [CrossRef]
- Vega, J.F.; Martínez-Salazar, J.; Trujillo, M.; Arnal, M.L.; Müller, A.J.; Bredeau, S.; Dubois, P. Rheology, Processing, Tensile Properties, and Crystallization of Polyethylene/Carbon Nanotube Nanocomposites. Macromolecules 2009, 42, 4719–4727. [Google Scholar] [CrossRef]
- Trujillo, M.; Arnal, M.L.; Müller, A.J.; Mujica, M.A.; Urbina de Navarro, C.; Ruelle, B.; Dubois, P. Supernucleation and crystallization regime change provoked by MWNT addition to poly(ε-caprolactone). Polymer 2012, 53, 832–841. [Google Scholar] [CrossRef]
- Vega, J.F.; Fernández-Alcázar, J.; López, J.V.; Michell, R.M.; Pérez-Camargo, R.A.; Ruelle, B.; Martínez-Salazar, J.; Arnal, M.L.; Dubois, P.; Müller, A.J. Competition between supernucleation and plasticization in the crystallization and rheological behavior of PCL/CNT-based nanocomposites and nanohybrids. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1310–1325. [Google Scholar] [CrossRef] [Green Version]
- Pötschke, P.; Abdel-Goad, M.; Alig, I.; Dudkin, S.; Lellinger, D. Rheological and dielectrical characterization of melt mixed polycarbonate-multiwalled carbon nanotube composites. Polymer 2004, 45, 8863–8870. [Google Scholar] [CrossRef]
- Koerner, H.; Liu, W.; Alexander, M.; Mirau, P.; Dowty, H.; Vaia, R. Deformation–morphology correlations in electrically conductive carbon nanotube—Thermoplastic polyurethane nanocomposites. Polymer 2005, 46, 4405. [Google Scholar] [CrossRef]
- Fernandez, I.; Santamaria, A.; Muñoz, M.E.; Castell, P. A rheological analysis of interactions in phenoxy/organoclay nanocomposites. Eur. Polym. J. 2007, 43, 3171–3176. [Google Scholar] [CrossRef]
- Park, K.-Y.; Lee, S.-E.; Kim, C.-G.; Han, J.-H. Fabrication and electromagnetic characteristics of electromagnetic wave absorbing sandwich structures. Comp. Sci. Technol. 2006, 66, 576. [Google Scholar] [CrossRef]
- Booij, H.C.; Palmen, J.H.M. Some aspects of linear and nonlinear viscoelastic behavior of polymer melts in shear. Rheol. Acta 1982, 21, 376–387. [Google Scholar] [CrossRef]
- Mavridis, H.; Shroff, R.N. Temperature dependence of polyolefin melt rheology. Polym. Eng. Sci. 1992, 32, 17778–17791. [Google Scholar] [CrossRef]
- Booij, H.C.; Palmen, J.H.M. Linear viscoelastic properties of a miscible polymer blend system. In Theoretical and Applied Rheology, Proceedings of the 11th International Congress on Rheology, Brussels, Belgium, 17–21 August 1992; Moldenaers, P., Keunings, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 321–323. [Google Scholar]
- Ferry, J.D. Viscoelastic Properties of Polymers; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Barrau, J.; Demont, P.; Peigney, A.; Laurent, C.; Lacabanne, C. DC and AC conductivity of carbon nanotubes–polyepoxy composites. Macromolecules 2003, 36, 5187–5194. [Google Scholar] [CrossRef] [Green Version]
- Devendra, R.; Hatzikiriakos, S.G.; Vogel, R. Rheology of metallocene polyethylene-based nanocomposites: Influence of graft modification. J. Rheol. 2006, 50, 415–434. [Google Scholar] [CrossRef]
- Clerc, J.P.; Giraud, G.; Laugier, J.M.; Luck, J.M. The electrical conductivity of binary disordered systems, percolation clusters, fractals and related models. Adv. Phys. 1990, 39, 191–309. [Google Scholar] [CrossRef]
- Bunde, A.; Havlin, S. Fractals and Disordered Systems; Springer: Berlin, Germany, 1996. [Google Scholar]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory; Taylor and Francis: London, UK, 1994. [Google Scholar]
- Kim, Y.J.; An, K.J.; Suh, K.S.; Choi, H.D.; Kwon, J.H.; Chung, Y.C.; Kim, W.N.; Lee, A.-K.; Choi, J.-I.; Yoon, H.G. Hybridization of oxidized MWNT and silver powder in polyurethane matrix for electromagnetic interference shielding application. IEEE Transact. Electromagn. C 2005, 47, 872–879. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- McClory, C.; McNally, T.; Baxendale, M.; Potschke, P.; Blau, W.; Ruether, M. Electrical and rheological percolation of PMMA/MWCNT nanocomposites as a function of CNT geometry and functionality. Eur. Polym. J. 2010, 46, 854–868. [Google Scholar] [CrossRef] [Green Version]
- Canales, J.; Muñoz, M.E.; Fernández, F.; Santamaria, A. Rheology, electtrical conductivity and crystallinity of a polyurethane/graphene composite: Implications for its use as hot-melt adhesive. Compos. Part A 2016, 84, 9–16. [Google Scholar] [CrossRef]
- Chapartegui, M.; Markaide, N.; Florez, S.; Elizetxea, C.; Fernández, M.; Santamaría, A. Specific rheological and electrical features of carbon nanotube dispersions in an epoxy matrix. Compos. Sci. Technol. 2010, 70, 879–884. [Google Scholar] [CrossRef]
- Lisunova, M.O.; Mamunya, Y.P.; Lebovka, N.I.; Melezhyk, A.V. Percolation behaviour of ultrahigh weight polyethylene/multi-walled carbon nanotubes composites. Eur. Polym. J. 2007, 43, 949–958. [Google Scholar] [CrossRef]
- Connor, M.T.; Roy, S.; Ezquerra, T.A.; Baltá Calleja, F.J. Broad band AC conductivity of conductor–polymer composites. Phys. Rev. B 1998, 57, 2286–2294. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, J.Z.; Velagala, B.S.; Shulte, K.; Bauhofer, W. Two percolation thresholds in carbon nanotube epoxy composites. Comp. Sci. Technol. 2007, 67, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Nayak, L.; Rahaman, M.; Aldalbahi, A.; Kumar Chaki, T.; Khastgir, D. Polyimide-Carbon nanotubes nanocomposites: Electrical ocnduction behavior under cryogenic condition. Polym. Engin. Sci. 2017, 57, 291–298. [Google Scholar] [CrossRef]
- Moriche, R.; Moreno-Avilés, M.A.; Jiménez-Suárez, A.; Prolongo, S.G.; Ureña, A. Graphene nanoplatelets electrical networks as highly efficient self-heating materials for glass fiber fabrics. J. Ind. Text. 2020. [Google Scholar] [CrossRef]
- Kim, B.W.; Park, S.H.; Bandaru, P.R. Anomalous decrease of the specific heat capacity at the electrical and thermal conductivity percolation threshold in nanocomposites. Appl. Phys. Lett. 2014, 105, 253108. [Google Scholar] [CrossRef]
- Sangroniz, L.; Sangroniz, A.; Fernández, M.; Etxeberria, A.; Müller, A.J.; Santamaria, A. Elaboration and characterization of conductive polymer nanocomposites with potential use as electrically driven membranes. Polymers 2019, 11, 1180. [Google Scholar] [CrossRef] [Green Version]
- Yum, S.-G.; Yin, H.; Jang, S.-H. Toward multi-functional road surface design with the nanocomposite coating of carbon nanotube modified polyurethane: Lab-scale experiments. Nanomaterials 2020, 10, 1905. [Google Scholar] [CrossRef] [PubMed]
- Willocq, B.; Bose, R.K.; Khelifa, F.; Garcia, S.J.; Dubois, P.H.; Raquez, J.M. Healing by the Joule effect of electrically conductive poly(ester-urethane)/ carbon nanotube nanocomposites. J. Mater. Chem. A 2016, 4, 4089–4097. [Google Scholar] [CrossRef]













| Applied Voltage (V) | Tmax (°C) | |||||
|---|---|---|---|---|---|---|
| 2 wt% | 3 wt% | 4 wt% | 5 wt% | 6 wt% | 8 wt% | |
| 10 | 23.4 | 21.8 | 25.6 | 27.4 | 29.0 | 30.2 |
| 15 | 24.6 | 23.6 | 31.5 | 35.0 | 41.7 | 43.3 |
| 20 | 26.5 | 25.8 | 39.5 | 50.3 | 60.9 | 62.1 |
| 25 | 29.0 | 29.3 | 48.9 | 67.8 | 78.4 | / |
| 30 | 32.3 | 34.7 | 60.2 | / | / | / |
| 35 | 36.0 | 41.3 | / | / | / | / |
| 40 | 40.3 | 52.8 | / | / | / | / |
| 45 | 46.1 | 59.5 | / | / | / | / |
| 50 | 49.8 | / | / | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangroniz, L.; Landa, M.; Fernández, M.; Santamaria, A. Matching Rheology, Conductivity and Joule Effect in PU/CNT Nanocomposites. Polymers 2021, 13, 950. https://doi.org/10.3390/polym13060950
Sangroniz L, Landa M, Fernández M, Santamaria A. Matching Rheology, Conductivity and Joule Effect in PU/CNT Nanocomposites. Polymers. 2021; 13(6):950. https://doi.org/10.3390/polym13060950
Chicago/Turabian StyleSangroniz, Leire, Maite Landa, Mercedes Fernández, and Antxon Santamaria. 2021. "Matching Rheology, Conductivity and Joule Effect in PU/CNT Nanocomposites" Polymers 13, no. 6: 950. https://doi.org/10.3390/polym13060950