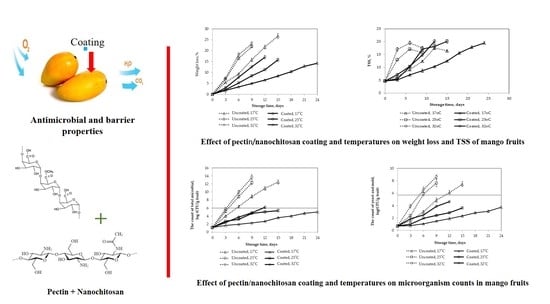
Effect of Pectin/Nanochitosan-Based Coatings and Storage Temperature on Shelf-Life Extension of “Elephant” Mango (Mangifera indica L.) Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Treatments
2.2.2. Determination of Weight Loss
2.2.3. Determination of Firmness
2.2.4. Determination of Color
2.2.5. Determination of Chemical Characteristics: Total Soluble Solids (TSS), Total Cidity (TA), Reducing Sugar and Vitamin C
2.2.6. Determination of Microbial Analysis
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Coating on Shelf-Life and Some Quality Traits of Mango Fruits
3.1.1. Weight Loss
3.1.2. Firmness
3.1.3. Color
3.1.4. Chemical Characteristics
3.2. Effects of Storage Temperature on Shelf-Life and Some Quality Traits of Mango Fruits
3.2.1. Weight Loss
3.2.2. Firmness
3.2.3. Color
3.2.4. Chemical Characteristics
3.2.5. Microbiological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musa, K.B.; Farzana, B.; Muhammad, S.J. Effect of coatings over the quality and shelf life of mango (Mangifera Indica, L.) fruit. J. Food Process. Preserv. 2013, 37, 66–73. [Google Scholar]
- Lakshminarayana, S.; Subramanyam, H. Carbon dioxide injury and fermentative decarboxylation in mango fruit at low temperature storage. J. Food Sci. Technol. 1970, 7, 148–152. [Google Scholar]
- Bender, R.J.; Brecht, J.K.; Baldwin, E.A.; Malundo, T.M.M. Aroma volatiles of mature-green and tree-ripe “Tommy Atkins” mangoes after controlled atmosphere versus air storage. HortScience 2000, 35, 684–686. [Google Scholar] [CrossRef] [Green Version]
- McGuire, R.G.; Hallman, G.J. Coating Guavas with Cellulose-or Carnauba-based Emulsions Interferes with Postharvest Ripening. HortScience 1995, 30, 294–295. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, E.A.; Nisperos, M.O.; Hagenmaier, R.H.; Baker, R.A. Use of lipids in edible coatings for food products. Food Technol. 1997, 51, 56–62. [Google Scholar]
- Baldwin, E.A.; Nisperos, M.O.; Shaw, P.E.; Burns, J.K. Effect of Coatings and Prolonged Storage Conditions on Fresh Orange Flavor Volatiles, Degrees Brix, and Ascorbic Acid Levels. J. Agric. Food Chem. 1995, 43, 1321–1331. [Google Scholar] [CrossRef]
- Vartiainen, R.M.; Kulonen, H.; Ratt, M.; Skytt, E.; Ahvenainen, R. Chitosan-coated paper: Effects of nisin and different acids on the antimicrobial activity. J. Appl. Polym. Sci. 2004, 94, 986–993. [Google Scholar] [CrossRef]
- Mucha, M.; Wankowicz, K.; Balcerzak, J. Analysis of water adsorption on chitosan and its blends with hy-droxy-propylcellulose. E-Polymers 2007, 16, 1–10. [Google Scholar]
- Park, S.Y.; Marsh, K.S.; Rhim, J.W. Characteristics of Different Molecular Weight Chitosan Films Affected by the Type of Organic Solvents. J. Food Sci. 2002, 67, 194–197. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Badii, F.; Shahamirian, M. Recent innovations in the area of edible films and coatings. Recent Pat. Food Nutr. Agric. 2013, 5, 201–213. [Google Scholar] [CrossRef]
- Ikono, R.; Vibriani, A.; Wibowo, I.; Saputro, K.E.; Muliawan, W.; Bachtiar, B.M.; Mardliyati, E.; Bachtiar, E.W.; Rochman, N.T.; Kagami, H.; et al. Nanochitosan antimicrobial activity against Streptococcus mutans and Candida albicans dual-species biofilms. BMC Res. Notes 2019, 12, 383. [Google Scholar] [CrossRef] [Green Version]
- Gláucia, M.C.S.; David, B.M.; Acácio, R.S.; Maria, H.M.C.; Natália, M.d.S.; Diederson, B.S.; Gisele, P.M. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Characteristics and Antimicrobial Properties of Active Edible Films Based on Pectin and Nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. [Google Scholar] [CrossRef] [Green Version]
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of Nanochitosan-Based Coating With and Without Copper Loaded on Physicochemical and Bioactive Components of Fresh Strawberry Fruit (Fragaria x ananassa Duchesne) During Storage. Food Bioprocess Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Seung, K.L.; Adel, A.K. Preharvest and Postharvest Factors Influencing Vitamin C Content of Horticultural Crops. Post-Harvest. Biol. Technol. 2000, 20, 207–220. [Google Scholar]
- Ngo, T.M.P.; Tran, T.X. Optimization of pectin extraction from vanang leaves and making pectin—Alginate films. J. Sci. Technol. Univ. Danang Vietnam 2016, 11, 170–174. [Google Scholar]
- Abdolali, H.S.K.; Rahim, K.; Saadat, S. Effect of chitosan coating and storage temperature on shelf-life and fruit quality of Ziziphus Mauritiana. Int. J. Fruit Sci. 2021, 21, 509–518. [Google Scholar]
- Allegra, A.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on postharvest maintenance of ‘Dottato’ fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Mohamed, C.; Jessica, P.; Didier, M.; Gérard, L.; Marie, N.; Ducamp, C. Preservation of mango quality by using functional chitosan-lactoperoxidase systems coatings. Postharvest Biol. Technol. 2015, 101, 10–14. [Google Scholar]
- AOAC. Official Methods of Analysis, 14th ed.; Williams, S., Ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 1984; pp. 844–846. [Google Scholar]
- ISO. Aerobic Plate Counts by the Surface Inoculation Method (Standard Plate Count Agar) According to ISO 4833; Standards and IFU No. 6; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- AOAC International. A.O.M. Yeast and Mold Counts in Food 2000; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- James, R.G.; Betty, H.P.; Rodrigo, A.C.; Adel, A.K. Quality changes in fresh-cut pear slices as affected by controlled at-mospheres and chemical preservatives. Postharvest Biol. Technol. 2002, 24, 271–278. [Google Scholar]
- Tumwesigye, K.S.; Sousa, A.R.; Oliveira, J.C.; Gallagher, M.J.S. Evaluation of novel bitter cassava film for equilibrium modified atmosphere packaging of cherry tomatoes. Food Packag. Shelf Life 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Ali, S.K.G.; Fojan, B.; Maryam, H.; Neda, M.; Ahmad, M.G.; Ali, Y.A. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. Food Sci. Technol. 2016, 70, 33–40. [Google Scholar]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Van Buren, J.P. The Chemistry of Texture in Fruits and Vegetables. J. Texture Stud. 1979, 10, 1–2. [Google Scholar] [CrossRef]
- Yaman, O.; Bayoιndιrlι, L. Effects of an edible coating and cold storage on shelf life and quality of cherries. LWT-Food Sci. Technol. 2002, 35, 146–150. [Google Scholar] [CrossRef]
- Kashappa, D.G.; Hyun, P.J. Study of gamma irradiation effects on chitosan microparticles. Drug Deliv. 2006, 13, 39–50. [Google Scholar]
- Abbasi, N.A.; Iqbal, Z.; Maqbool, M.; Hafiz, I.A. Postharvest quality of mango (Mangifera indica L.) fruit as affected by chitosan coating. Pak. J. Bot. 2009, 41, 343–357. [Google Scholar]
- Bautista-Baños, S.; Hernádez-López, M.; Bosquez-Molina, E.; Wilson, C.L. Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Prot. 2003, 22, 1087–1092. [Google Scholar] [CrossRef]
- Du, J.M.; Gemma, H.; Iwahori, S. Effects of chitosan coating on the storage of peach, Japanese pear, and kiwifruit. J. Jpn. Soc. Hortic. Sci. 1997, 66, 15–22. [Google Scholar] [CrossRef]
- Kittur, F.S.; Saroja, N.H.; Tharanathan, R.N.; Tharanathan, R. Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. Eur. Food Res. Technol. 2001, 213, 306–311. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Baskaran, R.; Ramesh, M.N.; Prashanth, K.V.; Tharanathan, R. Storage studies of mango packed using biodegradable chitosan film. Eur. Food Res. Technol. 2002, 215, 504–508. [Google Scholar]
- Ali, A.; Maqbool, M.; Ramachandran, S.; Alderson, P.G. Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2010, 58, 42–47. [Google Scholar] [CrossRef]
- Brizzolara, S.; Hertog, M.; Tosetti, R.; Nicolai, B.; Tonutti, P. Metabolic Responses to Low Temperature of Three Peach Fruit Cultivars Differently Sensitive to Cold Storage. Front. Plant. Sci. 2008, 9, 706. [Google Scholar] [CrossRef] [Green Version]
- Salunkhe, D.K.; Boun, H.R.; Reddy, N.R. Storage Processing and Nutritional Quality of Fruits and Vegetables; CRC Press Inc.: Boston, MA, USA, 1991; pp. 156–161. [Google Scholar]
- Medlicott, A.P.; Thompson, A.K. Analysis of sugars and organic acids in ripening mango fruits (Mangifera indica L. var Keitt) by high performance liquid chromatography. J. Sci. Food Agric. 1985, 36, 561–566. [Google Scholar] [CrossRef]
- Natalia, S.E.S.; Lanny, S.; Karsono, S. Improving shelf-life of Cavendish Banana Using Chitosan Edible Coating. Procedia Chem. 2014, 9, 113–120. [Google Scholar]
- Barman, K.; Asrey, R.; Pal, R.K. Putrescine and carnauba wax pretreatments alleviate chilling injury, enhance shelf life and preserve pomegranate fruit quality during cold storage. Sci. Hortic. 2011, 130, 795–800. [Google Scholar] [CrossRef]
- Kerry, C.H.; Milda, E.E. Edible Films and Coatings for Food Applications; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Zhu, X.; Wang, Q.; Cao, J.; Jiang, W. Effects of chitosan coating on postharvest quality of mango (Mangifera Indica, L.CV. Tainong) fruits. J. Food Process. Preserv. 2008, 35, 770–784. [Google Scholar] [CrossRef]



















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Do, T.V.T.; Reungsang, A.; Chaiwong, N.; Rachtanapun, P. Effect of Pectin/Nanochitosan-Based Coatings and Storage Temperature on Shelf-Life Extension of “Elephant” Mango (Mangifera indica L.) Fruit. Polymers 2021, 13, 3430. https://doi.org/10.3390/polym13193430
Ngo TMP, Nguyen TH, Dang TMQ, Do TVT, Reungsang A, Chaiwong N, Rachtanapun P. Effect of Pectin/Nanochitosan-Based Coatings and Storage Temperature on Shelf-Life Extension of “Elephant” Mango (Mangifera indica L.) Fruit. Polymers. 2021; 13(19):3430. https://doi.org/10.3390/polym13193430
Chicago/Turabian StyleNgo, Thi Minh Phuong, Thanh Hoi Nguyen, Thi Mong Quyen Dang, Thi Van Thanh Do, Alissara Reungsang, Nareekan Chaiwong, and Pornchai Rachtanapun. 2021. "Effect of Pectin/Nanochitosan-Based Coatings and Storage Temperature on Shelf-Life Extension of “Elephant” Mango (Mangifera indica L.) Fruit" Polymers 13, no. 19: 3430. https://doi.org/10.3390/polym13193430