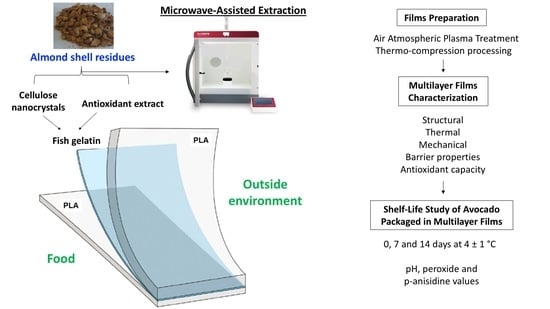
Multilayer Films Based on Poly(lactic acid)/Gelatin Supplemented with Cellulose Nanocrystals and Antioxidant Extract from Almond Shell By-Product and Its Application on Hass Avocado Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Additives Preparation from AS By-Products
2.2.1. Antioxidant Extract (ASE)
2.2.2. Cellulose Nanocrystals (CNCs)
2.3. Films Preparation
2.3.1. Fish Gelatin Films
2.3.2. Air Atmospheric Plasma Treatment
X-ray Photoelectron Spectroscopy Analysis (XPS)
Surface Tension
2.3.3. Trilayer PLA/FG/PLA Films
2.4. Multilayer Films Characterization
2.4.1. Thickness, Transparency and Colour Values
2.4.2. ATR-FTIR Analysis
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Thermal Analysis
2.4.5. Mechanical Properties
2.4.6. Barrier Properties
2.4.7. Antioxidant Activity and Total Phenolic Content (TPC) of Films
2.5. Shelf-Life Study of Avocado Packaged in Multilayer Films
2.5.1. Processing and Conditioning of Avocado Samples
2.5.2. Oxidative Stability Study of Packaged Avocado
2.6. Statistical Analysis
3. Results
3.1. Surface Properties of PLA Films after Corona Treatment
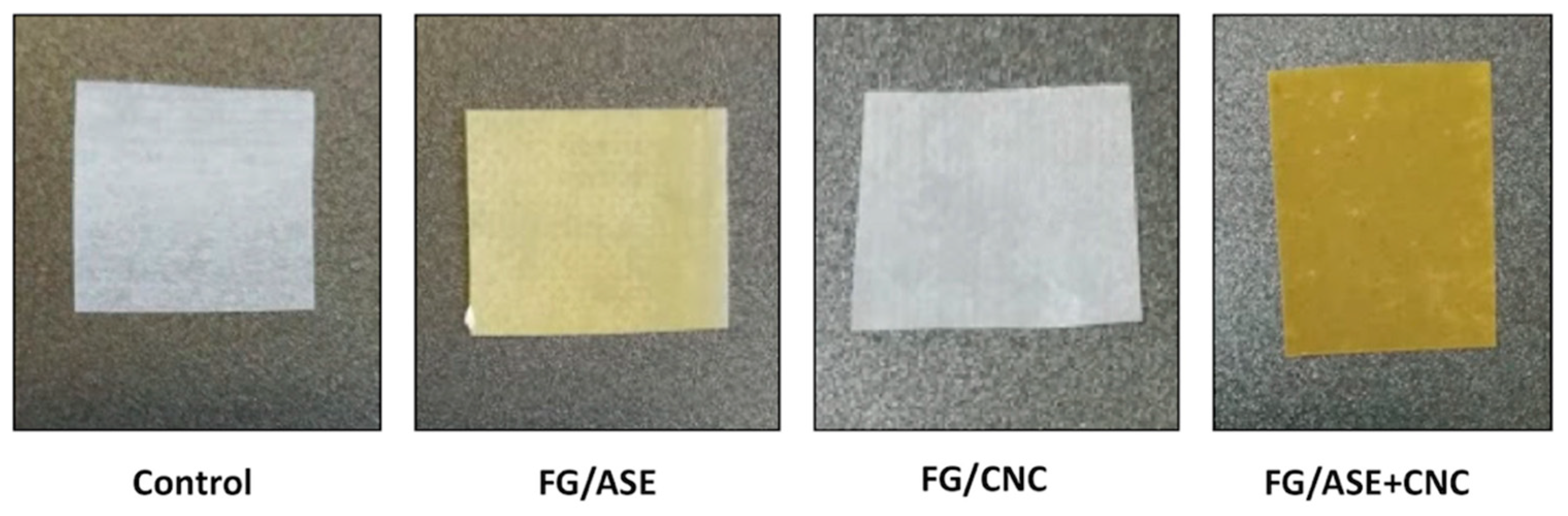
3.2. Thickness, Transparency and Colour
3.3. ATR-FTIR Analysis
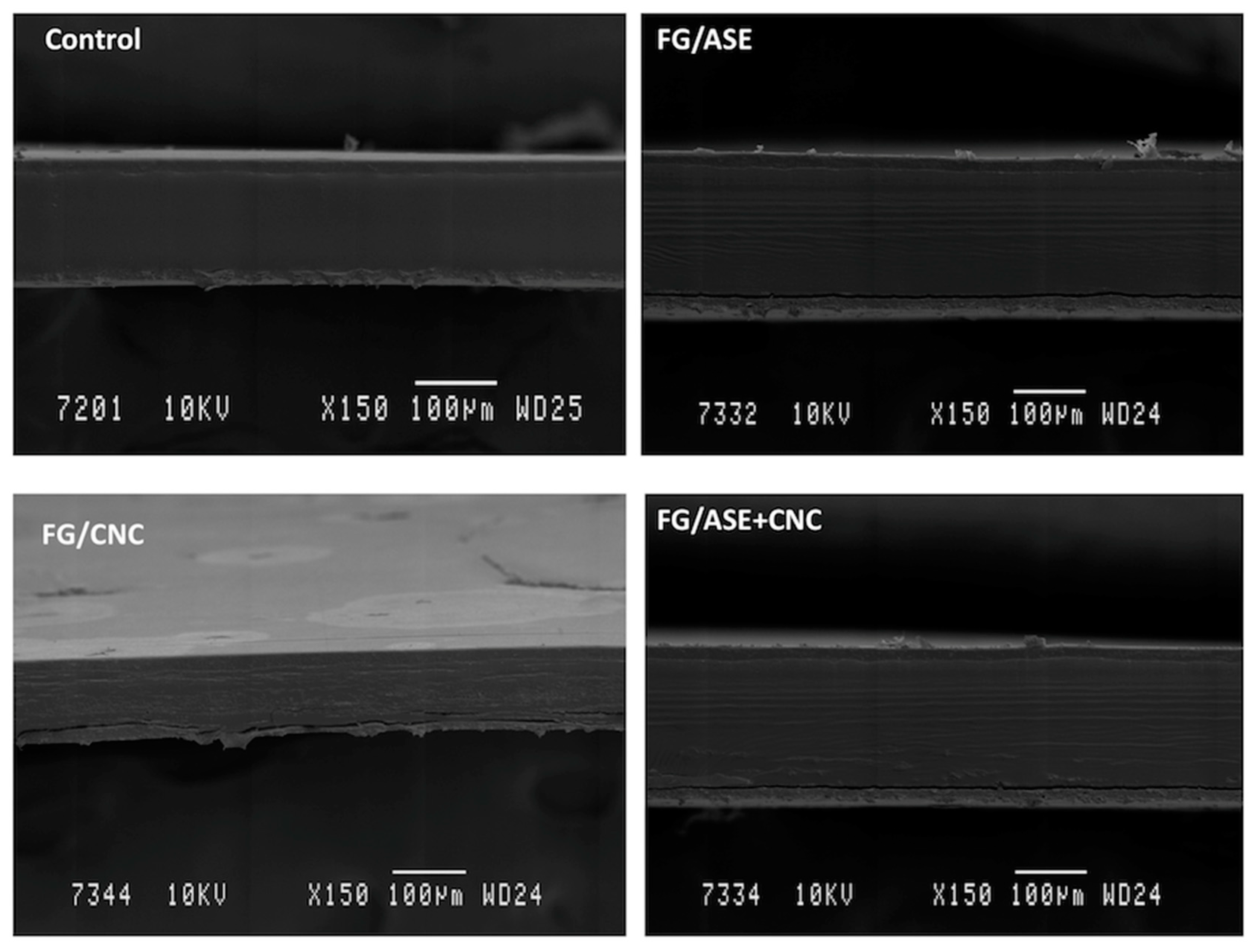
3.4. Morphological Characterization by SEM
3.5. Thermal Characterization
3.6. Mechanical Properties
3.7. Barrier Properties
3.8. Antioxidant Activity of Films
3.9. Oxidative Stability Study of Packaged Avocado in Multilayer Films
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Food and Agriculture Organization of the United States, FAOSTAT Database. Available online: http://www.fao.org/faostat/en/#data (accessed on 25 August 2021).
- Garcia, F.; Davidov-Pardo, G. Recent Advances in the Use of Edible Coatings for Preservation of Avocados: A Review. J. Food Sci. 2021, 86, 6–15. [Google Scholar] [CrossRef]
- USDA Database. Available online: Https://Fdc.Nal.Usda.Gov/ (accessed on 25 August 2021).
- Resende, L.M.B.; de Souza, V.R.; Ferreira, G.M.D.; Nunes, C.A. Changes in Quality and Phytochemical Contents of Avocado Oil under Different Temperatures. J. Food Sci. Technol. 2019, 56, 401–408. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Food Losses and Waste. Extent, Causes and Prevention. 2011. Available online: Http://Www.Fao.Org/3/a-I2697e.Pdf (accessed on 25 August 2021).
- Rai, S.; Dutta, P.K.; Mehrotra, G.K. Natural Antioxidant and Antimicrobial Agents from Agrowastes: An Emergent Need to Food Packaging. Waste Biomass Valorization 2020, 11, 1905–1916. [Google Scholar] [CrossRef]
- Pirayesh, H.; Khazaeian, A. Using Almond (Prunus Amygdalus, L.) Shell as a Bio-Waste Resource in Wood Based Composite. Compos. Part B Eng. 2012, 43, 1475–1479. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Vecchia, S.B.D.; Giovine, F.; Kbaier, H.B.H.; Bouzouita, N.; Pereira, L.B.; Zeppa, G. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus Dulcis, L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef] [Green Version]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. A Box-Behnken Design for Optimal Extraction of Phenolics from Almond By-Products. Food Anal. Methods 2019, 12, 2009–2024. [Google Scholar] [CrossRef]
- Urruzola, I.; Robles, E.; Serrano, L.; Labidi, J. Nanopaper from Almond (Prunus Dulcis) Shell. Cellulose 2014, 21, 1619–1629. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C. Recent Applications of Biopolymers Derived from Fish Industry Waste in Food Packaging. Polymers 2021, 13, 2337. [Google Scholar] [CrossRef]
- Sánchez, J.T.; Valdés, A.; Martínez-Abad, A.; Vilaplana, F.; Jiménez, A.; Garrigós, M.C. Physicochemical and Functional Properties of Active Fish Gelatin-Based Edible Films Added with Aloe Vera Gel. Foods 2020, 9, 1248. [Google Scholar] [CrossRef]
- Rocca-Smith, J.R.; Pasquarelli, R.; Lagorce-Tachon, A.; Rousseau, J.; Fontaine, S.; Aguié-Béghin, V.; Debeaufort, F.; Karbowiak, T. Toward Sustainable PLA-Based Multilayer Complexes with Improved Barrier Properties. ACS Sustain. Chem. Eng. 2019, 7, 3759–3771. [Google Scholar] [CrossRef]
- Nagarajan, M.; Prodpran, T.; Benjakul, S.; Songtipya, P. Properties and Characteristics of Multi-Layered Films from Tilapia Skin Gelatin and Poly(Lactic Acid). Food Biophys. 2017, 12, 222–233. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Javidi, Z.; Rezaei, M. Efficient Gas Barrier Properties of Multi-Layer Films Based on Poly(Lactic Acid) and Fish Gelatin. Int. J. Biol. Macromol. 2016, 92, 1205–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martucci, J.F.; Ruseckaite, R.A. Three-Layer Sheets Based on Gelatin and Poly(Lactic Acid), Part 1: Preparation and Properties. J. Appl. Polym. Sci. 2010, 118, 3102–3110. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation Behavior of Three-Layer Sheets Based on Gelatin and Poly (Lactic Acid) Buried under Indoor Soil Conditions. Polym. Degrad. Stab. 2015, 116, 36–44. [Google Scholar] [CrossRef]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Wildner, J.; Bazzichi, A.; Anguillesi, I.; Lazzeri, A. Whey Protein Layer Applied on Biodegradable Packaging Film to Improve Barrier Properties While Maintaining Biodegradability. Polym. Degrad. Stab. 2014, 108, 151–157. [Google Scholar] [CrossRef]
- Valdés, A.; Garcia-Serna, E.; Martínez-Abad, A.; Vilaplana, F.; Jimenez, A.; Garrigós, M.C. Gelatin-Based Antimicrobial Films Incorporating Pomegranate (Punica Granatum, L.) Seed Juice by-Product. Molecules 2020, 25, 166. [Google Scholar] [CrossRef] [Green Version]
- Rocca-Smith, J.R.; Karbowiak, T.; Marcuzzo, E.; Sensidoni, A.; Piasente, F.; Champion, D.; Heinz, O.; Vitry, P.; Bourillot, E.; Lesniewska, E.; et al. Impact of Corona Treatment on PLA Film Properties. Polym. Degrad. Stab. 2016, 132, 109–116. [Google Scholar] [CrossRef]
- Żenkiewicz, M.; Richert, J.; Rytlewski, P.; Moraczewski, K. Some Effects of Corona Plasma Treatment of Polylactide/Montmorillonite Nanocomposite Films. Plasma Process. Polym. 2009, 6, S387–S391. [Google Scholar] [CrossRef]
- Valdés, A.; Álvarez-Pérez, O.B.; Rojas, R.; Aguilar, C.N.; Garrigós, M.C. Impact of Olive Extract Addition on Corn Starch-Based Active Edible Films Properties for Food Packaging Applications. Foods 2020, 9, 1339. [Google Scholar] [CrossRef]
- García, A.V.; Santonja, M.R.; Sanahuja, A.B.; Selva, M.D.C.G. Characterization and Degradation Characteristics of Poly(ε-Caprolactone)-Based Composites Reinforced with Almond Skin Residues. Polym. Degrad. Stab. 2014, 108, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-Based Composite Edible Films Containing Origanum Vulgare, L. Essential Oil. Ind. Crop. Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- ISO 3960:2007. Corrected Version 2009-05-15. Animal and Vegetable Fats and Oils-Determination of Peroxide Value-Iodometric (Visual) Endpoint Determination. Available online: https://www.iso.org/standard/39158.html (accessed on 1 September 2021).
- Alden Press. Determination of the p-Anisidine Value, Method 2.504. In IUPAC Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th ed.; Alden Press: Oxford, UK, 1987; pp. 210–211. [Google Scholar]
- Sutheimer, S.; Caster, J.M.; Smith, S.H. Green Soap: An Extraction and Saponification of Avocado Oil. J. Chem. Educ. 2015, 92, 1763–1765. [Google Scholar] [CrossRef]
- Moraczewski, K.; Rytlewski, P.; Malinowski, R.; Zenkiewicz, M. Comparison of Some Effects of Modification of a Polylactide Surface Layer by Chemical, Plasma, and Laser Methods. Appl. Surf. Sci. 2015, 346, 11–17. [Google Scholar] [CrossRef]
- De Geyter, N. Influence of Dielectric Barrier Discharge Atmosphere on Polylactic Acid (PLA) Surface Modification. Surf. Coat. Technol. 2013, 214, 69–76. [Google Scholar] [CrossRef]
- Donate, R.; Alemán-Domínguez, M.E.; Monzón, M.; Yu, J.; Rodríguez-Esparragón, F.; Liu, C. Evaluation of Aloe Vera Coated Polylactic Acid Scaffolds for Bone Tissue Engineering. Appl. Sci. 2020, 10, 2576. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, F.; Nikiforov, A.; Morent, R.; de Geyter, N. Plasma Modification of Poly Lactic Acid Solutions to Generate High Quality Electrospun PLA Nanofibers. Sci. Rep. 2018, 8, 2241. [Google Scholar] [CrossRef] [Green Version]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface Modification of Polylactic Acid (PLA) by Air Atmospheric Plasma Treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Valdés, A.; Serrano, N.J.; Sanahuja, A.B.; Garrigós, M.C. Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds. Antioxidants 2020, 9, 629. [Google Scholar] [CrossRef]
- Da Cruz, M.R.; Morais, J.P.S.; Muniz, C.R.; Rosa, M.F.; Filho, M.S.M.S.; de Azeredo, H.M.C. Mesquite Seed Gum and Nile Tilapia Fish Gelatin Composite Films with Cellulose Nanocrystals. Pesqui. Agropecu. Bras. 2018, 53, 495–503. [Google Scholar] [CrossRef]
- Naduparambath, S.; Jinitha, T.V.; Shaniba, V.; Sreejith, M.P.; Balan, A.K.; Purushothaman, E. Isolation and Characterisation of Cellulose Nanocrystals from Sago Seed Shells. Carbohydr. Polym. 2018, 180, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Pandia-Estrada, S.; Romero-Santivañez, R.; Céspedes-Chombo, R.; Solari-Godiño, A. Edible Films Gelatin-Based Obtained from Mahi-Mahi Skin (Coryphaena Hippurus) and Oregano Extract: Physicochemical, Antimicrobial, Structural and Surface Characteristics. Sci. Agropecu. 2021, 12, 229–237. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties and Characteristics of Nanocomposite Films from Tilapia Skin Gelatin Incorporated with Ethanolic Extract from Coconut Husk. J. Food Sci. Technol. 2015, 52, 7669–7682. [Google Scholar] [CrossRef]
- Pan, L.; Li, P.; Tao, Y. Preparation and Properties of Microcrystalline Cellulose/Fish Gelatin Composite Film. Materials 2020, 13, 4370. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of Active/Intelligent Food Packaging Film Containing Amaranthus Leaf Extract for Shelf Life Extension of Chicken/Fish during Chilled Storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Kusznierewicz, B.; Malinowska-Pańczyk, E.; Sinkiewicz, I.; Gottfried, K.; Kołodziejska, I. Fish Gelatin Films Containing Aqueous Extracts from Phenolic-Rich Fruit Pomace. LWT 2020, 117, 108613. [Google Scholar] [CrossRef]
- Rattaya, S.; Benjakul, S.; Prodpran, T. Properties of Fish Skin Gelatin Film Incorporated with Seaweed Extract. J. Food Eng. 2009, 95, 151–157. [Google Scholar] [CrossRef]
- Bao, S.; Xu, S.; Wang, Z. Antioxidant Activity and Properties of Gelatin Films Incorporated with Tea Polyphenol-Loaded Chitosan Nanoparticles. J. Sci. Food Agric. 2009, 89, 2692–2700. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and Characterization of Active Gelatin-Based Films Incorporated with Natural Antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, Characterization, and Properties of Chitosan Films with Cinnamaldehyde Nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Shen, Y.; Ni, Z.-J.; Thakur, K.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Preparation and Characterization of Clove Essential Oil Loaded Nanoemulsion and Pickering Emulsion Activated Pullulan-Gelatin Based Edible Film. Int. J. Biol. Macromol. 2021, 181, 528–539. [Google Scholar] [CrossRef]
- Kwak, H.W.; Lee, H.; Park, S.; Lee, M.E.; Jin, H.-J. Chemical and Physical Reinforcement of Hydrophilic Gelatin Film with Di-Aldehyde Nanocellulose. Int. J. Biol. Macromol. 2020, 146, 332–342. [Google Scholar] [CrossRef]
- Pereda, M.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E. Polyelectrolyte Films Based on Chitosan/Olive Oil and Reinforced with Cellulose Nanocrystals. Carbohydr. Polym. 2014, 101, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M. Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films. Foods 2017, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lin, L.; Guo, Y.; Long, J.; Mu, R.-J.; Pang, J. Enhanced Functional Properties of Nanocomposite Film Incorporated with EGCG-Loaded Dialdehyde Glucomannan/Gelatin Matrix for Food Packaging. Food Hydrocoll. 2020, 108, 105863. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Gelatin/Agar-Based Functional Film Integrated with Pickering Emulsion of Clove Essential Oil Stabilized with Nanocellulose for Active Packaging Applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127220. [Google Scholar] [CrossRef]
- Rhim, J.-W. Preparation and Characterization of Vacuum Sputter Silver Coated PLA Film. LWT—Food Sci. Technol. 2013, 54, 477–484. [Google Scholar] [CrossRef]
- Lange, J.; Wyser, Y. Recent Innovations in Barrier Technologies for Plastic Packaging—A Review. Packag. Technol. Sci. 2003, 16, 149–158. [Google Scholar] [CrossRef]
- Stark, N.M. Opportunities for Cellulose Nanomaterials in Packaging Films: A Review and Future Trends. J. Renew. Mater. 2016, 4, 313–326. [Google Scholar] [CrossRef]
- Adilah, Z.M.; Jamilah, B.; Hanani, Z.A. Functional and Antioxidant Properties of Protein-Based Films Incorporated with Mango Kernel Extract for Active Packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Prgomet, I.; Goncalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottone, A.; Masullo, M.; Montoro, P.; Pizza, C.; Piacente, S. HR-LC-ESI-Orbitrap-MS Based Metabolite Profiling of Prunus Dulcis Mill. (Italian Cultivars Toritto and Avola) Husks and Evaluation of Antioxidant Activity. Phytochem. Anal. 2019, 30, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Lee, S.E.; Chang, Y.; Lacroix, M.; Han, J. Effect of Oxidized Phenolic Compounds on Cross-Linking and Properties of Biodegradable Active Packaging Film Composed of Turmeric and Gelatin. LWT 2018, 93, 427–433. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Pham, C.; Bilatto, S.; Azeredo, H.M.C.; Cranston, E.D.; Moreira, F.K.; Mattoso, L.H.C.; Bras, J. Effect of Tannic Acid and Cellulose Nanocrystals on Antioxidant and Antimicrobial Properties of Gelatin Films. ACS Sustain. Chem. Eng. 2021, 9, 8539–8549. [Google Scholar] [CrossRef]
- Guillén-Sánchez, J.; Paucar-Menacho, L.M. Oxidative Stability and Shelf Life of Avocado Oil Extracted Cold and Hot Using Discard Avocado (Persea Americana). Sci. Agropecu. 2020, 11, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Santana, I.; dos Reis, L.M.F.; Torres, A.G.; Cabral, L.M.C.; Freitas, S.P. Avocado (Persea Americana Mill.) Oil Produced by Microwave Drying and Expeller Pressing Exhibits Low Acidity and High Oxidative Stability. Eur. J. Lipid Sci. Technol. 2015, 117, 999–1007. [Google Scholar] [CrossRef]
- Berasategi, I.; Barriuso, B.; Ansorena, D.; Astiasarán, I. Stability of Avocado Oil during Heating: Comparative Study to Olive Oil. Food Chem. 2012, 132, 439–446. [Google Scholar] [CrossRef]
- Yaacoub, R.; Saliba, R.; Nsouli, B.; Khalaf, G.; Birlouez-Aragon, I. Formation of Lipid Oxidation and Isomerization Products during Processing of Nuts and Sesame Seeds. J. Agric. Food Chem. 2008, 56, 7082–7090. [Google Scholar] [CrossRef] [PubMed]
- Indriyani, L.; Rohman, A.; Riyanto, S. Physico-Chemical Characterization of Avocado (Persea Americana Mill.) Oil from Three Indonesian Avocado Cultivars. Res. J. Med. Plant 2016, 10, 67–78. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; Ventura-Sobrevilla, J.; Martínez-Vazquez, G.; Ruelas-Chacón, X.; Rojas, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Effects of a Natural Bioactive Coating on the Quality and Shelf Life Prolongation at Different Storage Conditions of Avocado (Persea Americana Mill.) Cv. Hass. Food Packag. Shelf Life 2017, 14, 102–107. [Google Scholar] [CrossRef]
- Gómez-López, V.M. Inhibition of Surface Browning, Cut Avocado. J. Food Qual. 2002, 25, 369–379. [Google Scholar] [CrossRef]
- Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from Agricultural Wastes: Products and Applications—A Review. Processes 2021, 9, 1594. [Google Scholar] [CrossRef]



 ), FG/ASE (
), FG/ASE (  ), FG/CNC (
), FG/CNC (  ) and FG/ASE + CNC (
) and FG/ASE + CNC (  ) films as a function of time at 4 °C. Mean ± SD, n = 3. Different letters (a,b,c) within the same multilayer film and parameter indicate statistically significant different values (p < 0.05).
) films as a function of time at 4 °C. Mean ± SD, n = 3. Different letters (a,b,c) within the same multilayer film and parameter indicate statistically significant different values (p < 0.05).
 ), FG/ASE (
), FG/ASE (  ), FG/CNC (
), FG/CNC (  ) and FG/ASE + CNC (
) and FG/ASE + CNC (  ) films as a function of time at 4 °C. Mean ± SD, n = 3. Different letters (a,b,c) within the same multilayer film and parameter indicate statistically significant different values (p < 0.05).
) films as a function of time at 4 °C. Mean ± SD, n = 3. Different letters (a,b,c) within the same multilayer film and parameter indicate statistically significant different values (p < 0.05).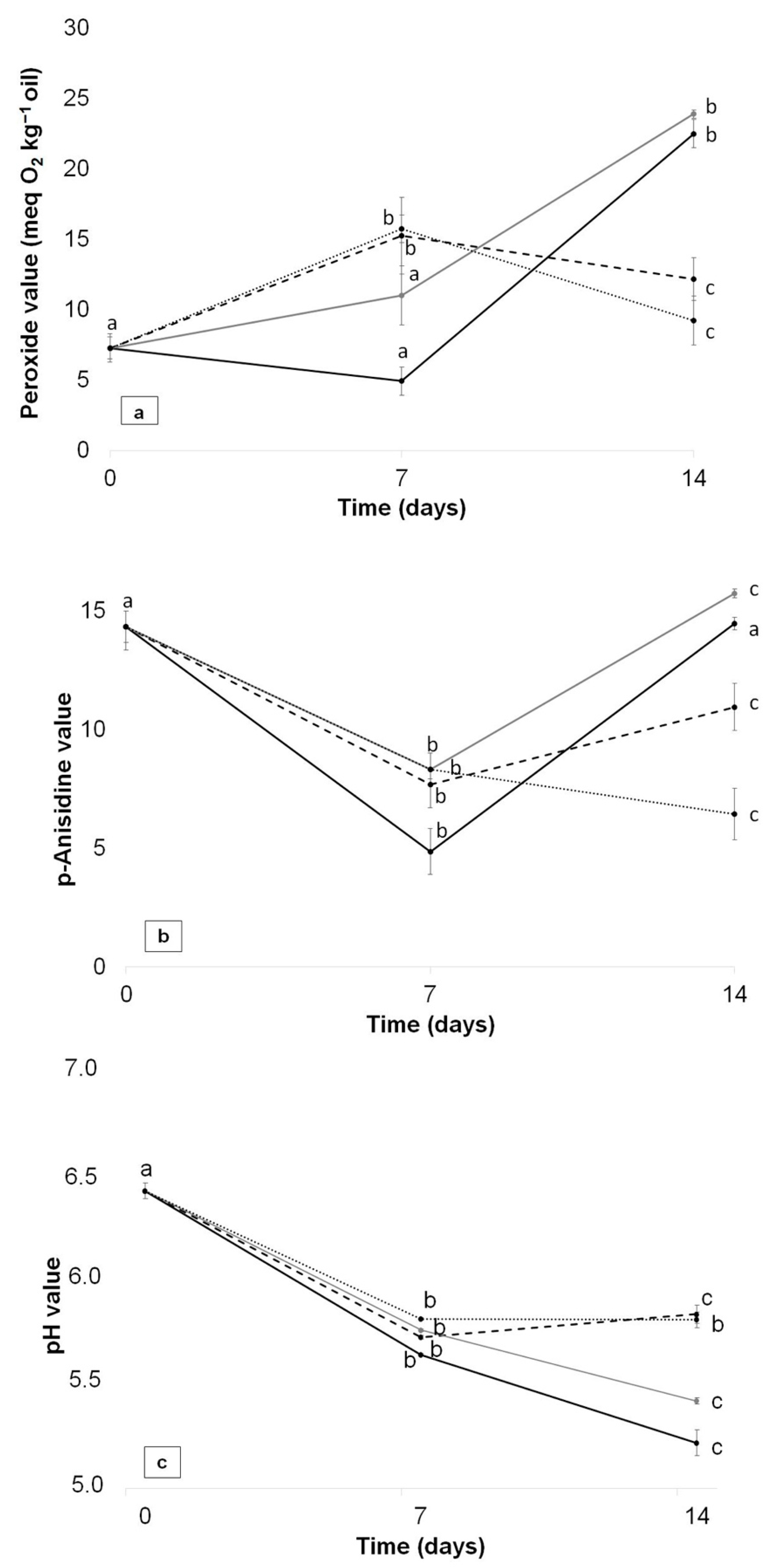
| Formulation | Codification | Content (wt.%) | |||
|---|---|---|---|---|---|
| FG | Glycerol | ASE | CNCs | ||
| PLA/FG/PLA | Control | 8 | 20 | 0 | 0 |
| PLA/FG + ASE/PLA | FG/ASE | 8 | 20 | 6 | 0 |
| PLA/FG + CNCs/PLA | FG/CNC | 8 | 20 | 0 | 4.5 |
| PLA/FG + ASE + CNCs/PLA | FG/ASE + CNC | 8 | 20 | 6 | 4.5 |
| PLA Surface Treatment (s) | Elemental Composition (%) 1 | Surface Tension (mN m−1) 2 | ||||
|---|---|---|---|---|---|---|
| C | O | O/C | γps | γds | γs | |
| 0 | 85 ± 1 a | 15 ± 1 a | 0.18 ± 0.02 a | 3.1 | 17.9 | 21.0 |
| 20 | 81 ± 3 a,b | 19 ± 3 a,b | 0.24 ± 0.05 a,b | 4.3 | 21.9 | 26.3 |
| 40 | 78 ± 1 b | 22 ± 1 b | 0.28 ± 0.02 b | 11.8 | 16.2 | 27.9 |
| 60 | 79 ± 2 b | 21 ± 1 b | 0.27 ± 0.06 b | 18.9 | 21.4 | 40.3 |
| 80 | 73 ± 1 c | 27 ± 1 c | 0.37 ± 0.02 c | 26.5 | 20.5 | 47.1 |
| Parameter | Control | FG/ASE | FG/CNC | FG/ASE + CNC |
|---|---|---|---|---|
| Thickness (mm) | 0.105 ± 0.011 a | 0.109 ± 0.008 a | 0.105 ± 0.004 a | 0.118 ± 0.014 a |
| Transparency (%) | 7.89 ± 0.36 a | 5.92 ± 0.15 b | 6.67 ± 0.55c | 5.62 ± 0.12 b |
| L* | 83.95 ± 0.02 a | 60.09 ± 0.02 b | 83.94 ± 0.06 a | 50.21 ± 0.02 c |
| a* | −2.58 ± 0.03 a | 10.35 ± 0.04 b | −2.65 ± 0.29 a | 14.28 ± 0.02 c |
| b* | 5.23 ± 0.02 a | 36.77 ± 0.04 b | 8.85 ± 0.02 c | 31.93 ± 0.05 d |
| ΔE | - | 41.60 ± 0.05 a | 3.61 ± 0.04 b | 46.03 ± 0.29 c |
| WI | 83.75 ± 0.02 a | 46.38 ± 0.06 b | 83.43 ± 0.26 a | 36.31 ± 0.44 c |
| Parameter | Control | FG/ASE | FG/CNC | FG/ASE + CNC |
|---|---|---|---|---|
| Tini (°C) | 243 ± 2 a | 249 ± 1 b | 245 ± 2 a | 256 ± 1 c |
| Tmax (°C) | 364 ± 4 a | 366 ± 2 a | 365 ± 3 a | 371 ± 3 a |
| OOT (°C) | 122 ± 3 a | 132 ± 1 b | 124 ± 1 a | 144 ± 1 c |
| OIT (°C) | 11.43 ± 0.27 a | 16.81 ± 0.35 b | 12.74 ± 0.27 c | 20.92 ± 0.64 d |
| Tensile strength (MPa) | 23.69 ± 0.48 a | 36.71 ± 0.11 b | 10.48 ± 0.03 c | 34.34 ± 0.02 d |
| Elastic modulus (MPa) | 6.33 ± 0.59 a | 8.63 ± 0.81 b | 9.77 ± 0.05 c | 8.54 ± 0.51 b |
| Elongation at break (%) | 3.26 ± 0.16 a | 4.60 ± 0.02 b | 0.86 ± 0.29 c | 4.69 ± 0.05 b |
| OTR.e (cm3 mm m−2 day) | 14.10 ± 0.86 a | 57.11 ± 4.73 b | 3.04 ± 0.18 c | 40.87 ± 5.20 d |
| Solubility (%) | 43.41 ± 2.68 a | 52.62 ± 1.25 b | 36.69 ± 2.95 c | 39.19 ± 0.16 c |
| Antioxidant Essay | Control | FG/ASE | FG/CNC | FG/ASE + CNC |
|---|---|---|---|---|
| DPPH | nd | 17 ± 1 a | nd | 28 ± 3 b |
| ABTS | nd | 268 ± 26 a | nd | 377 ± 15 b |
| FRAP | nd | 258 ± 1 a | nd | 409 ± 4 b |
| TPC | nd | 361 ± 9 a | nd | 566 ± 15 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, A.; Martínez, C.; Garrigos, M.C.; Jimenez, A. Multilayer Films Based on Poly(lactic acid)/Gelatin Supplemented with Cellulose Nanocrystals and Antioxidant Extract from Almond Shell By-Product and Its Application on Hass Avocado Preservation. Polymers 2021, 13, 3615. https://doi.org/10.3390/polym13213615
Valdés A, Martínez C, Garrigos MC, Jimenez A. Multilayer Films Based on Poly(lactic acid)/Gelatin Supplemented with Cellulose Nanocrystals and Antioxidant Extract from Almond Shell By-Product and Its Application on Hass Avocado Preservation. Polymers. 2021; 13(21):3615. https://doi.org/10.3390/polym13213615
Chicago/Turabian StyleValdés, Arantzazu, Carmen Martínez, Mari Carmen Garrigos, and Alfonso Jimenez. 2021. "Multilayer Films Based on Poly(lactic acid)/Gelatin Supplemented with Cellulose Nanocrystals and Antioxidant Extract from Almond Shell By-Product and Its Application on Hass Avocado Preservation" Polymers 13, no. 21: 3615. https://doi.org/10.3390/polym13213615