2. Materials and Methods
2.1. Reagents
Stearoyl chloride, 1,3-dibromo-2-propanol, pentaerythritol tribromide and sodium hydride (NaH) were purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). Poly(ethylene oxide) monomethyl ethers (PEO) with 550, 750, 2000, and 5000 g/mol of number-average molecular weight Mn were purchased from Sigma-Aldrich Co. Ltd. (St. Louis, MO, USA). According to Mn of PEO (Mn,PEO), PEO are described as PEO550, PEO750, PEO2k and PEO5k, respectively. Pyridine and dehydrated tetrahydrofuran (THF) were purchased from Fujifilm Wako Chemicals Co. Ltd. (Tokyo, Japan). All reagents were used as obtained. Star-like amphiphilic polymers consisting of plural PEO chains and one stearyl chain were synthesized by the methods shown below. The amphiphilic polymers are denoted as (PEOx)y-C18, where x and subscription y are Mn,PEO and number of PEO chains in a star-like polymer, respectively.
2.2. Synthesis of 1,3-dibromo-2-propyl Stearate (1)
1,3-dibromo-2-propanol (1.17 g, 5.40 mmol) was dissolved in THF in a round-bottom flask capped with a rubber septum under dry nitrogen atmosphere. Pyridine (0.42 g, 5.40 mmol) was added into the flask and stirred at ambient temperature. Then, the solution was allowed to cool by ice-cold water bath. Stearoyl chloride (1.5 g, 4.90 mmol) was added dropwise to the solution. After the solution was stirred at ambient temperature for 24 h, precipitate was filtered off. Then, the resulting solution was extracted with water and hexane. The organic layer was dried with MgSO4 and evaporated to dryness under reduced pressure. The crude product was purified by column chromatography, eluting with hexane to give 1 as a white solid: yield 32%; 1H NMR (CDCl3) δ 5.05 (m, 1H, CO2–CH–), 3.52 (s, 4H, Br–CH2–), 2.26(t, 2H, OCO–CH2–), 1.58 (m, 2H, OCO–CH2–CH2–), 1.35 (m, 28H, CH2–CH2–CH2), 0.82 (t, 3H, CH2–CH3).
2.3. Synthesis of 3-bromo-2,2-dibromomethyl-1-propyl Stearate (2)
Pentaerythritol tribromide (2.3 g, 6.9 mmol) was dissolved in THF in a round-bottom flask capped with a rubber septum under dry nitrogen atmosphere. Pyridine (0.54 g, 6.9 mmol) was added to the flask and stirred at ambient temperature. Then, the solution allowed to cool by ice-cold water bath. Stearoyl chloride (2.1 g, 6.9 mmol) was added dropwise to the solution. After the solution was stirred at ambient temperature for 24 h, precipitate was filtered off. Then, the solution was extracted with water and hexane. The organic layer was dried with MgSO4 and evaporated to dryness under reduced pressure. The crude product was purified by column chromatography, eluting with hexane to give 2 as a white solid: yield 33%; 1H NMR (CDCl3) δ 4.12 (s, 2H, CO2–CH2), 3.11 (s, 6H, Br–CH2–), 2.31 (t, 2H, OCO–CH2–), 1.62 (m, 2H, OCO-CH2–CH2–), 1.35 (m, 28H, CH2–CH2–CH2), 0.82 (t, 3H, CH2–CH3).
2.4. Synthesis of (PEOx)1-C18
In the round-bottom flask, PEO was dissolved in THF. After the flask was capped with a rubber septum and filled with N2, pyridine (1 equiv. to PEO) was added into the flask and stirred at ambient temperature. Then, the solution allowed to cool by ice-cold water bath. Stearoyl chloride (1 equiv. to PEO) was added dropwise to the solution. The reaction mixture was stirred overnight at ambient temperature. After precipitate was filtered off, the resulting solution was extracted with water and hexane, and aqueous layer was dialyzed for 5 days to remove unreacted PEG. The solution was dried by freeze drying to give (PEOx)1-C18 as a white powder.
(PEO550)1-C18. Yield = 26 %; 1H-NMR (CDCl3) δ 4.14 (m, 2H COO–CH2), 3.76 (m, 2H, COO–CH2–CH2–O), 3.60 (m, 45H, O–CH2–CH2–O), 3.40 (s, 3H, O–CH3), 2.28 (m, 2H, OCO–CH2), 1.64 (m, 2H, OCO–CH2–CH2), 1.24 (m, 28H, CH2–CH2–CH2), 0.85 (t, 3H CH2–CH3).
(PEO750)1-C18. Yield = 32 %; 1H-NMR (CDCl3) δ 4.18 (m, 2H COO–CH2), 3.75 (m, 2H, COO–CH2–CH2–O), 3.60 (m, 62H, O–CH2–CH2–O), 3.38 (s, 3H, O–CH3), 2.26 (m, 2H, OCO–CH2), 1.64 (m, 2H, OCO–CH2–CH2), 1.22 (m, 28H, CH2–CH2–CH2), 0.83 (t, 3H CH2–CH3).
(PEO2k)1-C18. Yield = 22 %; 1H-NMR (CDCl3) δ 4.15 (m, 2H COO–CH2), 3.76 (m, 2H, COO–CH2–CH2), 3.62 (m, 180H, O–CH2–CH2–O), 3.40 (s, 3H, O–CH3), 2.30 (m, 2H, OCO–CH2), 1.65 (m, 2H, OCO–CH2–CH2), 1.24 (m, 28H, CH2–CH2–CH2), 0.85 (t, 3H CH2–CH3).
(PEO5k)1-C18. Yield = 17 %; 1H-NMR (CDCl3) δ 4.14 (m, 2H COO–CH2), 3.76 (m, 2H, COO–CH2–CH2–O), 3.60 (m, 440H, O–CH2–CH2–O), 3.38 (s, 3H, O–CH3), 2.28 (m, 2H, OCO–CH2), 1.64 (m, 2H, OCO–CH2–CH2), 1.24 (m, 28H, CH2–CH2–CH2), 0.85 (t, 3H CH2–CH3).
2.5. Synthesis of (PEOx)2-C18
(PEO
x)
2-C18s were synthesized according to
Scheme 1. NaH was dispersed in THF in a two-neck flask, and the flask was filled with N
2. PEO (1 equiv. to NaH) was dissolved in THF in another flask under N
2 atmosphere, and then the THF solution was added dropwise to the two-neck flask. The reaction mixture was stirred at ambient temperature for 30 min to be completely deprotonated.
1 (0.45 equiv. to PEO) was dissolved in THF, and then added dropwise to the two-neck flask. The reaction mixture was refluxed for 5 days. After precipitates were filtered off, the filtrate was extracted with water and hexane and aqueous layer was dialyzed to remove unreacted PEO. The solution was dried by freeze driying to give (PEO
x)
2-C18 as a white powder.
(PEO550)2–C18. Yield = 21 %; 1H-NMR (CDCl3) δ 4.19 (m, 1H COO–CH), 3.82–3.55 (m, 110H, CH–CH2-O and O–CH2-CH2–O), 3.38 (s, 6H, CH2-O–CH3), 2.25 (m, 2H, OCO-CH2–CH2), 1.62 (m, 2H, OCO-CH2–CH2-CH2), 1.24 (m, 28H, CH2–CH2–CH2), 0.83 (t, 3H CH2–CH3).
(PEO750)2-C18. Yield = 36 %; 1H-NMR (CDCl3) δ 4.20 (m, 1H COO–CH), 3.82–3.55 (m, 142H, CH–CH2-O and O–CH2–CH2–O), 3.40 (s, 6H, CH2-O–CH3), 2.28 (m, 2H, OCO–CH2–CH2), 1.60 (m, 2H, OCO–CH2–CH–CH2), 1.23 (m, 28H, CH2–CH2-CH2), 0.86 (t, 3H CH2–CH3).
(PEO2K)2–C18. Yield = 42 %; 1H-NMR (CDCl3) δ 4.20 (m, 1H COO-CH), 3.82–3.55 (m, 370H, CH–CH2–O and O–CH2–CH2–O), 3.40 (s, 6H, CH2–O–CH3), 2.26 (m, 2H, OCO–CH2–CH2), 1.62 (m, 2H, OCO–CH2-CH2–CH2), 1.24 (m, 28H, CH2–CH2–CH2), 0.85 (t, 3H CH2–CH3).
(PEO5K)2–C18. Yield = 43 %; 1H-NMR (CDCl3) δ 4.18 (m, 1H COO–CH), 3.82–3.55 (m, 920H, CH–CH2–O and O–CH2-CH2–O), 3.40 (s, 6H, CH2–O–CH3), 2.25 (m, 2H, OCO-CH2–CH2), 1.61 (m, 2H, OCO–CH2–CH2–CH2), 1.24 (m, 28H, CH2–CH2–CH2), 0.83 (t, 3H CH2–CH3).
2.6. Synthesis of (PEOx)3-C18
(PEO
x)
3-C18s were synthesized according to
Scheme 2. NaH was dispersed in THF in a two-neck flask, and the flask was filled with N
2. PEO (1 equiv. to NaH) was dissolved in THF in another flask under N
2 atmosphere, and then the THF solution was added dropwise to the two-neck flask. The reaction mixture was stirred at ambient temperature for 30 min to be completely deprotonated.
2 (0.3 equiv. to PEO) was dissolved in THF, and then added dropwise the two-neck flask. The reaction mixture was refluxed for 5 days. After precipitates were filtered off, the filtrate was extracted with water and hexane and aqueous layer was dialyzed to remove unreacted PEO. The solution was dried by freeze drying to give (PEO
x)
3-C18 as a white powder.
(PEO550)3–C18. Yield 46%; 1H-NMR (CDCl3) δ 4.12 (s, 2H, CO2–CH2), 3.75–3.50 (s, 162H, C–CH2–O and O–CH2–CH2–O), 2.28 (t, 2H, OCO–CH2–), 1.78 (m, 2H, OCO–CH2–CH2–), 1.26 (m, 28H, CH2–CH3–CH2), 0.82 (t, 3H, CH2–CH3).
(PEO750)3–C18. Yield 38%; 1H NMR (CDCl3) δ 4.11 (s, 2H, CO2–CH2), 3.75–3.50 (s, 210H, C–CH2–O and O–CH2–CH2–O), 2.28 (t, 2H, OCO–CH2–), 1.83 (m, 2H, OCO–CH2–CH2–), 1.25 (m, 28H, CH2–CH2–CH2), 0.84 (t, 3H, CH2–CH3).
(PEO2K)3–C18. Yield 33%; 1H-NMR (CDCl3) δ 4.12 (s, 2H, CO2–CH2), 3.80–3.50 (s, 560H, C–CH2–O and O–CH2–CH2–O), 2.32 (t, 2H, OCO–CH2–), 1.84 (m, 2H, OCO–CH2–CH2–), 1.25 (m, 28H, CH2–CH2–CH2), 0.85 (t, 3H, CH2–CH3).
(PEO5K)3–C18. Yield 35%; 1H-NMR (CDCl3) δ 4.15 (s, 2H, CO2–CH2), 3.80–3.50 (s, 1380H, C–CH2–O and O–CH2–CH2–O), 2.29 (t, 2H, OCO–CH2–), 1.80 (m, 2H, OCO–CH2–CH2–), 1.25 (m, 28H, CH2–CH2–CH2), 0.85 (t, 3H, CH2–CH3).
2.7. Preparation of Polymer Micelles
Amphiphilic polymers were dispersed in nanopure water, which was prepared by Barnstead Nanopure System, at a desired concentration. The solutions were homogenized by using a bath-type ultrasonic homogenizer. The resulting micelle solutions were further clarified by centrifugation and filtrated through a membrane filter with 0.2 μm of pore size.
2.8. Characterization
1H-NMR spectra were recorded on a JEOL JNM ECP-500 NMR spectrometer (Tokyo, Japan). Chemical shifts were referenced to TMS and CHCl
3 slightly contained in CDCl
3. Gel permeation chromatography (GPC) eluted with THF was conducted on a system equipped with an isocratic pump model PU-4180, a differential refractometer model RI-4030 (JASCO, Tokyo, Japan), and a PSgel-packed column model KF-805 (Shodex). Number-average molecular weight (
Mn) of polymers was determined by the proton ratio of CH
2 of PEO to CH
2 of C18. Weight-average molecular weights (
Mw) of polymers were determined by using
Mn from
1H-NMR and polydispersity from GPC measurements. Critical micelle concentration (CMC) was determined by fluorescence probe method using sodium 8-anilino-1-naphthalene sulfonate (ANS-Na). Aqueous solutions of amphiphilic polymers were in the range of concentration from 1.0 × 10
−4 to 1.0 mg/mL, in which ANS-Na was contained at the concentration of 2.0 × 10
−5 M. The fluorescence spectra of ANS-Na from 400 to 600 nm were recorded on a Hitachi F-4500 spectrometer (Tokyo, Japan) with 390 nm of excitation wavelength. Fluorescence intensity at 490 nm was monitored to determine CMC. Concentration dependence of fluorescence spectra and intensity at 490 nm of aqueous (PEG2k)
2-C18 solution containing ANS-Na were shown in
Figure 1 as a typical example. The concentration, at which the slope of concentration dependence of the intensity at 490 nm was drastically changed, was defined as CMC. Aggregation numbers (
Nagg) of micelles were determined by field flow fractionation coupled with multiangle light scattering (FFF-MALS) using an Eclipse 3+ separation system with a Dawn Heleos II MALS detector (Wyatt Technology Europe GmbH, Dernbach, Germany). A Wyatt channel (Eclipse 3 channel LC) was used, which had a tip-to-tip length of 17.4 cm and a nominal thickness of 250 μm and a membrane (Nadir cellulose membrane 10 kDa LC) was attached to the bottom of the channel. The
Nagg of polymer micelles, which is the number of polymers in one micelle, was determined by dividing the weight average molar masses of the micelles obtained from Zimm plots of FFF-MALS by
Mw of polymers.
2.9. Small-Angle X-ray Scattering (SAXS)
For structural analyses of polymer micelles, SAXS measurements were performed at BL-40B2 station of SPring-8, Hyogo, Japan. Aqueous micelle solutions were injected into 2 mm ϕ quartz tubes and placed in the SAXS apparatus. A 30 x 30 cm2 imaging plate (Rigaku R-AXIS VII, Tokyo, Japan) was placed 1 m from the sample. The wavelength of the incident X-ray (λ) was adjusted to 0.10 nm. This setup provided a q range of 0.1–4 nm−1, where q is the magnitude of the scattering vector defined as q = (4π/λ)sinθ with a scattering angle of 2θ. The X-ray transmittance of the samples was determined by using ion chambers placed in front of and behind the sample. The 2-dimensional SAXS images were converted to one dimensional SAXS profiles of SAXS intensity I(q) vs. q.
3. Results and Discussion
The
Mn,
Mw, CMC and
Nagg of synthesized amphiphilic polymers were summarized in
Table 1, and CMCs and
Naggs were plotted against
Mn of PEO (
Mn,PEO) in
Figure 2. As described above, the components of all polymers are the same and the only differences between polymers are the molecular architectures. Therefore, the differences in self-assemblies of these polymers should be derived from the differences of molecular architectures. The CMC of PEO-C18 is monotonically increased with increasing
Mn,PEO. In the case that
Mn,PEO ≥ 750, (PEO
x)
2-C18 and (PEO
x)
3-C18 show similar manner of
Mn,PEO dependence of CMC. These results can be explained due to increment of hydrophilicity with increasing
Mn,PEO and number of PEO chain. Contrary to this tendency, CMCs of (PEO550)
2-C18 and (PEO550)
3-C18 are much higher than those of (PEO750)
2-C18 and (PEO750)
3-C18, respectively, although CMC of (PEO550)
1-C18 follows the general trend of
Mn,PEO dependence of CMC. In
Mn,PEO dependences of
Nagg, (PEO550)
2-C18 and (PEO550)
3-C18 also show peculiarity. As shown in
Figure 2, regardless of the number of PEO chain,
Naggs of micelles are simply decreased with increasing
Mn,PEO, except for (PEO550)
2-C18 and (PEO550)
3-C18. Therefore, it is considered that the unique associating behavior in the star-like amphiphilic polymers having multiple hydrophilic PEO chains should remarkably appear in the region where the chain length of PEO is relatively short. This suggests that structures of micelles of (PEO
x)
2-C18 and (PEO
x)
3-C18 should drastically change between 550 and 750 g/mol of
Mn,PEG. Thus, SAXS measurements were carried out for (PEO
x)
y-C18 micelles.
Figure 3 compares SAXS profiles of (PEO
x)
y-C18 micelles. (PEO2k)
1-C18, (PEO750)
2-C18 and (PEO550)
3-C18 are approximately equal mole fractions of PEO in (PEO
x)
y-C18, while (PEG550)
1-C18, (PEG550)
2-C18 and (PEG550)
3-C18 are equal chain length of PEO. When
q < 0.2 nm
−1,
I(
q)s of all samples are almost independent of
q. This means polymer micelles in this study form spherically symmetric shapes. In addition, this means there are no secondary aggregations of polymer micelles. On the other hand, SAXS profiles in
q > 0.5 nm
−1 show significant differences depending on the molecular structures. The SAXS profiles in this region reflect the internal composition distribution in the polymer micelles. To elucidate the origin of such differences in the SAXS profiles, numerical analyses using theoretical scattering function must be required. The solid lines in
Figure 3 are theoretical SAXS curves calculated by the following core–corona model [
16,
17,
18,
19,
20].
where
βcore and
βcorona are excess scattering lengths of hydrophobic core and PEO chain, respectively.
βcore =
Vcore(
ρcore−
ρ0) and
βcoroae =
Vcorona(
ρcorona−
ρ0), where
ρcore,
ρcorona and
ρ0 are electron densities of core, PEO and solvent, respectively, and
Vcore and
Vcorona are volumes of core and corona, respectively. Here, we used 357 e
−/nm
3 for corona and 333 e
−/nm
3 for solvent. Thus,
Vcore,
Vcorona, and
ρcoron are the unknown parameters.
Fcore(
q) is scattering amplitude of hydrophobic core regarded as sphere given by the following equation.
where
Rc is radius of core and
σ is thickness of interface between core and corona. The
Rc gives
Vcore. We use the Debye function for
Fchain(
q) given by the following equation [
20].
where
Rg is the radius of gyration of PEO. The
Rg gives the
Vcorona. The third term in Equation (1) is the cross term between hydrophobic core and corona given by the following equation.
where
Achain(q) is the scattering amplitude of PEO chain given by the following equation.
The S
chain-chain is the cross term between different chains within the corona layer given by the following equation.
The P’(0) is the scattering of PEO chain at q = 0. Thus, by using N
agg, R
c, R
g, and ρ
corona as adjustable parameters, we performed the fitting analyses for the experimental SAXS data to yield the smallest residues. Here, the N
aggs from FFF-MALS were used as the initial values on N
aggs in the fitting procedures. The solid lines are the best-fit results of numerical calculations.
Table 2 summarizes the characteristics of (PEOx)
y-C18 micelles obtained from the numerical calculations. Since the N
aggs obtained from SAXS analyses well agree with those from FFF-MALS, the numerical analyses for SAXS by the core–corona model are reliable enough. From the results of numerical analyses, R
core and thickness of corona layer of (PEOx)
y-C18 micelles are obtained.
Figure 4 shows plots of
Rcore and thickness of corona layer against the
Mn,PEG.
Rcores are almost constant regardless of
Mn,PEO and molecular architecture because stearyl group is employed as hydrophobic chains for all (PEO
x)
y-C18. In addition, the thicknesses of the corona layers become predictably thick as the
Mn,PEO increases. Moreover, the slopes of
Mn,PEO dependence of the corona thickness are almost the same even if the molecular architecture is different. Therefore, significant specificity owing to differences of molecular architecture does not appear in the
Mn,PEO dependences of
Rcore and corona thickness. However, the corona thicknesses of (PEO
x)
2-C18 and (PEO
x)
3-C18 micelles always thicker than that of (PEO
x)
1-C18 micelles. This means that the characteristic feature of the (PEO
x)
2-C18 and (PEO
x)
3-C18 micelles derived from the differences in molecular architecture only appears in the difference in the thicknesses of corona layers in (PEO550)
2-C18 and (PEO550)
3-C18 micelles. This result is consistent with the results obtained from the
Mn,PEO dependences of CMC and
Nagg as shown in
Figure 2. The difference in the number of PEO chains should affect the number density of PEO chains or the occupied area of one PEO chain on the core–corona interface of (PEO
x)
y-C18 micelles. Since these parameters indicate the degree of crowding of hydrophilic chains on the interface [
21,
22,
23,
24], they are significantly important to characterize the polymer micelles.
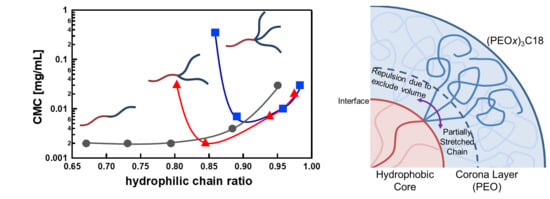
Figure 5 shows the occupied area of one PEO chain (
σ) defined as 4
πRcore/
Nagg against
Mn,PEO. The
σ decreases as the number of PEO chains in one (PEO
x)
y-C18 molecule increases. That is, the larger the number of PEO chains in one (PEO
x)
y-C18 molecule, the more crowded the PEO chains at the core–corona interface in (PEO
x)
y-C18 micelles. Therefore, it is considered that the partial PEO chains of (PEO
x)
2-C18 and (PEO
x)
3-C18 near the core–corona interface locally take an unfavorable conformation due to the excluded volumes of PEO as schematically shown in
Figure 6. When the PEO chains are long enough, most parts of the PEO chains can take stable conformation [
18,
20,
21]. Since the effect of the PEO crowding near the interface in (PEO
x)
2-C18 and (PEO
x)
3-C18 micelles is negligible, these polymers should show similar associating behavior to (PEO
x)
1-C18. However, as the chain length of PEO becomes shorter, the PEO crowding near the interface have significant effect on the micelle formation of (PEO
x)
2-C18 and (PEO
x)
3-C18. Since the boundary
Mn,PEO is located between 550 and 750 g/mol, the curvatures of the interfaces become large and the
Naggs become small in (PEO550)
2-C18 and (PEO550)
3-C18 micelles in order to eliminate the unfavorable conformation of PEO chains near the core–corona interface.