Micellar Drug Delivery Systems Based on Natural Biopolymers
Abstract
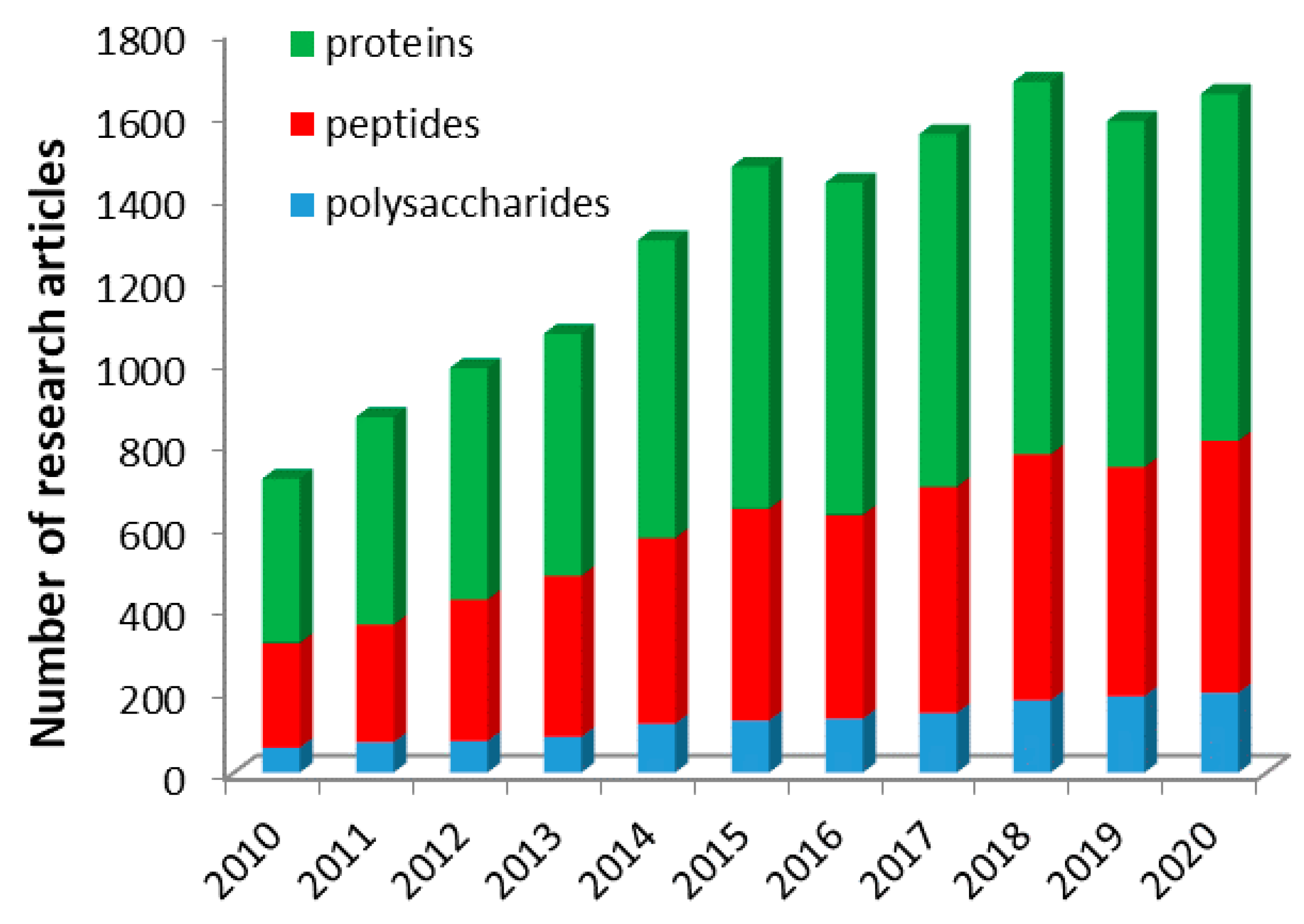
:1. Introduction
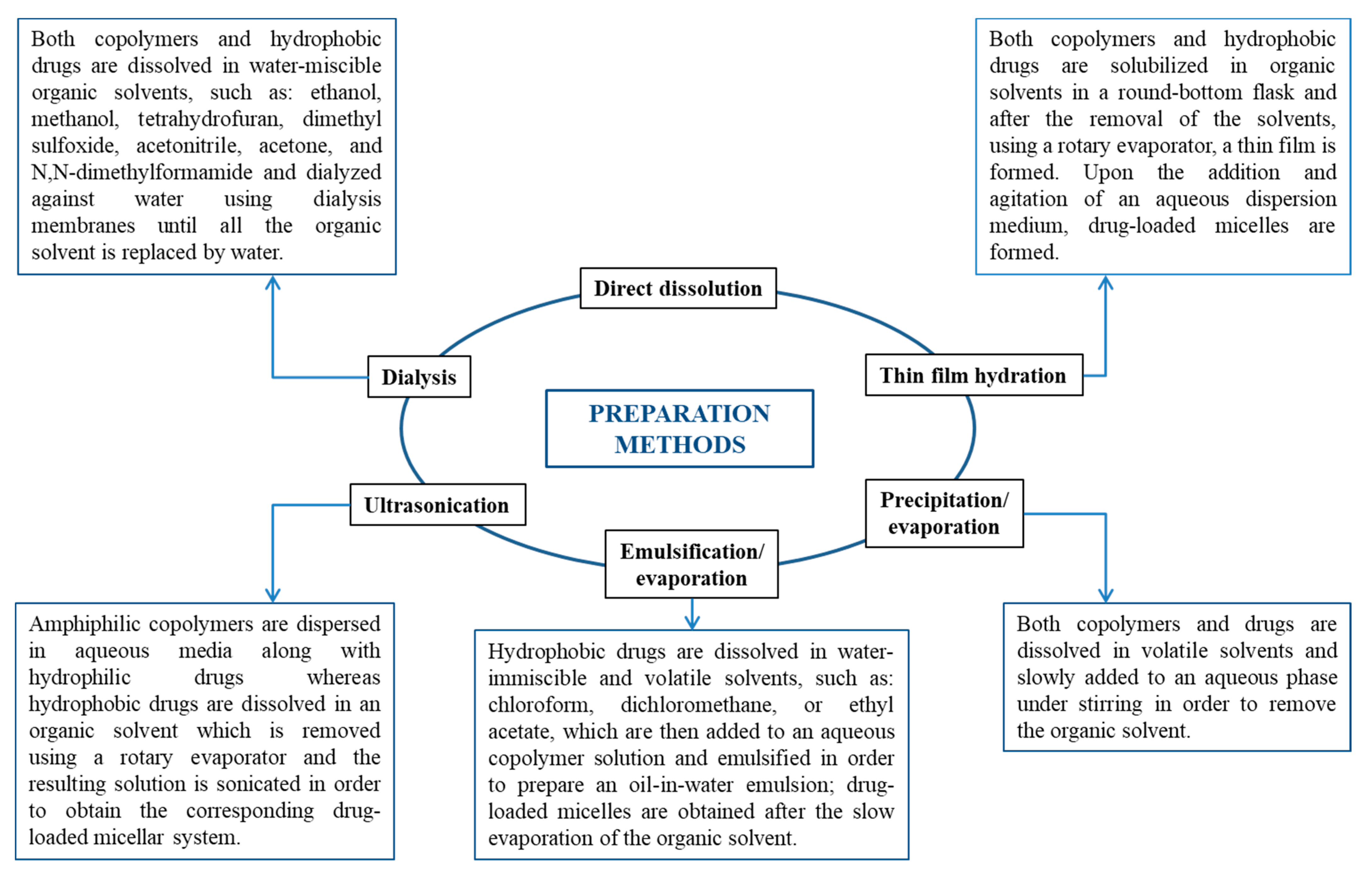
2. Self-Assembly and Drug-Loading Methods
3. Characterization Methods of Drug-Loaded Micellar Systems
4. Micellar Drug Delivery Systems Based on Polysaccharides
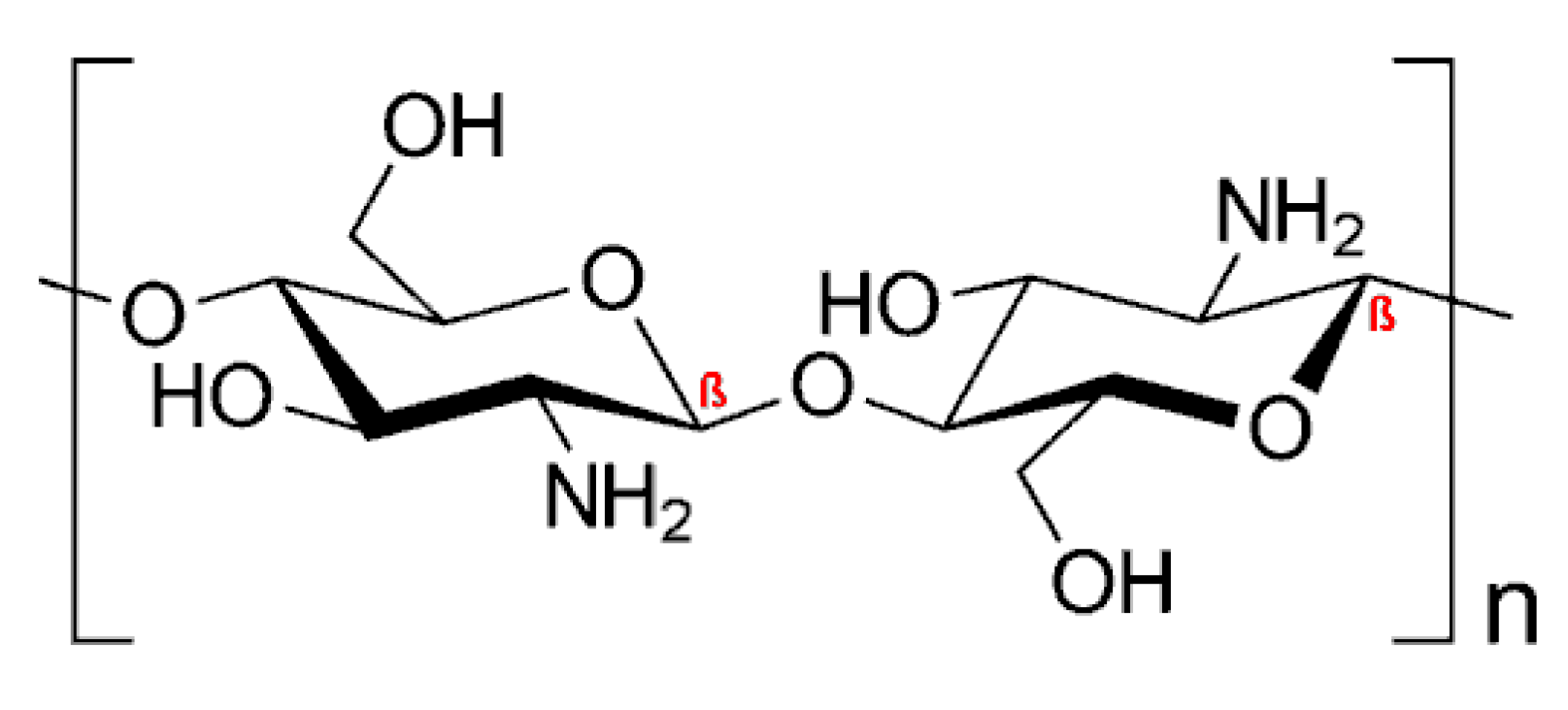
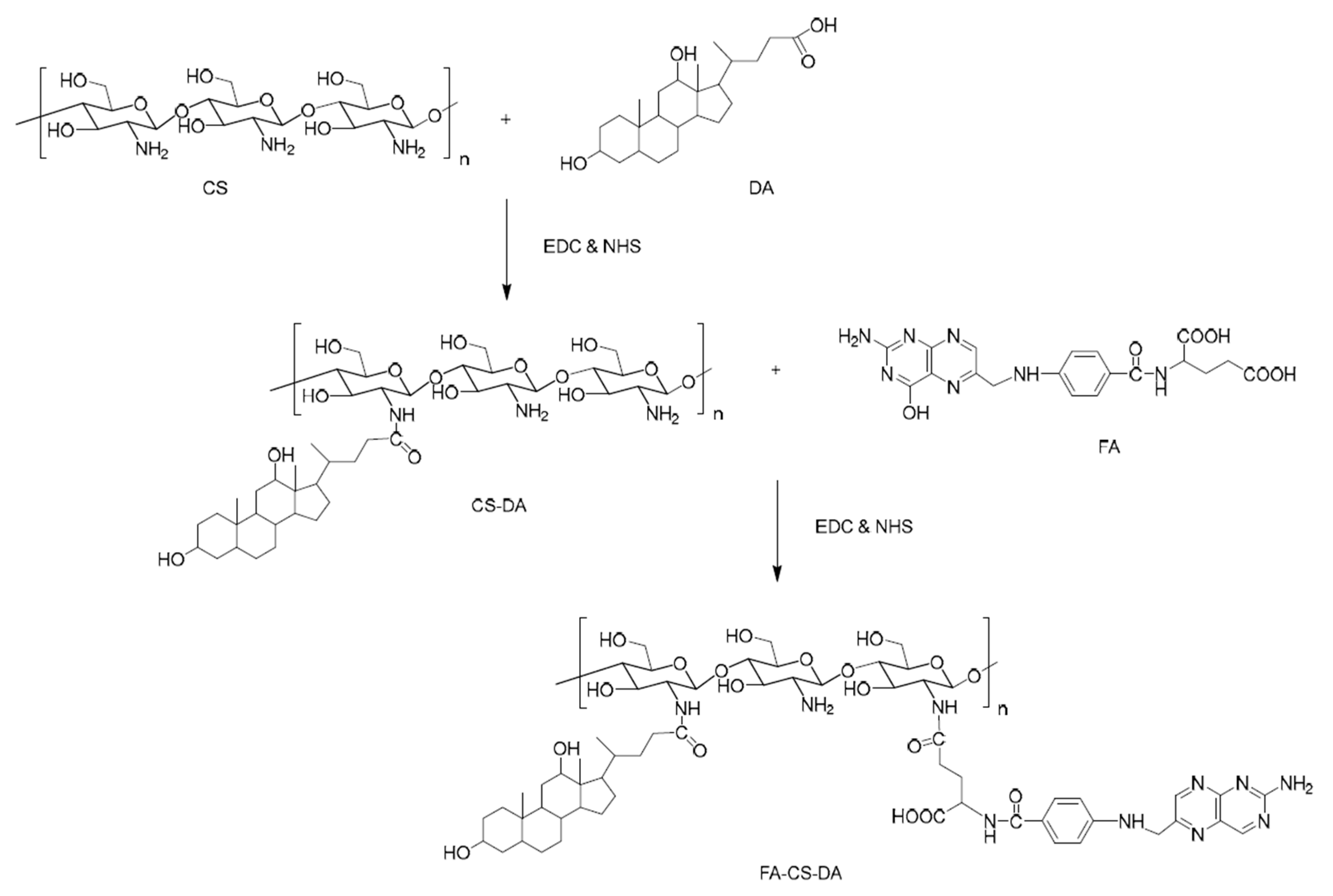
4.1. Drug Delivery Systems Based on Cationic Polysaccharides
4.2. Drug Delivery Systems Based on Anionic Polysaccharides
4.3. Drug Delivery Systems Based on Non-Ionic Polysaccharides
4.4. Drug Delivery Systems Based on Miscellaneous Polysaccharides
5. Micellar Drug Delivery Systems Based on Peptides
6. Micellar Drug Delivery Systems Based on Proteins
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, Y.H.A.; Youssry, M. Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs. Materials 2018, 11, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, D. Polymers and Polymer Nanocomposites for Cancer Therapy. Appl. Sci. 2019, 9, 3899. [Google Scholar] [CrossRef] [Green Version]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers 2019, 11, 1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.-M.; Gherghel, D.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef]
- Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization. Polymers 2020, 12, 1018. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Sukumaran, N.P.; Haponiuk, J.T.; Thomas, S. Biopolymers and Their Composites for Drug Delivery: A Brief Review. Macromol. Symp. 2018, 380, 1800114. [Google Scholar] [CrossRef]
- Atanase, L.; Desbrieres, J.; Riess, G. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Self-Assembly of Block and Graft Copolymers in Organic Solvents: An Overview of Recent Advances. Polymers 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samrot, A.V.; Sean, T.C.; Kudaiyappan, T.; Bisyarah, U.; Mirarmandi, A.; Faradjeva, E.; Mohamed, A.A.; Ali, H.H.; Angalene, J.L.A.; Kumar, S.S. Production, characterization and application of nanocarriers made of polysaccharides, proteins, bio-polyesters and other biopolymers: A review. Int. J. Biol. Macromol. 2020, 165, 3088–3105. [Google Scholar] [CrossRef] [PubMed]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef] [Green Version]
- Kacar, G. Molecular understanding of interactions, structure, and drug encapsulation efficiency of Pluronic micelles from dissipative particle dynamics simulations. Colloid Polym. Sci. 2019, 297, 1037–1051. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Abbad, S.; Pan, R.-R.; Waddad, A.Y.; Hou, L.-L.; Lv, H.-X.; Zhou, J.-P. N-Octyl-N-Arginine Chitosan Micelles as an Oral Delivery System of Insulin. J. Biomed. Nanotechnol. 2013, 9, 601–609. [Google Scholar] [CrossRef]
- Pang, X.; Lu, Z.; Du, H.; Yang, X.; Zhai, G. Hyaluronic acid-quercetin conjugate micelles: Synthesis, characterization, in vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2014, 123, 778–786. [Google Scholar] [CrossRef]
- Li, J.; Shin, G.H.; Chen, X.; Park, H.J. Modified curcumin with hyaluronic acid: Combination of pro-drug and nano-micelle strategy to address the curcumin challenge. Food Res. Int. 2015, 69, 202–208. [Google Scholar] [CrossRef]
- Tian, C.; Asghar, S.; Xu, Y.; Chen, Z.; Zhang, M.; Huang, L.; Ye, J.; Ping, Q.; Xiao, Y.-Y. The effect of the molecular weight of hyaluronic acid on the physicochemical characterization of hyaluronic acid-curcumin conjugates and in vitro evaluation in glioma cells. Colloids Surf. B Biointerfaces 2018, 165, 45–55. [Google Scholar] [CrossRef]
- Cheng, Z.; Lin, H.; Wang, Z.; Yang, X.; Zhang, M.; Liu, X.; Wang, B.; Wu, Z.; Chenabe, D. Preparation and characterization of dissolving hyaluronic acid composite microneedles loaded micelles for delivery of curcumin. Drug Deliv. Transl. Res. 2020, 10, 1520–1530. [Google Scholar] [CrossRef]
- Wu, Y.; Li, F.; Zhang, X.; Li, Z.; Zhang, Q.; Wang, W.; Pan, D.; Zheng, X.; Gu, Z.; Zhang, H.; et al. Tumor microenvironment-responsive PEGylated heparin-pyropheophorbide-a nanoconjugates for photodynamic therapy. Carbohydr. Polym. 2021, 255, 117490. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Liu, Y.; Zhang, H.; Zhang, Z.; Gao, H.; Cun, X. Antitumor and Antimetastasis Activities of Heparin-based Micelle Served as Both Carrier and Drug. ACS Appl. Mater. Interfaces 2016, 8, 9577–9589. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Sreenivasan, K. Conjugation of curcumin onto alginate enhances aqueous solubility and stability of curcumin. Carbohydr. Polym. 2014, 99, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.; James, N.R.; Pr, A.K.; Raj, D.K. Galactosylated alginate-curcumin micelles for enhanced delivery of curcumin to hepatocytes. Int. J. Biol. Macromol. 2016, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, D.; Karabasz, A.; Bzowska, M.; Szuwarzyński, M.; Karewicz, A.; Nowakowska, M. Blood-compatible, stable micelles of sodium alginate—Curcumin bioconjugate for anti-cancer applications. Eur. Polym. J. 2019, 113, 208–219. [Google Scholar] [CrossRef]
- Karabasz, A.; Lachowicz, D.; Karewicz, A.; Mezyk-Kopec, R.; Stalińska, K.; Werner, E.; Cierniak, A.; Dyduch, G.; Bereta, J.; Bzowska, M. Analysis of toxicity and anticancer activity of micelles of sodium alginate-curcumin. Int. J. Nanomed. 2019, 14, 7249–7262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raveendran, R.; Bhuvaneshwar, G.; Sharma, C.P. Hemocompatible curcumin–dextran micelles as pH sensitive pro-drugs for enhanced therapeutic efficacy in cancer cells. Carbohydr. Polym. 2016, 137, 497–507. [Google Scholar] [CrossRef]
- Ji, W.; Wang, B.; Fan, Q.; Xu, C.; He, Y.-W.; Chen, Y. Chemosensitizing indomethacin-conjugated dextran-based micelles for effective delivery of paclitaxel in resistant breast cancer therapy. PLoS ONE 2017, 12, e0180037. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Tang, R.; Zheng, Y.; Yan, G.; Wang, X.; Wang, J.; Tang, R. Indomethacin-grafted and pH-sensitive dextran micelles for overcoming inflammation-mediated multidrug resistance in breast cancer. Carbohydr. Polym. 2020, 237, 116139. [Google Scholar] [CrossRef]
- Chen, S.; Wu, J.; Tang, Q.; Xu, C.; Huang, Y.; Huang, D.; Luo, F.; Wu, Y.; Huang, Y.; Weng, Z.; et al. Nano-micelles based on hydroxyethyl starch-curcumin conjugates for improved stability, antioxidant and anticancer activity of curcumin. Carbohydr. Polym. 2020, 228, 115398. [Google Scholar] [CrossRef]
- Wong, S.; Zhao, J.; Cao, C.; Wong, C.K.; Kuchel, R.P.; De Luca, S.; Hook, J.M.; Garvey, C.J.; Smith, S.; Ho, J.; et al. Just add sugar for carbohydrate induced self-assembly of curcumin. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Lu, H.; Chen, F.; Callari, M.; Pourgholami, M.; Morris, D.L.; Barner-Kowollik, C. PEGylated Albumin-Based Polyion Complex Micelles for Protein Delivery. Biomacromolecules 2016, 17, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, F.; Zhao, M.; Zhu, X.; Ke, C.; Yu, J.; Yan, Z.; Zhang, F.; Sun, Y.; Chen, D.; et al. A Redox-Sensitive Micelle-Like Nanoparticle Self-Assembled from Amphiphilic Adriamycin-Human Serum Albumin Conjugates for Tumor Targeted Therapy. BioMed Res. Int. 2015, 2015, 987404. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.; Cao, Z.; Zhou, W.; Zhang, Y.; Chen, Q.; Lu, Y.; Chen, X.; Guo, Q.; Li, C.; et al. Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials 2018, 183, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, M.H. The Trojan Horse Goes Wild: The Effect of Drug Loading on the Behavior of Nanoparticles. Angew. Chem. Int. Ed. 2021, 60, 2202–2206. [Google Scholar] [CrossRef]
- Zhang, N.; Wardwell, P.R.; Bader, R. Polysaccharide-Based Micelles for Drug Delivery. Pharmers 2013, 5, 329–352. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Liu, F.; Guo, J.; Xue, J.; Qian, Z.; Gu, Y. Folate-modified chitosan micelles with enhanced tumor targeting evaluated by near infrared imaging system. Carbohydr. Polym. 2011, 86, 1118–1129. [Google Scholar] [CrossRef]
- Kansom, T.; Dumkliang, E.; Patrojanasophon, P.; Sajomsang, W.; Saeeng, R.; Zhu, W.M.; Opanasopit, P. Folate-Functionalized Amphiphilic Chitosan Polymeric Micelles Containing Andrographolide Analogue (3A.1) for Colorectal Cancer. Key Eng. Mater. 2019, 819, 15–20. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, L.-J.; Du, Y.-Z.; Hu, F.-Q. Stearic Acid-g-chitosan Polymeric Micelle for Oral Drug Delivery: In Vitro Transport and in Vivo Absorption. Mol. Pharm. 2010, 8, 225–238. [Google Scholar] [CrossRef]
- Moazeni, E.; Gilani, K.; Najafabadi, A.R.; Rouini, M.; Mohajel, N.; Amini, M.; Barghi, M.A. Preparation and evaluation of inhalable itraconazole chitosan based polymeric micelles. DARU J. Pharm. Sci. 2012, 20, 85. [Google Scholar] [CrossRef] [Green Version]
- Fattahi, A.; Golozar, M.A.; Varshosaz, J. Retinoic acid-oligomeric chitosan micelles as novel gene delivery carrier; in vitro transfection study. J. Rep. Pharm. Sci. 2013, 2, 125–130. [Google Scholar]
- Xu, J.; Yu, J.; Xu, X.; Wang, L.; Liu, Y.; Li, L.; Zhao, J.; He, M. Development, Characterization, and Evaluation of PSMA-Targeted Glycol Chitosan Micelles for Prostate Cancer Therapy. J. Nanomater. 2014, 2014, 462356. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Kumari, P.; Ray, E.; Ghosh, B.; Biswas, S. Curcumin-loaded chitosan–cholesterol micelles: Evaluation in monolayers and 3D cancer spheroid model. Nanomedicine 2017, 12, 1435–1453. [Google Scholar] [CrossRef] [PubMed]
- Muddineti, O.S.; Shah, A.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Cholesterol-grafted chitosan micelles as a nanocarrier system for drug-siRNA co-delivery to the lung cancer cells. Int. J. Biol. Macromol. 2018, 118, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Parvathy, J.; Nair, A.S. Evaluation of micellar architecture based on functionalized chitosan for the in vitro release of an antibiotic. Des. Monomers Polym. 2016, 19, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wang, H.; Dong, L.; Zhang, P.; Mu, Y.; Cui, X.; Zhou, J.; Huo, M.; Yin, T. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int. J. Nanomed. 2019, 14, 4649–4666. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.-J.; Jin, Y.; Xie, M.-Q.; Ye, Y.-J.; Qin, D.-D.; Lou, K.-Y.; Chen, Y.-Z.; Gao, F. Preparation and characterization of mPEG grafted chitosan micelles as 5-fluorouracil carriers for effective anti-tumor activity. Chin. Chem. Lett. 2014, 25, 1435–1440. [Google Scholar] [CrossRef]
- Zhao, X.; Yao, Y.; Tian, K.; Zhou, T.; Jia, X.; Li, J.; Liu, P. Leakage-free DOX/PEGylated chitosan micelles fabricated via facile one-step assembly for tumor intracellular pH-triggered release. Eur. J. Pharm. Biopharm. 2016, 108, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Chen, Y.; Yang, H.; Xu, W.; Zhang, M.; Ma, Y.; Achilefu, S.; Gu, Y. A dual-targeting nanocarrier based on modified chitosan micelles for tumor imaging and therapy. Polym. Chem. 2014, 5, 4734–4746. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Jiang, Y.; Xie, Y.; Qiu, L.-L.; Yang, Q.; Lu, H.-Y. Novel amphiphilic folic acid-cholesterol-chitosan micelles for paclitaxel delivery. Oncotarget 2016, 8, 3315–3326. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Sun, S.; Gong, X.; Li, Q.; Yan, P.; Cui, F. Polymeric micelles based on modified glycol chitosan for paclitaxel delivery: Preparation, characterization and evaluation. Int. J. Mol. Sci. 2018, 19, 1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Liangb, N.; Wang, D.; Yana, P.; Kawashimac, Y.; Cui, F.; Suna, S. Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel. Int. J. Mol. Sci. 2018, 19, 3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, G.; Hou, S.; Qu, D.; Tian, C.; Zhu, J.; Xue, L.; Ju, C.; Zhang, C. Self-assembled micelles based on N-octyl-N’-phthalyl-O-phosphoryl chitosan derivative as an effective oral carrier of paclitaxel. Carbohydr. Polym. 2019, 207, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Feng, J.; Zhang, Q.; Wu, W.; Mo, H.; Huang, L.; Zhang, W. l-Carnitine conjugated chitosan-stearic acid polymeric micelles for improving the oral bioavailability of paclitaxel. Drug Deliv. 2020, 27, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Liangb, N.; Yana, P.; Kawashimac, Y.; Cui, F.; Suna, S. A Chitosan-Based Micellar System as Nanocarrier For the Delivery of Paclitaxel. Polymers 2020, 12, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Huo, M.; Wang, J.; Zhou, J.; Mohammad, J.M.; Zhang, Y.; Zhu, Q.; Waddad, A.Y.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef]
- Šmejkalová, D.; Nešporová, K.; Hermannová, M.; Huerta-Angeles, G.; Čožíková, D.; Vištejnová, L.; Safrankova, B.; Novotny, J.; Kučerík, J.; Velebny, V. Paclitaxel isomerisation in polymeric micelles based on hydrophobized hyaluronic acid. Int. J. Pharm. 2014, 466, 147–155. [Google Scholar] [CrossRef]
- Liu, J.; Liang, N.; Li, S.; Han, Y.; Yan, P.; Kawashima, Y.; Cui, F.; Suna, S. Tumor-targeting and redox-sensitive micelles based on hyaluronic acid conjugate for delivery of paclitaxel. J. Biomater. Appl. 2020, 34, 1458–1469. [Google Scholar] [CrossRef]
- Deng, L.; Wang, G.; Ren, J.; Zhang, B.; Yan, J.; Li, W.; Khashab, N.M. Enzymatically triggered multifunctional delivery system based on hyaluronic acid micelles. RSC Adv. 2012, 2, 12909–12914. [Google Scholar] [CrossRef]
- Saadat, E.; Amini, M.; Dinarvand, R.; Dorkoosh, F.A. Polymeric micelles based on hyaluronic acid and phospholipids: Design, characterization, and cytotoxicity. J. Appl. Polym. Sci. 2014, 131, 40944–40952. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, M.; Huang, Y.; Gong, K.; Chen, J. Mechanisms of cellular uptake with hyaluronic acid—octadecylamine micelles as drug delivery nanocarriers. RSC Adv. 2016, 6, 39896–39902. [Google Scholar] [CrossRef]
- Gao, Q.-Q.; Zhang, C.-M.; Zhang, E.-X.; Chen, H.-Y.; Zhen, Y.-H.; Zhang, S. Zwitterionic pH-responsive hyaluronic acid polymer micelles for delivery of doxorubicin. Colloids Surf. B Biointerfaces 2019, 178, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Wang, L.; Wang, X.; Tu, P. Comparison of hyaluronic acid-based micelles and polyethylene glycol-based micelles on reversal of multidrug resistance and enhanced anticancer efficacy in vitro and in vivo. Drug Deliv. 2018, 25, 330–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Han, J.; Jin, Z.; Kim, C.-S.; Park, S.; Kim, K.-P.; Park, J.-O.; Choi, E. Dual tumor-targeted multifunctional magnetic hyaluronic acid micelles for enhanced MR imaging and combined photothermal-chemotherapy. Colloids Surf. B Biointerfaces 2018, 164, 424–435. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Meng, F.; Cheng, L.; Feijen, J.; Zhong, Z. Reduction-responsive core-crosslinked hyaluronic acid-b-poly(trimethylene carbonate-co-dithiolane trimethylene carbonate) micelles: Synthesis and CD44-mediated potent delivery of docetaxel to triple negative breast tumor in vivo. J. Mater. Chem. B 2018, 6, 3040–3047. [Google Scholar] [CrossRef]
- Bongiovì, F.; Fiorica, C.; Palumbo, F.S.; Di Prima, G.; Giammona, G.; Pitarresi, G. Imatinib-Loaded Micelles of Hyaluronic Acid Derivatives for Potential Treatment of Neovascular Ocular Diseases. Mol. Pharm. 2018, 15, 5031–5045. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef] [Green Version]
- Ahn, D.-G.; Lee, J.; Park, S.-Y.; Kwark, Y.-J.; Lee, K.Y. Doxorubicin-Loaded Alginate-g-Poly(N-isopropylacrylamide) Micelles for Cancer Imaging and Therapy. ACS Appl. Mater. Interfaces 2014, 6, 22069–22077. [Google Scholar] [CrossRef]
- Yurong, G.; Li, G.; Gao, Y.; Jiang, H.; Tao, Q. Thermo-sensitive complex micelles from sodium alginate- graft -poly(N -isopropylacrylamide) for drug release. Int. J. Biol. Macromol. 2016, 86, 296–301. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Zhang, R.; Yuan, S.; Lu, Q.; Yu, Y. Synthesis and micelle properties of the hydrophobic modified alginate. Int. J. Polym. Mater. 2017, 66, 742–747. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, K.; Li, J.; Feng, Y.; Yu, G.; Wang, L.; Zhao, X.; Peng, Y.; Zhang, Q. Electrolyte and pH-sensitive amphiphilic alginate: Synthesis, self-assembly and controlled release of acetamiprid. RSC Adv. 2018, 8, 32193–32199. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zhang, L.; Gan, W.; Zhou, J.; Zhang, L. Self-assembled micelles based on hydrophobically modified quaternized cellulose for drug delivery. Colloids Surf. B Biointerfaces 2011, 83, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Songsurang, K.; Siraleartmukul, K.; Muangsin, N. Mucoadhesive drug carrier based on functional-modified cellulose as poorly water-soluble drug delivery system. J. Microencapsul. 2015, 32, 450–459. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, J.; Guo, Y.; Ge, W.; Sun, R.; Chen, M.-W.; Wang, X.; Wang, L. Multi-color light-emitting amphiphilic cellulose/conjugated polymers nanomicelles for tumor cell imaging. Cellulose 2016, 24, 889–902. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Lu, S.; Li, C.; Zhao, W.; Zhao, Y.; Yu, S.; Wang, T.; Sun, T. Nano micelles of cellulose-graft-poly (l-lactic acid) anchored with epithelial cell adhesion antibody for enhanced drug loading and anti-tumor effect. Mater. Today Commun. 2020, 22, 100764. [Google Scholar] [CrossRef]
- Sun, H.; Guo, B.; Li, X.; Cheng, R.; Meng, F.; Liu, H.; Zhong, Z. Shell-Sheddable Micelles Based on Dextran-SS-Poly(ε-caprolactone) Diblock Copolymer for Efficient Intracellular Release of Doxorubicin. Biomacromolecules 2010, 11, 848–854. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Hu, P.; Hou, M.; Dong, Y.; Wei, Y. Antifouling zwitterionic dextran micelles for efficient loading DOX. Carbohydr. Polym. 2018, 191, 136–141. [Google Scholar] [CrossRef]
- Du, Y.; Weng, Q.; Yuan, H.; Hu, F.-Q. Synthesis and Antitumor Activity of Stearate-g-dextran Micelles for Intracellular Doxorubicin Delivery. ACS Nano 2010, 4, 6894–6902. [Google Scholar] [CrossRef]
- Varshosaz, J.; Hassanzadeh, F.; Sadeghi, H.M.M.; Firozian, F.; Mirian, M. Effect of Molecular Weight and Molar Ratio of Dextran on Self-Assembly of Dextran Stearate Polymeric Micelles as Nanocarriers for Etoposide. J. Nanomater. 2012, 2012, 265657. [Google Scholar] [CrossRef]
- Lin, B.; Su, H.; Jin, R.; Li, D.; Wu, C.; Jiang, X.; Xia, C.; Gong, Q.; Song, B.; Ai, H. Multifunctional dextran micelles as drug delivery carriers and magnetic resonance imaging probes. Sci. Bull. 2015, 60, 1272–1280. [Google Scholar] [CrossRef] [Green Version]
- Situ, J.-Q.; Wang, X.-J.; Zhu, X.-L.; Xu, X.-L.; Kang, X.-Q.; Hu, J.-B.; Lu, C.-Y.; Ying, X.-Y.; Yu, R.-S.; You, J.; et al. Multifunctional SPIO/DOX-loaded A54 Homing Peptide Functionalized Dextran-g-PLGA Micelles for Tumor Therapy and MR Imaging. Sci. Rep. 2016, 6, 35910. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.S.; Liu, S.; Chen, Y.Y.; Meerasa, A.; Gu, F.X. Size-tunable nanoparticles composed of dextran-b-poly(D,L-lactide) for drug delivery applications. Nano Res. 2011, 5, 49–61. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Z.; Chen, L.; Cao, Y.; He, C.; Zhu, X. Biodegradable Stereocomplex Micelles Based on Dextran-block-polylactide as Efficient Drug Deliveries. Langmuir 2013, 29, 13072–13080. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, D.H.; Chung, C.W.; Yoo, J.J.; Choi, K.H.; Kim, C.H.; Ha, S.H.; Kang, D.H. Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly(DL-lactide-co-glycolide) copolymer. Int. J. Nanomed. 2011, 6, 1415–1427. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhou, Y.; Chen, W.; Ren, J.; Zhang, L.; Lu, L.; Luo, G.; Huang, H. Preparation, Characterization and Evaluation of α-Tocopherol Succinate-Modified Dextran Micelles as Potential Drug Carriers. Materials 2015, 8, 6685–6696. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, X. Binding, stability, and antioxidant activity of curcumin with self-assembled casein–dextran conjugate micelles. Int. J. Food Prop. 2017, 20, 3295–3307. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yi, J.; Li, W.; Wang, L.; Wang, Z. Synthesis and characterization of three novel amphiphilic dextran self-assembled micelles as potential drug delivery system. J. Mater. Sci. 2017, 52, 12593–12607. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Concheiro, A.; Makwana, H.; Fernandez-Trillo, F.; Alexander, C.; Alvarez-Lorenzo, C. Dually sensitive dextran-based micelles for methotrexate delivery. RSC Adv. 2017, 7, 14448–14460. [Google Scholar] [CrossRef] [Green Version]
- Das Karmakar, P.; Seesala, V.S.; Pal, A.; Dhara, S.; Chatterjee, S.; Pal, S. Synthesis of RAFT-Mediated Amphiphilic Graft Copolymeric Micelle Using Dextran and Poly (Oleic Acid) toward Oral Delivery of Nifedipine. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2354–2363. [Google Scholar] [CrossRef]
- Fan, Y.; Picchioni, F. Modification of starch: A review on the application of “green” solvents and controlled functionalization. Carbohydr. Polym. 2020, 241, 116350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Z.; Shi, F.; Ding, J.; Xiao, C.; Zhuang, X.; He, C.; Chen, L.; Zhu, X. Disulfide crosslinked PEGylated starch micelles as efficient intracellular drug delivery platforms. Soft Matter 2013, 9, 2224–2233. [Google Scholar] [CrossRef]
- Wu, C.; Yang, J.; Xu, X.; Gao, C.; Lü, S.; Liu, M. Redox-responsive core-cross-linked mPEGylated starch micelles as nanocarriers for intracellular anticancer drug release. Eur. Polym. J. 2016, 83, 230–243. [Google Scholar] [CrossRef]
- Wen, N.; Gao, C.; Lü, S.; Xu, X.; Bai, X.; Wu, C.; Ning, P.; Zhang, S.; Liu, M. Novel amphiphilic glucose-responsive modified starch micelles for insulin delivery. RSC Adv. 2017, 7, 45978–45986. [Google Scholar] [CrossRef] [Green Version]
- Wen, N.; Lü, S.; Gao, C.; Xu, X.; Bai, X.; Wu, C.; Ning, P.; Liu, M. Glucose-responsive zwitterionic dialdehyde starch-based micelles with potential anti-phagocytic behavior for insulin delivery. Chem. Eng. J. 2018, 335, 52–62. [Google Scholar] [CrossRef]
- Kou, Z.; Dou, D.; Zhu, J.; Mai, Y.; Yi, H.; Lan, L.; Lan, P. Release Mechanism and pH Responsiveness of Starch-Based Polymers. Nano 2019, 14, 1950145. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhao, L. Sorafenib-loaded hydroxyethyl starch-TG100-115 micelles for the treatment of liver cancer based on synergistic treatment. Drug Deliv. 2019, 26, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Liang, R.; Zhong, F.; Ye, A.M.; Hemar, Y.; Yang, Z.; Singh, H. Self-Assembled Micelles Based on OSA-Modified Starches for Enhancing Solubility of β-Carotene: Effect of Starch Macromolecular Architecture. J. Agric. Food Chem. 2019, 67, 6614–6624. [Google Scholar] [CrossRef]
- Maiti, S.; Mukherjee, S. Controlled drug delivery attributes of co-polymer micelles and xanthan-O-carboxymethyl hydrogel particles. Int. J. Biol. Macromol. 2014, 70, 37–43. [Google Scholar] [CrossRef]
- Garhwal, R.; Shady, S.F.; Ellis, E.J.; Ellis, J.Y.; Leahy, C.D.; McCarthy, S.P.; Crawford, K.S.; Gaines, P. Sustained Ocular Delivery of Ciprofloxacin Using Nanospheres and Conventional Contact Lens Materials. Investig. Opthalmol. Vis. Sci. 2012, 53, 1341–1352. [Google Scholar] [CrossRef]
- Wang, J.; Cui, S.; Bao, Y.; Xing, J.; Hao, W. Tocopheryl pullulan-based self assembling nanomicelles for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 43, 614–621. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Ji, F.; Bao, Y.; Wang, J.; Wang, X.; Guo, L.; Li, Y. New bifunctional-pullulan-based micelles with good biocompatibility for efficient co-delivery of cancer-suppressing p53 gene and doxorubicin to cancer cells. RSC Adv. 2015, 5, 94719–94731. [Google Scholar] [CrossRef]
- Hassanzadeh, F.; Varshosaz, J. Biotin-encoded Pullulan-Retinoic Acid Engineered Nanomicelles: Preparation, Optimization and In Vitro Cytotoxicity Assessment in MCF-7 Cells. Indian J. Pharm. Sci. 2016, 78, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Ji, F.; Bao, Y.; Xia, J.; Guo, L.; Wang, J.; Li, Y. Biocompatible cationic pullulan-g-desoxycholic acid-g-PEI micelles used to co-deliver drug and gene for cancer therapy. Mater. Sci. Eng. C 2017, 70, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, F.; Mahmoudi, E.; Varshosaz, J.; Khodarahmi, G.A.; Rostami, M.; Ghanadian, M.; Dana, N. Novel NGR anchored pullulan micelles for controlled and targeted delivery of doxorubicin to HeLa cancerous cells. Iran. Polym. J. 2018, 27, 263–274. [Google Scholar] [CrossRef]
- Constantin, M.; Bucatariu, S.; Ursu, L.; Butnaru, M.; Daraba, O.M.; Burlui, A.M.; Fundueanu, G. Novel Cationic and Hydrophobic Pullulan Derivatives as DNA Nanoparticulate Carriers. Cellul. Chem. Technol. 2019, 53, 695–707. [Google Scholar] [CrossRef]
- Yuan, H.; Zhong, W.; Wang, R.; Zhou, P.; Nie, Y.; Hu, W.; Tao, X.; Yang, P. Preparation of Cholesteryl-Modified Aminated Pullulan Nanoparticles to Evaluate Nanoparticle of Hydrophobic Degree on Drug Release and Cytotoxicity. J. Nanomater. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Synthesis and characterization of redox-sensitive heparin-β-sitosterol micelles: Their application as carriers for the pharmaceutical agent, doxorubicin, and investigation of their antimetastatic activities in vitro. Mater. Sci. Eng. C 2017, 75, 1326–1338. [Google Scholar] [CrossRef]
- Emami, J.; Kazemi, M.; Hasanzadeh, F.; Minaiyan, M.; Mirian, M.; Lavasanifar, A. Novel pH-triggered biocompatible polymeric micelles based on heparin–α-tocopherol conjugate for intracellular delivery of docetaxel in breast cancer. Pharm. Dev. Technol. 2020, 25, 492–509. [Google Scholar] [CrossRef]
- Peng, N.; Yang, M.; Tang, Y.; Zou, T.; Guo, F.; Wu, K.; Wang, X.; Li, X.; Li, X. Amphiphilic hexadecyl-quaternized chitin micelles for doxorubicin delivery. Int. J. Biol. Macromol. 2019, 130, 615–621. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Pinheiro, A.C.; Pastrana, L.M.; Vicente, A.A. Amphiphilic Modified Galactomannan as a Novel Potential Carrier for Hydrophobic Compounds. Front. Sustain. Food Syst. 2019, 3, 17. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, L.; Wu, J.; Liu, Y.; Zhang, Z.; Guan, Q. Doxorubicin-loaded folate-mediated pH-responsive micelle based on Bletilla striata polysaccharide: Release mechanism, cellular uptake mechanism, distribution, pharmacokinetics, and antitumor effects. Int. J. Biol. Macromol. 2020, 164, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.D.J.; Chaves, L.L.; Ribeiro, F.D.O.S.; De Lima, L.R.M.; Oliveira, T.C.; García-Villén, F.; Viseras, C.; De Paula, R.C.; Rolim-Neto, P.J.; Hallwass, F.; et al. Microwave-initiated rapid synthesis of phthalated cashew gum for drug delivery systems. Carbohydr. Polym. 2021, 254, 117226. [Google Scholar] [CrossRef] [PubMed]
- Negahban, Z.; Shojaosadati, S.A.; Hamedi, S. A novel self-assembled micelles based on stearic acid modified schizophyllan for efficient delivery of paclitaxel. Colloids Surf. B Biointerfaces 2021, 199, 111524. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Lu, C.; Wang, Z.; Du, Y.; Xu, X.; Xu, M.; Zhao, Z.; Chen, M.; Dai, Y.; Weng, Q.; et al. Fucoidan-based micelles as P-selectin targeted carriers for synergistic treatment of acute kidney injury. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102342. [Google Scholar] [CrossRef]
- Cao, C.; Zhao, J.; Lu, M.; Garvey, C.J.; Stenzel, M.H. Correlation between Drug Loading Content and Biological Activity: The Complexity Demonstrated in Paclitaxel-Loaded Glycopolymer Micelle System. Biomacromolecules 2019, 20, 1545–1554. [Google Scholar] [CrossRef]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Banerjee, A.; Onyuksel, H. Peptide delivery using phospholipid micelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 562–574. [Google Scholar] [CrossRef]
- Wu, X.L.; Kim, J.H.; Koo, H.; Bae, S.M.; Shin, H.; Kim, M.S.; Lee, B.-H.; Park, R.-W.; Choi, K.; Kwon, I.C.; et al. Tumor-Targeting Peptide Conjugated pH-Responsive Micelles as a Potential Drug Carrier for Cancer Therapy. Bioconjugate Chem. 2010, 21, 208–213. [Google Scholar] [CrossRef]
- Dong, H.; Dube, N.; Shu, J.Y.; Seo, J.W.; Mahakian, L.M.; Ferrara, K.W.; Xu, T. Long-Circulating 15 nm Micelles Based on Amphiphilic 3-Helix Peptide–PEG Conjugates. ACS Nano 2012, 6, 5320–5329. [Google Scholar] [CrossRef]
- Lee, J.; Hyun, H.; Kim, H.A.; Lee, M. Dexamethasone loaded r3v6 peptide micelles for gene delivery. J. Control. Release 2011, 152, e151–e152. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.; Lee, M. Peptide Micelles for Anti-cancer Drug Delivery in an Intracranial Glioblastoma Animal Model. Bull. Korean Chem. Soc. 2014, 35, 3030–3034. [Google Scholar] [CrossRef]
- Han, J.; Oh, J.; Ihm, S.-H.; Lee, M. Peptide micelle-mediated curcumin delivery for protection of islet β-cells under hypoxia. J. Drug Target. 2016, 24, 618–623. [Google Scholar] [CrossRef]
- Morelli, G.; Accardo, A.; Vitiello, M.T.; Tesauro, D.; Galdiero, M.; Finamore, E.; Mansi, R.; Ringhieri, P.; Martora, F. Self-assembled or mixed peptide amphiphile micelles from Herpes simplex virus glycoproteins as potential immunomodulatory treatment. Int. J. Nanomed. 2014, 9, 2137–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, C.; Chowdhuri, S.; Kuo, C.-H.; Fang, Y.; Alenghat, F.J.; Hyatt, D.; Kani, K.; Gross, M.E.; Chung, E.J. Protein Mimetic and Anticancer Properties of Monocyte-Targeting Peptide Amphiphile Micelles. ACS Biomater. Sci. Eng. 2017, 3, 3273–3282. [Google Scholar] [CrossRef]
- Joo, J.; Poon, C.; Yoo, S.P.; Chung, E.J. Shape Effects of Peptide Amphiphile Micelles for Targeting Monocytes. Molecules 2018, 23, 2786. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.D.; Cardwell, L.N.; Porciani, D.; A Nguyen, J.; Zhang, R.; Gallazzi, F.; Tata, R.R.; Burke, D.H.; Daniels, M.A.; Ulery, B.D. Aptamer-displaying peptide amphiphile micelles as a cell-targeted delivery vehicle of peptide cargoes. Phys. Biol. 2018, 15, 065006. [Google Scholar] [CrossRef]
- Quader, S.; Liu, X.; Chen, Y.; Mi, P.; Chida, T.; Ishii, T.; Miura, Y.; Nishiyama, N.; Cabral, H.; Kataoka, K. cRGD peptide-installed epirubicin-loaded polymeric micelles for effective targeted therapy against brain tumors. J. Control. Release 2017, 258, 56–66. [Google Scholar] [CrossRef]
- Soria-Carrera, H.; Lucía, A.; De Matteis, L.; Aínsa, J.A.; De La Fuente, J.M.; Martín-Rapún, R. Polypeptidic Micelles Stabilized with Sodium Alginate Enhance the Activity of Encapsulated Bedaquiline. Macromol. Biosci. 2019, 19, 1800397. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, F.; Wu, L.; Tian, Y.; Zhou, B.; Zhang, X.; Huang, R.; Yu, C.; He, G.; Yang, L. Novel Self-Assembled Micelles Based on Cholesterol-Modified Antimicrobial Peptide (DP7) for Safe and Effective Systemic Administration in Animal Models of Bacterial Infection. Antimicrob. Agents Chemother. 2018, 62, 11. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lei, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmers 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Stenzel, M. Drug Delivery Vehicles Based on Albumin-Polymer Conjugates. Macromol. Biosci. 2016, 16, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lu, H.; Dag, A.; Hart-Smith, G.; Stenzel, M.H. Albumin-polymer conjugate nanoparticles and their interactions with prostate cancer cells in 2D and 3D culture: Comparison between PMMA and PCL. J. Mater. Chem. B 2016, 4, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lu, H.; Khine, Y.Y.; Dag, A.; Stenzel, M.H. Polyion Complex Micelle Based on Albumin–Polymer Conjugates: Multifunctional Oligonucleotide Transfection Vectors for Anticancer Chemotherapeutics. Biomacromolecules 2014, 15, 4195–4205. [Google Scholar] [CrossRef] [PubMed]
- Noorani, L.; Stenzel, M.H.; Liang, R.; Pourgholami, M.H.; Morris, D.L. Albumin nanoparticles increase the anticancer efficacy of albendazole in ovarian cancer xenograft model. J. Nanobiotechnol. 2015, 13, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Noorani, L.; Jiang, Y.; Du, A.W.; Stenzel, M.H. Penetration and drug delivery of albumin nanoparticles into pancreatic multicellular tumor spheroids. J. Mater. Chem. B 2017, 5, 9591–9599. [Google Scholar] [CrossRef]
- Jiang, Y.; Wong, S.; Chen, F.; Chang, T.; Lu, H.; Stenzel, M.H. Influencing Selectivity to Cancer Cells with Mixed Nanoparticles Prepared from Albumin–Polymer Conjugates and Block Copolymers. Bioconjugate Chem. 2017, 28, 979–985. [Google Scholar] [CrossRef]
- Taguchi, K.; Lu, H.; Jiang, Y.; Jiang, Y.; Hung, T.T.; Stenzel, M.H. Safety of nanoparticles based on albumin-polymer conjugates as a carrier of nucleotides for pancreatic cancer therapy. J. Mater. Chem. B 2018, 6, 6278–6287. [Google Scholar] [CrossRef]
- Wu, Y.; Shih, E.K.; Ramanathan, A.; Vasudevan, S.; Weil, T. Nano-Sized Albumin-Copolymer Micelles for Efficient Doxorubicin Delivery. Biointerphases 2012, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Liang, M.; Svejkar, D.; Hart-Smith, G.; Lu, H.; Scarano, W.; Stenzel, M.H. Albumin-micelles via a one-pot technology platform for the delivery of drugs. Chem. Commun. 2014, 50, 6394. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Stenzel, M.H. Polyion complex micelles for protein delivery. Aust. J. Chem. 2018, 71, 768–780. [Google Scholar] [CrossRef]
- Li, H.; Yan, K.; Shang, Y.; Shrestha, L.; Liao, R.; Liu, F.; Li, P.; Xu, H.; Xu, Z.; Chu, P.K. Folate-bovine serum albumin functionalized polymeric micelles loaded with superparamagnetic iron oxide nanoparticles for tumor targeting and magnetic resonance imaging. Acta Biomater. 2015, 15, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, M.G.; Jang, Y.L.; Lee, M.S.; Kim, N.W.; Yin, Y.; Lee, J.H.; Lim, S.Y.; Park, J.W.; Kim, J.; et al. Self-assembled PEGylated albumin nanoparticles (SPAN) as a platform for cancer chemotherapy and imaging. Drug Deliv. 2018, 25, 1570–1578. [Google Scholar] [CrossRef]
- Guo, M.; Wang, G. Milk Protein Polymer and Its Application in Environmentally Safe Adhesives. Polymers 2016, 8, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapira, A.; Markman, G.; Assaraf, Y.G.; Livney, Y.D. β-casein–based nanovehicles for oral delivery of chemotherapeutic drugs: Drug-protein interactions and mitoxantrone loading capacity. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 547–555. [Google Scholar] [CrossRef]
- Shapira, A.; Davidson, I.; Avni, N.; Assaraf, Y.G.; Livney, Y.D. β-Casein nanoparticle-based oral drug delivery system for potential treatment of gastric carcinoma: Stability, target-activated release and cytotoxicity. Eur. J. Pharm. Biopharm. 2012, 80, 298–305. [Google Scholar] [CrossRef]
- Bachar, M.; Mandelbaum, A.; Portnaya, I.; Perlstein, H.; Even-Chen, S.; Barenholz, Y.; Danino, D. Development and characterization of a novel drug nanocarrier for oral delivery, based on self-assembled β-casein micelles. J. Control. Release 2012, 160, 164–171. [Google Scholar] [CrossRef]
- Perlstein, H.; Bavli, Y.; Turovsky, T.; Rubinstein, A.; Danino, D.; Stepensky, D.; Barenholz, Y. Beta-casein nanocarriers of celecoxib for improved oral bioavailability. Eur. J. Nanomed. 2014, 6, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Perlstein, H.; Turovsky, T.; Gimeson, P.; Cohen, R.; Rubinstein, A.; Danino, D.; Barenholz, Y. Thermotropic behavior of celecoxib-loaded beta-casein micelles: Relevance to the improved bioavailability. Eur. J. Nanomed. 2015, 7, 303–312. [Google Scholar] [CrossRef]
- Xv, L.; Qian, X.; Wang, Y.; Yu, C.; Qin, D.; Zhang, Y.; Jin, P.; Du, Q. Structural Modification of Nanomicelles through Phosphatidylcholine: The Enhanced Drug-Loading Capacity and Anticancer Activity of Celecoxib-Casein Nanoparticles for the Intravenous Delivery of Celecoxib. Nanomaterials 2020, 10, 451. [Google Scholar] [CrossRef] [Green Version]
- Trejo, R.; Dokland, T.; Jurat-Fuentes, J.; Harte, F. Cryo-transmission electron tomography of native casein micelles from bovine milk. J. Dairy Sci. 2011, 94, 5770–5775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Zeev, M.; Assaraf, Y.G.; Livney, Y.D. β-casein nanovehicles for oral delivery of chemotherapeutic drug combinations overcoming P-glycoprotein-mediated multidrug resistance in human gastric cancer cells. Oncotarget 2016, 7, 23322–23334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Zeev, M.; Kelmansky, D.; Assaraf, Y.G.; Livney, Y.D. β-Casein micelles for oral delivery of SN-38 and elacridar to overcome BCRP-mediated multidrug resistance in gastric cancer. Eur. J. Pharm. Biopharm. 2018, 133, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fokkink, R.; Ni, Y.; Kleijn, J.M. Bovine beta-casein micelles as delivery systems for hydrophobic flavonoids. Food Hydrocoll. 2019, 96, 653–662. [Google Scholar] [CrossRef]
- Malekhosseini, P.; Alami, M.; Khomeiri, M.; Esteghlal, S.; Nekoei, A.-R.; Hosseini, S.M.H. Development of casein-based nanoencapsulation systems for delivery of epigallocatechin gallate and folic acid. Food Sci. Nutr. 2019, 7, 519–527. [Google Scholar] [CrossRef]
- Picchio, M.L.; Cuggino, J.C.; Nagel, G.; Wedepohl, S.; Minari, R.J.; Igarzabal, C.I.A.; Gugliotta, L.M.; Calderón, M. Crosslinked casein-based micelles as a dually responsive drug delivery system. Polym. Chem. 2018, 9, 3499–3510. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Su, C.-M.; Huang, C.-Y.; Chen, Y.-L.; Ger, T.-R. pH-responsive magnetic micelles gelatin-g-poly(NIPAAm-co-DMAAm-co-UA)-g-dextran/Fe3O4 as a hydrophilic drug carrier. RSC Adv. 2016, 7, 28207–28212. [Google Scholar] [CrossRef] [Green Version]
- Raja, I.S.; Thangam, R.; Fathima, N.N. Polymeric Micelle of a Gelatin-Oleylamine Conjugate: A Prominent Drug Delivery Carrier for Treating Triple Negative Breast Cancer Cells. ACS Appl. Bio Mater. 2018, 1, 1725–1734. [Google Scholar] [CrossRef]
- Fan, D.; Yu, J.; Yan, R.; Xu, X.; Wang, Y.; Xie, X.; Liu, C.; Liu, Y.; Huang, H. Preparation and Evaluation of Doxorubicin-Loaded Micelles Based on Glycyrrhetinic Acid Modified Gelatin Conjugates for Targeting Hepatocellular Carcinoma. J. Nanomater. 2018, 2018, 8467169. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Dinh, V.T.; Dang, L.H.; Dang, N.N.; Bach, L.G.; Nguyen, C.T.; Nguyen, T.B.T.; Van Thu, L.; Tran, N.Q. Dual Interactions of Amphiphilic Gelatin Copolymer and Nanocurcumin Improving the Delivery Efficiency of the Nanogels. Polymers 2019, 11, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beibei, D.; Tiantang, F.; Jiafeng, L.; Li, G.; Qin, Z.; Wuyou, Y.; Hongyun, T.; Wenxin, W.; Fan, Z. PLLA-Grafted Gelatin Amphiphilic Copolymer and Its Self-Assembled Nano Carrier for Anticancer Drug Delivery. Macromol. Chem. Phys. 2019, 220, 1800528. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Wang, Q. Zein-based micro- and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131, 40696. [Google Scholar] [CrossRef]
- Podaralla, S.; Averineni, R.; Alqahtani, M.; Perumal, O.P. Synthesis of Novel Biodegradable Methoxy Poly(ethylene glycol)–Zein Micelles for Effective Delivery of Curcumin. Mol. Pharm. 2012, 9, 2778–2786. [Google Scholar] [CrossRef]
- Song, R.; Zhou, Y.; Li, Y.; Yang, Z.; Li, F.; Huang, Q.; Shi, T.; Zhang, G. Preparation and characterization of mPEG-g-α-zein biohybrid micelles as a nano-carrier. J. Appl. Polym. Sci. 2015, 132, 42555. [Google Scholar] [CrossRef]
- Sabra, S.A.; Elzoghby, A.O.; Sheweita, S.A.; Haroun, M.; Helmy, M.W.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Rohani, S. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur. J. Pharm. Biopharm. 2018, 128, 156–169. [Google Scholar] [CrossRef]
- Sabra, S.A.; Sheweita, S.A.; Haroun, M.; Ragab, D.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Elzoghby, A.O.; Rohani, S. Magnetically Guided Self-Assembled Protein Micelles for Enhanced Delivery of Dasatinib to Human Triple-Negative Breast Cancer Cells. J. Pharm. Sci. 2019, 108, 1713–1725. [Google Scholar] [CrossRef]


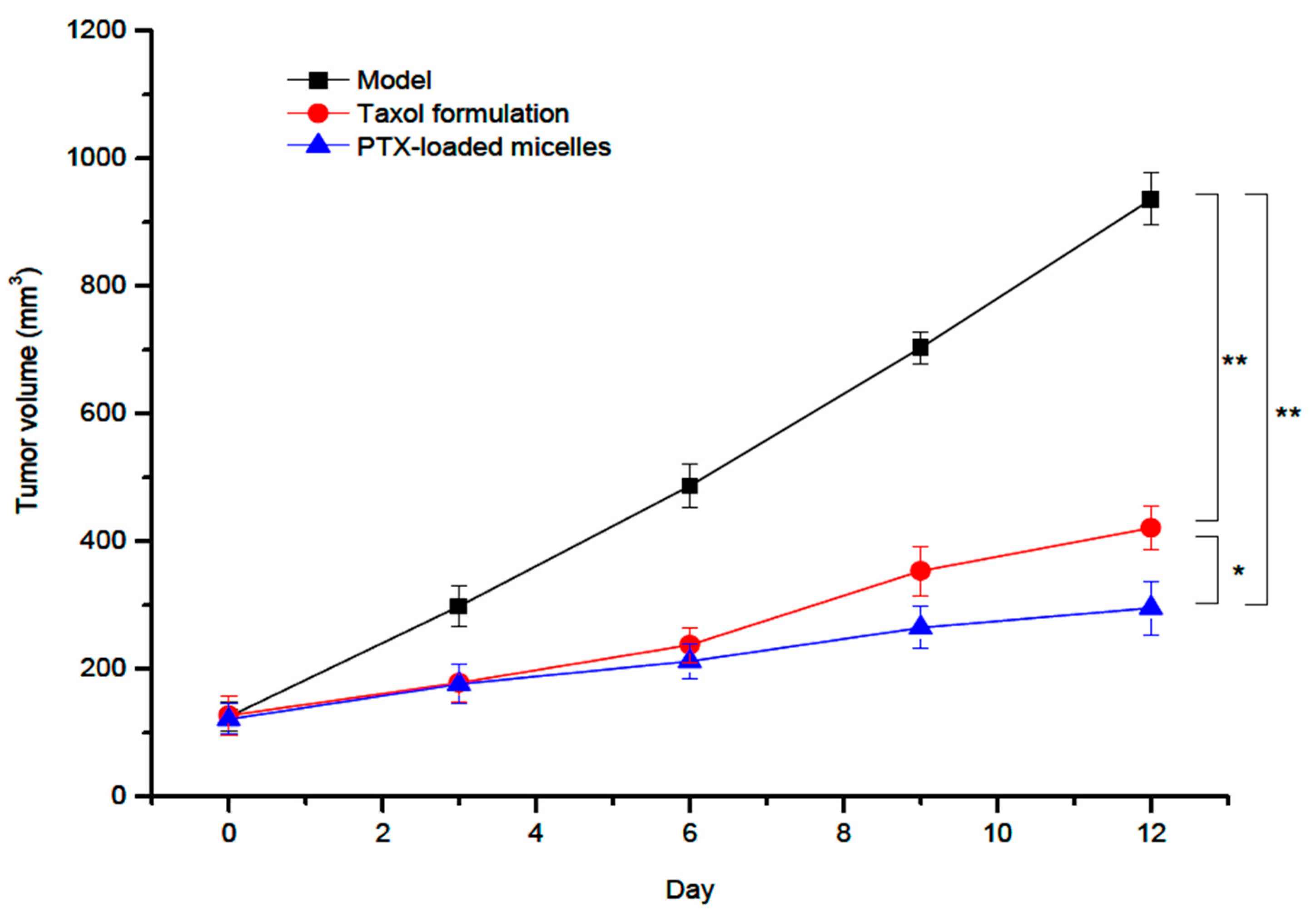
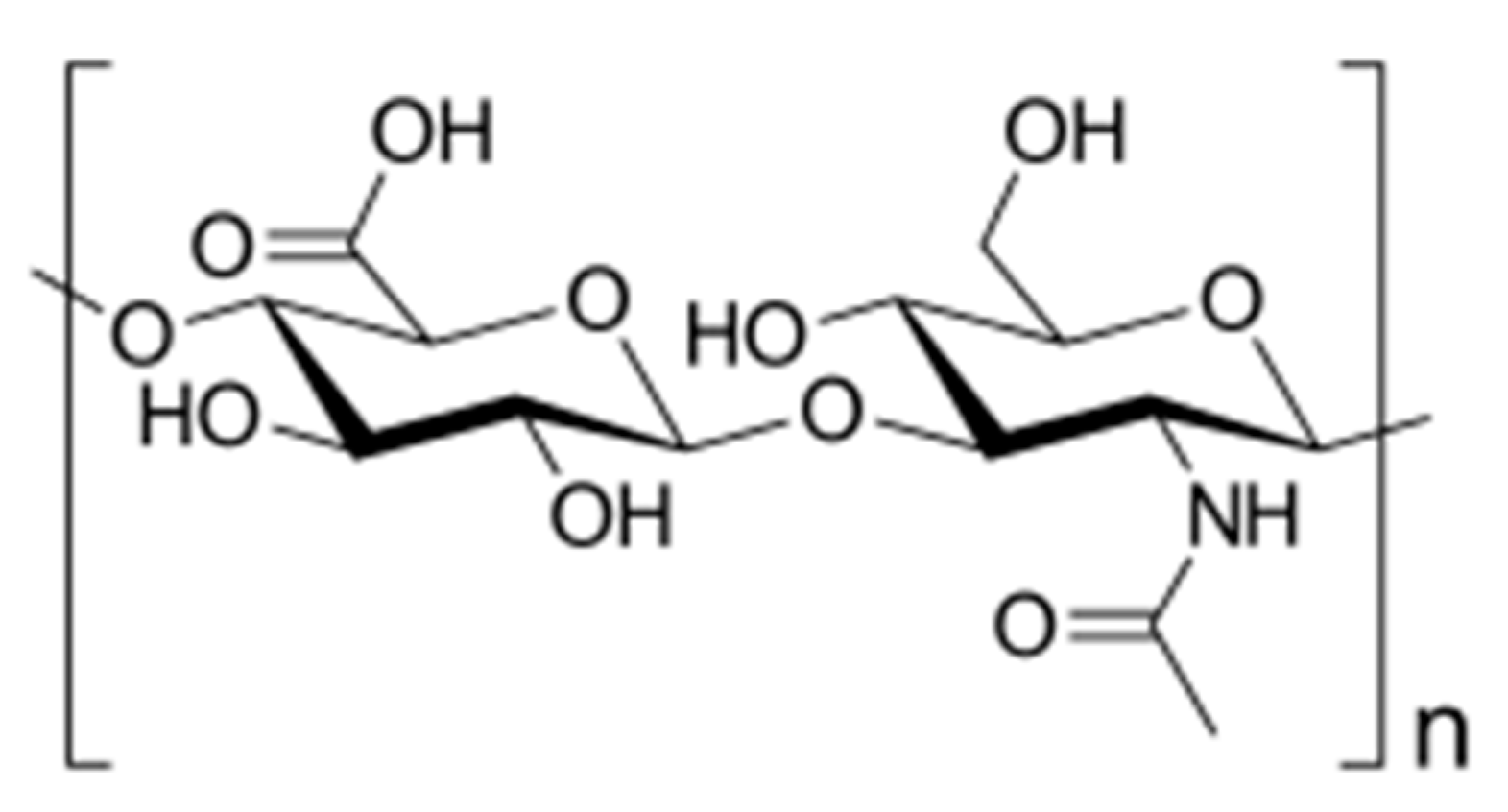
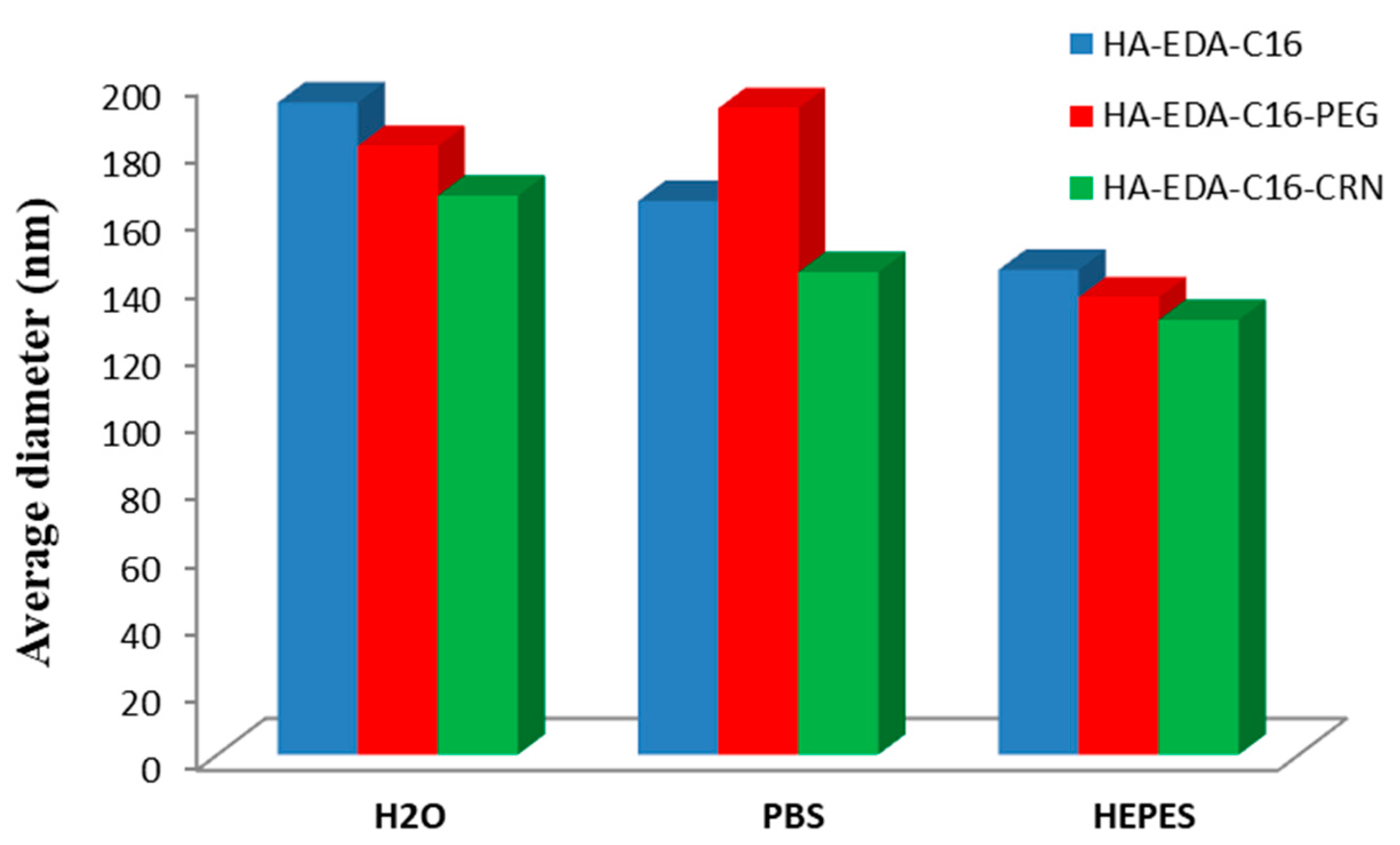


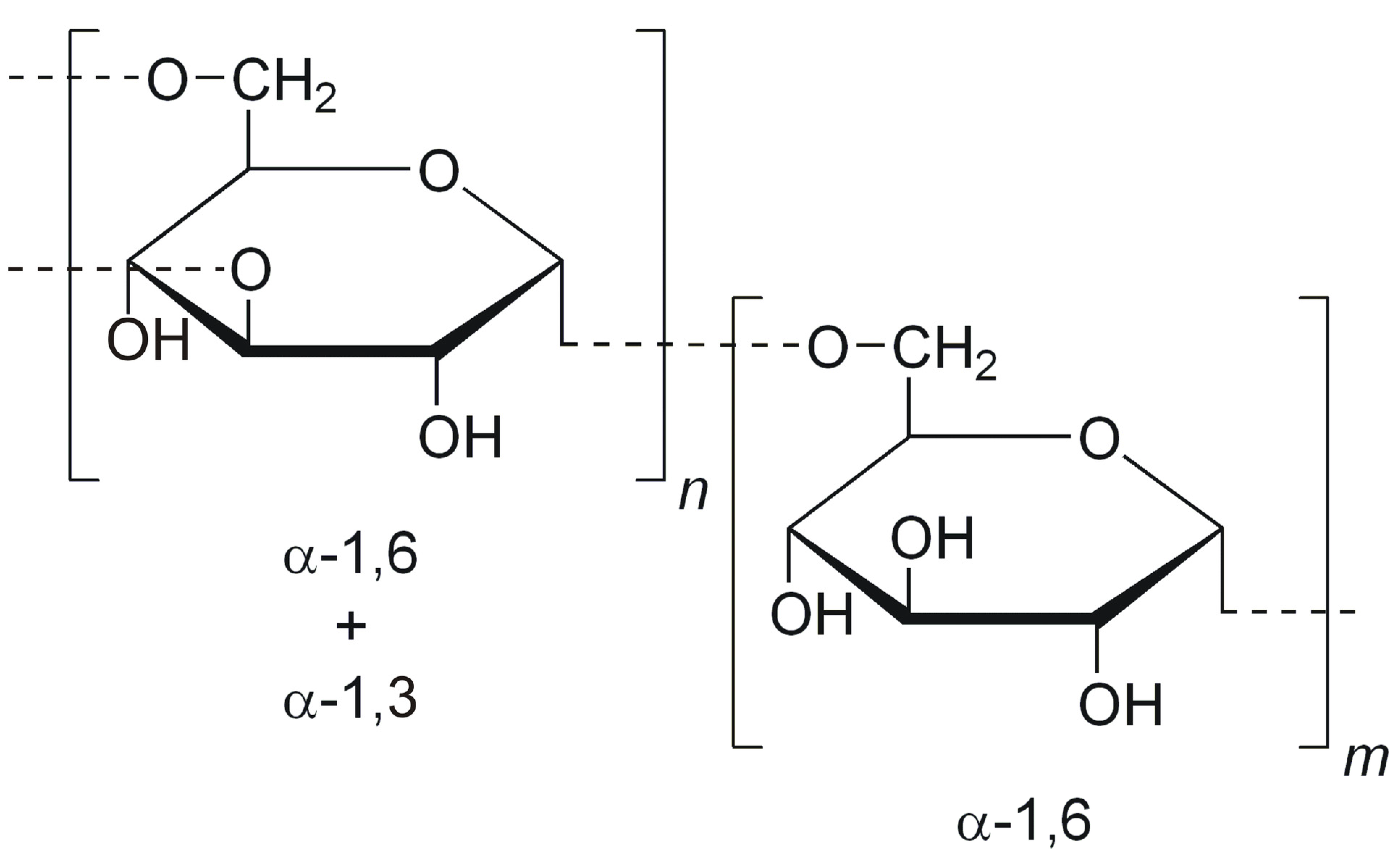
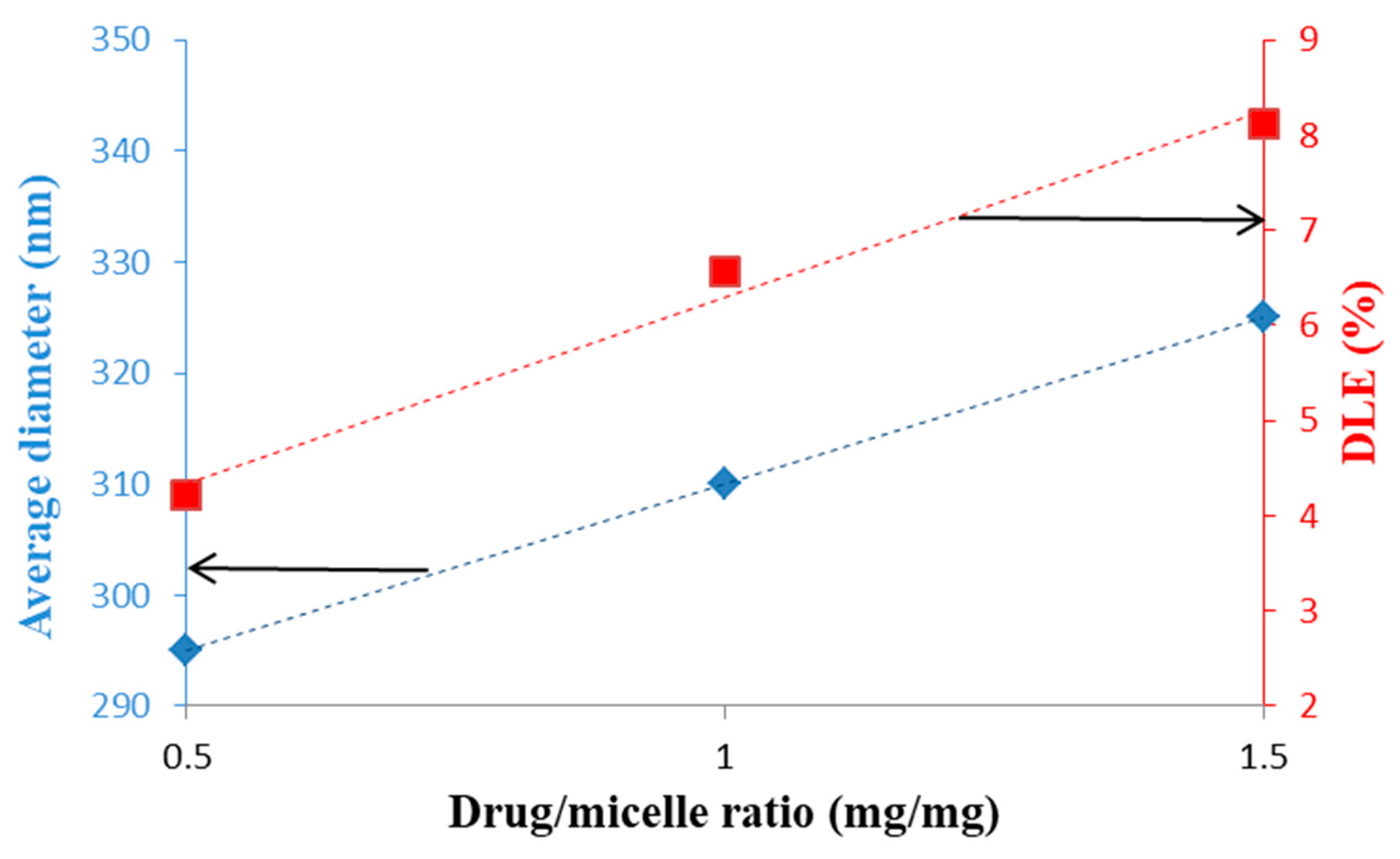
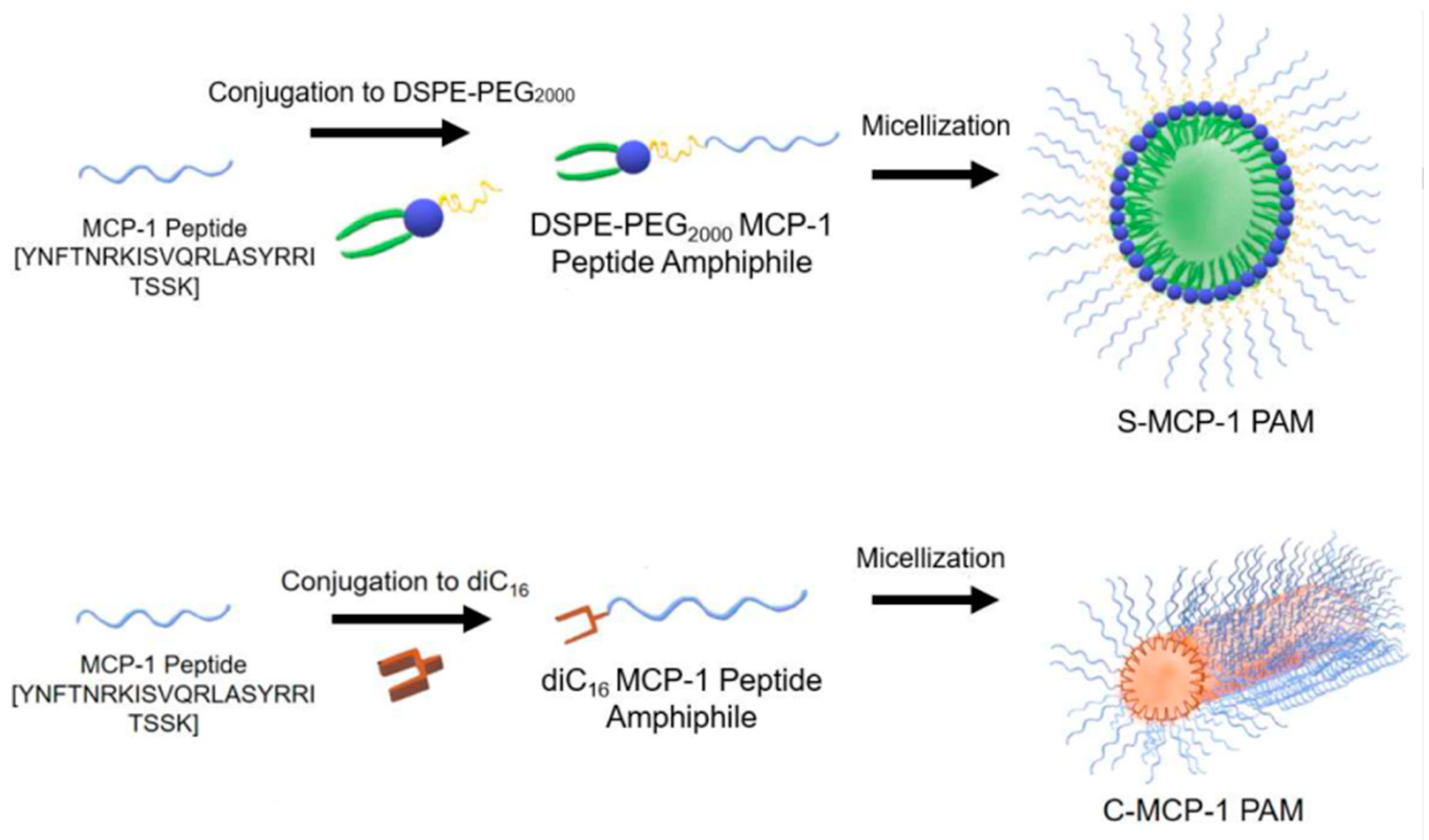

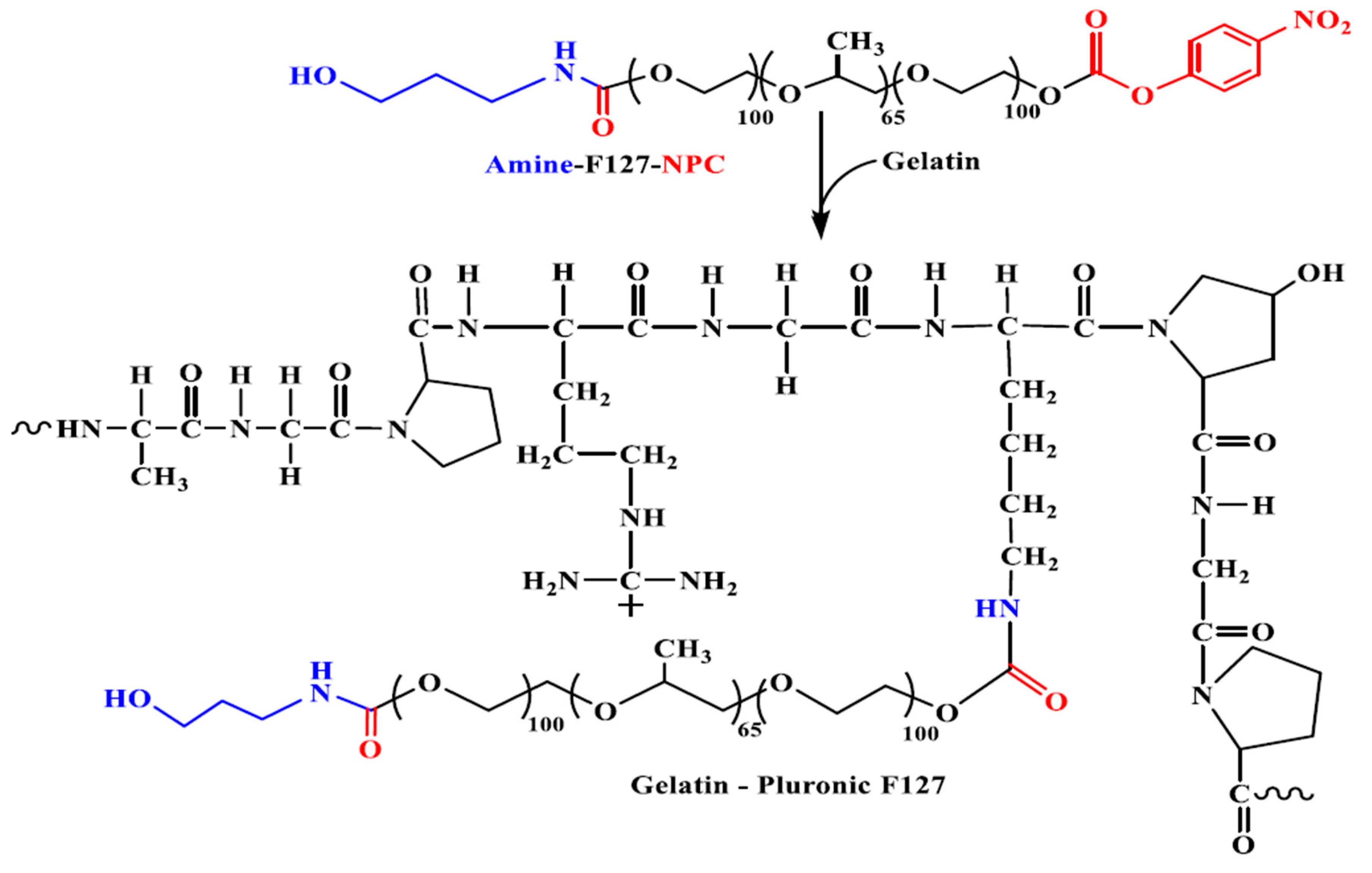
| Biopolymer | Conjugated Drug | Micellar Size (nm) | ZP Value (mV) | Preparation Method | Reference |
|---|---|---|---|---|---|
| N-octyl-N arginine chitosan (+) | Insulin (−) | 257 ÷ 327 | 4.61 ÷ 6.53 | Ionic complexation | [16] |
| Adipic dihydrazole hyaluronic acid | Quercetin | 172.1 | −20.3 | Direct dissolution | [17] |
| Hyaluronic acid | Curcumin | 84 ÷ 131 | −19.43 ÷ 24.27 | Sonication | [18] |
| Hyaluronic acid | Curcumin | 209 ÷ 926 | −25 ÷ −35 | Direct dissolution | [19] |
| Dithiodipropionic acid hyaluronic acid | Quercetin + Curcumin | 172.6 | −33.71 | Dialysis | [20] |
| Heparin-graft-deocycholate | Doxorubicin | 154.7 166.0 | −32.7 −36.7 | Sonication | [21] |
| Pegylated heparin | Pyropheophorbide-a | 97.31 | −14.63 | Not indicated | [22] |
| Alginate | Curcumin | 459 | −45 | Direct dissolution | [23] |
| Galactosylated alginate | Curcumin | 235 | −29 | Direct dissolution | [24] |
| Alginate | Curcumin | 200 | −53 | Direct dissolution | [25,26] |
| Dextran | Curcumin | 222 | −15.2 | Direct dissolution | [27] |
| Dextran | Indomethacin | 68.3 | n.d. | Dialysis | [28] |
| Dextran | Indomethacin | 200 | −1.8 | Dialysis | [29] |
| Hydroxyethyl starch | Curcumin | <100 | −27.8 | Precipitation/dialysis | [30] |
| Fructose | Curcumin | 100 ÷ 150 | - | Solution mixing | [31] |
| Bovine serum albumin functionalized poly(oligo (ethylene glycol) methyl ether methacrylate) | Sprouty 1 (C-12) (Spry1) protein | 15 ÷ 25 | −9 ÷ 2 | Direct dissolution | [32] |
| Human serum albumin | Adriamycin | 100 | 5 ÷ 10 | Thin film hydration | [33] |
| ABD035 peptide | Paclitaxel | 29 | 8.7 | Dialysis | [34] |
| Characterization Technique | Characteristic |
|---|---|
| Dynamic and static light scattering (DLS and SLS) | Size Polydispersity Aggregation number Nagg |
| Atomic force microscopy (AFM), scanning and transmission electronic microscopy (SEM and TEM, cryo-TEM) | Morphology Size |
| Fluorescence and surface tension | Critical micellar and association concentrations (CAC and C.M.C.) |
| Electrophoretic Light Scattering (ELS) | Surface charge (zeta potential ZP) |
| Differential scanning calorimetry (DSC), X-ray diffraction, Fourier transform infrared spectroscopy (FTIR), Nuclear magnetic resonance (NMR) | Critical micellization temperature (CMT) Degree of crystallinityDrug/polymer interactions |
| UV spectroscopy, High performance liquid chromatography (HPLC), Liquid chromatography-Mass spectroscopy analysis | Drug loading efficiency (DLE)Drug encapsulation efficiency (DEE)Release kinetics |
| Small angle X-ray and neutron scattering (SAXS), (SANS) | Size Structural properties |
| Copolymer | Drug | Size (nm) | ZP (mV) | Preparation Method | Reference |
|---|---|---|---|---|---|
| Xanthan–graft–C16 alkyl chain | Glibenclamide | 652.8 | −27.6 | Precipitation/evaporation | [99] |
| Pullulan–graft–PCL | ciprofloxacin | 142 | - | Dialysis | [100] |
| Pullulan–graft–α-tocopherol | CPT | 171.5 ÷ 257.7 | - | Dialysis | [101] |
| Pullulan–graft-SA | Dox | 188.75 | 17.83 | Dialysis | [102] |
| Pullulan–graft–retinoic acid+biotin | Dox | 191 | −9.45 | Direct dissolution | [103] |
| Pullulan–graft–desaxycholic acid–graft–PEI | Dox DNA | 160.8 | 28 | Dialysis | [104] |
| Pulullan–graft–retinoic acid | Dox | 124.5 | −3.65 | Direct dissolution | [105] |
| Pullulan–graft–(dibuthyl amino) propyl carbamate | DNA | 246.3 ÷ 1214 | 24.7 ÷ 47 | Dialysis | [106] |
| Pullulan–graft–cholesterol | methatrexate | 97.8 144.4 178.0 | 4.29 3.392.56 | Dialysis | [107] |
| Heparin–graft–β-sitosterol cysteamine | Dox | 93.3 | −41.8 | Sonication and dialysis | [108] |
| Heparin–graft–α-tocopherol | Docetaxel | 139.7 | −24.6 | Dialysis | [109] |
| Chitin–graft–hexadecyl | Dox | 332.4 ÷ 385.0 | 38.2 ÷ 44.8 | Dialysis | [110] |
| Guar gum galactomannan–graft–acetic anhydride | Cur | 113.9 ÷ 152.8 | −14.8 ÷ −18.1 | Precipitation/solvent diffusion | [111] |
| Bletilla striata–graft–SA | Dox | 124.17 ÷ 145.93 | −13.17 ÷ −18.97 | Dialysis | [112] |
| Phthalated cashew gum | Benznidazole | 288.8 | −31.8 | Precipitation | [113] |
| Schizophyllan–graft–SA | PTX | 156 ÷ 175 | −28 ÷ 35.14 | Sonication | [114] |
| Fucoidan–graft–octenyl succinic anhydride | Cur | 103.9 | −43.57 | Not indicated | [115] |
| Poly(1-O-methacryloyl-β-d-fructopyranose)-block-poly(methyl methacylate) | PTX | 25 ÷ 46 | −9.9 ÷ −22.3 | Dialysis | [116] |
| Protein | Source | Composition | Molecular Weight |
|---|---|---|---|
| Gelatin | Collagen | 4-hydroxylysine, hydroxyproline, glycine, alanine, and proline | 15–250 KDa |
| Albumin | Plasma protein | Single polypeptide with 585 amino acids | 66 KDa |
| Whey protein | Milk protein | α-lactoglobulin (LG), β-LG, lactalbumin, immunoglobulin, lactoferrin | β-LG-18 KDa α-LG-14 KDa |
| Casein | Milk protein | Proline- α s1, α s2, β and k subunits | α s1-23 KDa α s2-25 KDa β-24 KDa k-19 KDa |
| Zein | Zea mays L. | High proportion of glutamine and proline | α-zein-22–24 KDa, β-zein-44 KDa, γ-zein-14 KDa |
| Gliadin | Wheat flour | Glutamine, Proline | 28–55 KDa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanase, L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477. https://doi.org/10.3390/polym13030477
Atanase LI. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers. 2021; 13(3):477. https://doi.org/10.3390/polym13030477
Chicago/Turabian StyleAtanase, Leonard Ionut. 2021. "Micellar Drug Delivery Systems Based on Natural Biopolymers" Polymers 13, no. 3: 477. https://doi.org/10.3390/polym13030477






