Analysis of Mechanical Properties and Mechanism of Natural Rubber Waterstop after Aging in Low-Temperature Environment
Abstract
:1. Introduction
2. Experiment Flow Chart
3. Experimental Program
Materials
4. Sample Preparation and Aging Environment
5. Equipment
6. Testing Methods
6.1. Mechanical Properties Test
6.2. Scanning Electron Microscopy with X-Ray Microanalysis (SEM-EDS) Test
6.3. Fourier Infrared Spectroscopy-Attenuated Total Reflection Total Reflection (FTIR-ATR) Test
7. Results and Discussion
Mechanical Properties
8. Failure Modes
Surface Damage
9. Cross-Sectional Damage of Specimens after Tensile Test
10. Chemical Analysis
10.1. Energy-Dispersive Spectrometer Analysis
10.2. Fourier Infrared Spectroscopy-Attenuated Total Refection Analysis
11. Conclusions
- Increase in low-temperature aging time leads to a decrease in the tensile strength, elongation, tear strength and compression permanent deformation and an increase in the hardness of the rubber waterstop.
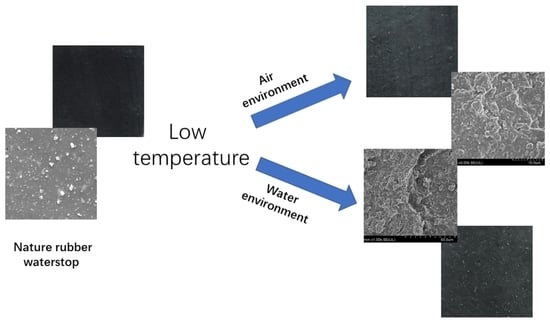
- SEM observation reveals that with the increase in aging time, the surface additive particles disappear, many protrusions appear and gradually increase in the low-temperature air environment. In the low-temperature water environment, the surface protrusions become rough, and there are only a small number of additive particles in the cross-section at 90 days, which reduces the adhesion of the rubber matrix and affects the strength of the rubber waterstop.
- FTIR-ATR analysis shows that the infrared spectra of the low-temperature air environment and low-temperature water environment are almost the same, the decrease in functional groups used to characterize the basic structure of polyisoprene is not obvious during the aging process, and the peak amplitude produced by the hydroxyl carboxyl group and other functional groups during the oxidation process is small, and the oxidation is slow.
- EDS results show that with the increase in aging time, there is an increase in O element and a decrease in C element in the low-temperature air environment. In the low-temperature water environment, the rubber matrix still maintains good adhesion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, L.Q. Application of waterstop in water conservancy engineering. Hebei Water Resour. 2018, 5, 41. [Google Scholar]
- Zhang, L.B. Talking about the application of rubber waterstop in hydraulic engineering. Technol. Enterp. 2012, 9, 241. [Google Scholar]
- Shen, H.Q. Application of rubber waterstop in water conservancy project construction. Technol. Enterp. Henan Water Resour. South North Water Diversion 2018, 8, 91–92. [Google Scholar]
- Tan, L.Y. Talking about the application of rubber waterstop in water conservancy projects. ChengShi Jianshe LiLun Yan Jiu 2011, 24, 1–2. [Google Scholar]
- Thorsten, P.; Ines, J.; Werner, M. Hydrolytic degradation and functional stability of a segmented shape memory poly (ester urethane). Polym. Degrad. Stab. 2008, 1, 61–73. [Google Scholar]
- Liu, S.M.; Yu, L.; Gao, J.X.; Zhang, X.D. Durability of rubber waterstop in extreme environment: Effect and mechanisms of ultraviolet aging. Polym. Bull. 2020, 1, 14. [Google Scholar] [CrossRef]
- Xue, Z.H.; Liu, P. Research on the recycling and reuse of waste plastics. Plast. Sci. Technol. 2021, 49, 107–110. [Google Scholar]
- Liu, C.W. How to Reduce Marine Plastic Waste and Reduce Economic and Social Costs; China Finance Press: Beijing, China, 2021. [Google Scholar]
- Zeng, X.; Huang, H.S. Development status and Prospect of natural rubber technology in China. Trop. Agric. China 2021, 1, 25–30. [Google Scholar]
- Deng, J.; Zheng, Y.; Pan, Z.C. Study on thermal oxygen aging properties of different rubber materials. Spec. Rubber Prod. 2019, 40, 17–20. [Google Scholar]
- Li, B.; Li, S.X.; Zhang, Z.N. Effect of thermal oxygen aging on mechanical properties and tribological behavior of nitrile butadiene rubber. J. Mater. Eng. 2021, 49, 114–121. [Google Scholar]
- Beatty, J.R.; Davies, J.M. Time and Stress Effects in the Behavior of Rubber at Low Temperature. J. Rubber Chem. Technol. 1950, 23, 54–66. [Google Scholar] [CrossRef]
- GeSang, Z.M.; NiMa, L.Z. Analysis on climate characteristics of Shigatse in 2014. Tibet Sci. Technol. 2017, 12, 66–70. [Google Scholar]
- YangJin, Z.M.; JiaYong, C.C. Study on cloud and precipitation probability in flood season in Xigaze, Xizang. J. Beijing Agric. 2015, 15, 153–156. [Google Scholar]
- Li, S.J.; Liu, L.H.; Gao, Y.F. Architecture Material; Chongqing University Press: Chongqing, China, 2014. [Google Scholar]
- Yan, Y.Y. Reflection on the present situation and development of highland barley production in Xigaze area. Tibet J. Agric. Sci. 2011, 33, 10–13. [Google Scholar]
- GB/T 528–2009 Petroleum and Chemical Industry Association of China, Rubber, Vulcanizedor Thermoplastic-Determination of Tensile Stress-Strain Properties; Chinese Code Press: Beijing, China, 2009.
- GB/T 529–2008 Petroleum and Chemical Industry Association of China, Rubber, Vulcanizedor Thermoplastic-Determination of Tear Strength (Trouser, Angle and Crescent Test Pieces); ChineseCode Press: Beijing, China, 2008.
- GB/T 531.1-2008 Petroleum and Chemical Industry Association of China, Rubber, Vulcanizedor Thermoplastic-Determination of Indentation Hardness-Part 1: Duromerer Method (Shore Hardness); Chinese Code Press: Beijing, China, 2008.
- GB/T 7759.1-2015 Petroleum and Chemical Industry Association of China, Rubber, Vulcanized or Thermoplastic-Determination of Compression Set-Part 1: At Ambient or Elevated Temperatures; Chinese Code Press: Beijing, China, 2015.











| Time(d) | Tensile Strength (MPa) | Elongation (%) | Tear Strength (kN/m) | Hardness (HA) | Compression Set (%) |
|---|---|---|---|---|---|
| 0 | 15.2 | 494.9 | 55.9 | 56 | 20 |
| 18 | 12.8 | 482.1 | 52.2 | 60 | 19 |
| 36 | 11.8 | 391.8 | 51.0 | 60 | 17 |
| 54 | 11.3 | 342.0 | 48.6 | 60 | 15 |
| 72 | 10.6 | 339.4 | 46.9 | 61 | 13 |
| 90 | 9.9 | 335.2 | 42.1 | 61 | 13 |
| Time(d) | Tensile Strength (MPa) | Elongation (%) | Tear Strength (kN/m) | Hardness (HA) | Compression Set (%) |
|---|---|---|---|---|---|
| 0 | 15.2 | 494.9 | 55.9 | 56 | 20 |
| 18 | 12.9 | 466.7 | 47.8 | 59 | 17 |
| 36 | 12.5 | 414.7 | 46.9 | 59 | 15 |
| 54 | 12.1 | 398.0 | 45.4 | 59 | 12 |
| 72 | 11.7 | 360.1 | 44.6 | 59 | 14 |
| 90 | 12.3 | 359.4 | 42.9 | 60 | 13 |
| Time (d) | 0 | 18 | 36 | 54 | 72 | 90 | |
|---|---|---|---|---|---|---|---|
| Element | |||||||
| C(Air) | 88.0 | 87.3 | 86.0 | 85.6 | 83.8 | 75.7 | |
| O(Air) | 8.0 | 8.5 | 9.8 | 9.8 | 11.6 | 20.0 | |
| C(Water) | 88.9 | 88.2 | 85.3 | 85.9 | 85.7 | 85.2 | |
| O(Water) | 6.7 | 7.1 | 10.9 | 14.6 | 10.2 | 10.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Liu, S.; Yang, W.; Liu, M. Analysis of Mechanical Properties and Mechanism of Natural Rubber Waterstop after Aging in Low-Temperature Environment. Polymers 2021, 13, 2119. https://doi.org/10.3390/polym13132119
Yu L, Liu S, Yang W, Liu M. Analysis of Mechanical Properties and Mechanism of Natural Rubber Waterstop after Aging in Low-Temperature Environment. Polymers. 2021; 13(13):2119. https://doi.org/10.3390/polym13132119
Chicago/Turabian StyleYu, Lin, Shiman Liu, Weiwei Yang, and Mengying Liu. 2021. "Analysis of Mechanical Properties and Mechanism of Natural Rubber Waterstop after Aging in Low-Temperature Environment" Polymers 13, no. 13: 2119. https://doi.org/10.3390/polym13132119