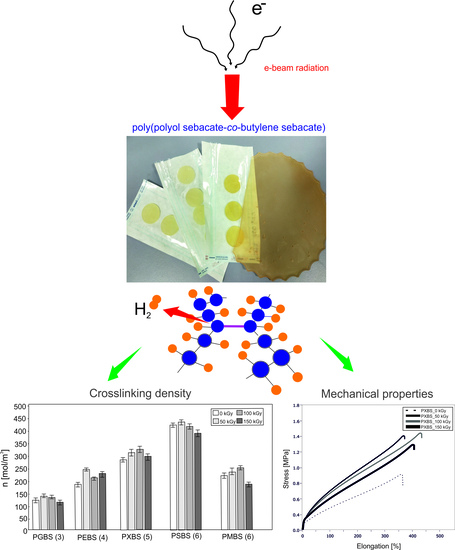
Effect of E-Beam Irradiation on Thermal and Mechanical Properties of Ester Elastomers Containing Multifunctional Alcohols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Elastomers
2.2. Irradiation
2.3. Experimental Methods
2.3.1. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Mechanical Properties
2.3.6. Water Contact Angle
2.3.7. Cross-Link Density
2.3.8. Gel Permeation Chromatography (GPC)
3. Results and Discussion
3.1. Nuclear Magnetic Resonance Spectroscopy (NMR)
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Thermal Properties: Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
3.4. Mechanical Properties
3.5. Cross-Link Density
3.6. Water Contact Angle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Drobny, J.G. Ionizing Radiation and Polymers: Principles, Technology, and Applications; William Andrew: Norwich, NY, USA, 2012. [Google Scholar]
- Rouif, S. Radiation cross−linked polymers: Recent developments and new applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 236, 68–72. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, X.; Zhao, Z.; Mao, L.; Zhang, L.; Coates, P. Effects of wide−range γ−irradiation doses on the structures and properties of 4,4−dicyclohexyl methane diisocyanate based poly(carbonate urethane)s. J. Appl. Polym. Sci. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Murray, K.A.; Kennedy, J.E.; McEvoy, B.; Vrain, O.; Ryan, D.; Cowman, R.; Higginbotham, C.L. The influence of electron beam irradiation conducted in air on the thermal, chemical, structural and surface properties of medical grade polyurethane. Eur. Polym. J. 2013, 49, 1782–1795. [Google Scholar] [CrossRef]
- Pinto, C.; Andrade e Silva, L.G. Study of ionizing radiation on the properties of polyamide 6 with fiberglass reinforcement. Radiat. Phys. Chem. 2007, 76, 1708–1710. [Google Scholar] [CrossRef]
- Mizera, A.; Manas, M.; Holik, Z.; Manas, D.; Stanek, M.; Cerny, J.; Bednarik, M.; Ovsik, M. Properties of selected polymers after radiation cross−linking. Int. J. Math. Comput. Simul. 2012, 6, 592–599. [Google Scholar]
- Seefried, A.; Fuchs, M.; Drummer, D. Radiation crosslinking of semicrystalline thermoplastics: A novel approach to modifying a material’s thermoformability. Plast. Eng. 2012, 68, 14–22. [Google Scholar] [CrossRef]
- Marinović-Cincović, M.; Marković, G.; Samaržija-Jovanović, S.; Budinski-Simendić, J.; Jovanović, V. The influence of γ radiation on the properties of elastomers based on ethylene propylene diene terpolymer and chlorosulfonated polyethylene rubber. J. Thermoplast. Compos. Mater. 2015, 28, 1361–1372. [Google Scholar] [CrossRef]
- Bik, J.; Głuszewski, W.; Rzymski, W.M.; Zagórski, Z.P. EB radiation crosslinking of elastomers. Radiat. Phys. Chem. 2003, 67, 421–423. [Google Scholar] [CrossRef]
- Mizera, A.; Manas, M.; Manas, D.; Holik, Z.; Stanek, M.; Navratil, J.; Bednarik, M. Temperature stability of modified PBT by radiation cross−linking. Adv. Mater. Res. 2014, 1025–1026, 256–260. [Google Scholar]
- Zhu, S.; Shi, M.; Tian, M.; Qu, L.; Chen, G. Effects of irradiation on polyethyleneterephthalate(PET) fibers impregnated with sensitizer. J. Text. Inst. 2018, 109, 294–299. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, M.; Tian, M. Burning behavior of irradiated PET flame−retardant fabrics impregnated with sensitizer. Mater. Lett. 2015, 160, 58–60. [Google Scholar] [CrossRef]
- Manas, D.; Mizera, A.; Navratil, M.; Manas, M.; Ovsik, M.; Sehnalek, S.; Stoklasek, P. The electrical, mechanical and surface properties of thermoplastic polyester elastomer modified by electron beta radiation. Polymers 2018, 10, 1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quynh, T.M.; Mai, H.H.; Lan, P.N. Properties of radiation−induced crosslinking stereocomplexes derived from poly(L−Lactide) and different poly(D−Lactide). Radiat. Phys. Chem. 2013, 83, 105–110. [Google Scholar] [CrossRef]
- Nagasawa, N.; Kasai, N.; Yagi, T.; Yoshii, F.; Tamada, M. Radiation−induced crosslinking and post−processing of poly(L−lactic acid) composite. Radiat. Phys. Chem. 2011, 80, 145–148. [Google Scholar] [CrossRef]
- Sugane, K.; Takahashi, H.; Shimasaki, T.; Teramoto, N.; Shibata, M. Stereocomplexation, thermal and mechanical properties of conetworks composed of star−shaped L−lactide, D−lactide and ε−caprolactone oligomers utilizing sugar alcohols as core molecules. Polymers 2017, 9, 582. [Google Scholar] [CrossRef] [Green Version]
- Phong, L.; Han, E.S.C.; Xiong, S.; Pan, J.; Loo, S.C.J. Properties and hydrolysis of PLGA and PLLA cross−linked with electron beam radiation. Polym. Degrad. Stab. 2010, 95, 771–777. [Google Scholar] [CrossRef]
- Huang, Y.; Gohs, U.; Müller, M.T.; Zschech, C.; Wiessner, S. Electron beam treatment of polylactide at elevated temperature in nitrogen atmosphere. Radiat. Phys. Chem. 2019, 159, 166–173. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, Q.; Qin, R.; Yan, H.; Liang, G. Effect of γ−radiation on crystallization of polycaprolactone. Radiat. Phys. Chem. 2005, 74, 42–50. [Google Scholar] [CrossRef]
- Zhu, G.; Liang, G.; Xu, Q.; Yu, Q. Shape−memory effects of radiation crosslinked Poly(ε−caprolactone). J. Appl. Polym. Sci. 2003, 90, 1589–1595. [Google Scholar] [CrossRef]
- Suhartini, M.; Mitomo, H.; Nagasawa, N.; Yoshii, F.; Kume, T. Radiation crosslinking of poly(butylene succinate) in the presence of low concentrations of trimethallyl isocyanurate and its properties. J. Appl. Polym. Sci. 2003, 88, 2238–2246. [Google Scholar] [CrossRef]
- Rai, R.; Tallawi, M.; Roether, J.A.; Detsch, R.; Barbani, N.; Rosellini, E.; Kaschta, J.; Schubert, D.W.; Boccaccini, A.R. Sterilization effects on the physical properties and cytotoxicity of poly(glycerol sebacate). Mater. Lett. 2013, 105, 32–35. [Google Scholar] [CrossRef]
- Sun, Z.J.; Chen, C.; Sun, M.Z.; Ai, C.H.; Lu, X.L.; Zheng, Y.F.; Yang, B.F.; Dong, D.L. The application of poly (glycerol−sebacate) as biodegradable drug carrier. Biomaterials 2009, 30, 5209–5214. [Google Scholar] [CrossRef] [PubMed]
- Zaky, S.H.; Lee, K.W.; Gao, J.; Jensen, A.; Verdelis, K.; Wang, Y.; Almarza, A.J.; Sfeir, C. Poly (glycerol sebacate) elastomer supports bone regeneration by its mechanical properties being closer to osteoid tissue rather than to mature bone. Acta Biomater. 2017, 54, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, J.M.; Hollister, S.J. Tailoring the mechanical properties of 3D−designed poly(glycerol sebacate) scaffolds for cartilage applications. J. Biomed. Mater. Res.-Part A 2010, 94, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Sundback, C.A.; Shyu, J.Y.; Wang, Y.; Faquin, W.C.; Langer, R.S.; Vacanti, J.P.; Hadlock, T.A. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials 2005, 26, 5454–5464. [Google Scholar] [CrossRef]
- Motlagh, D.; Yang, J.; Lui, K.Y.; Webb, A.R.; Ameer, G.A. Hemocompatibility evaluation of poly(glycerol−sebacate) in vitro for vascular tissue engineering. Biomaterials 2006, 27, 4315–4324. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Bismarck, A.; Hansen, U.; Junaid, S.; Tran, M.Q.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials 2008, 29, 47–57. [Google Scholar] [CrossRef]
- Neeley, W.L.; Redenti, S.; Klassen, H.; Tao, S.; Desai, T.; Young, M.J.; Langer, R. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials 2008, 29, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Bruggeman, J.P.; Bettinger, C.J.; Langer, R. Biodegradable xylitol−based elastomers: In vivo behavior and biocompatibility. J. Biomed. Mater. Res.-Part A 2010, 95, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Bruggeman, J.P.; Bettinger, C.J.; Nijst, C.L.E.; Kohane, D.S.; Langer, R. Biodegradable xylitol−based polymers. Adv. Mater. 2008, 20, 1922–1927. [Google Scholar] [CrossRef]
- Bruggeman, J.P.; de Bruin, B.J.; Bettinger, C.J.; Langer, R. Biodegradable poly(polyol sebacate) polymers. Biomaterials 2008, 29, 4726–4735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, Z.Y.; Zhang, Q.S.; Wu, Q.P.; Li, Y.Z.; Ma, D.X.; Chen, J.Z. Efficient synthesis of hydroxyl functioned polyesters from natural polyols and sebacic acid. Chin. Chem. Lett. 2011, 22, 635–638. [Google Scholar] [CrossRef]
- Dasgupta, Q.; Chatterjee, K.; Madras, G. Combinatorial approach to develop tailored biodegradable poly(xylitol dicarboxylate) polyesters. Biomacromolecules 2014, 15, 4302–4313. [Google Scholar] [CrossRef] [PubMed]
- Kavimani, V.; Jaisankar, V. Synthesis and characterisation of sorbitol based copolyesters for biomedical applications. J. Phys. Sci. Appl. 2014, 4, 507–515. [Google Scholar]
- Piątek-Hnat, M.; Bomba, K. The influence of of cross−linking process on the physicochemical properties of new copolyesters containing xylitol. Mater. Today Commun. 2020, 20, 100734. [Google Scholar] [CrossRef]
- Piątek-Hnat, M.; Bomba, K.; Pęksiński, J. Synthesis and selected properties of ester elastomer containing sorbitol. Appl. Sci. 2020, 10, 1628. [Google Scholar] [CrossRef] [Green Version]
- Piatek-Hnat, M.; Bomba, K.; Pęksiński, J. Structure and properties of biodegradable poly (Xylitol Sebacate−Co−Butylene Sebacate) copolyester. Molecules 2020, 25, 1541. [Google Scholar] [CrossRef] [Green Version]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]








| Pre-Polymer | Molar Composition [mol] | Mw [g/mol] | PDI | Composition (PBS to PPS Segments) by 1H NMR | ||
|---|---|---|---|---|---|---|
| PGBS | SA | GL | BG | 40,000 | 2.1 | 1.84:1 |
| 2 | 1 | 1 | ||||
| PEBS | SA | ER | BG | 42,000 | 1.7 | 1.64:1 |
| 2 | 1 | 1 | ||||
| PXBS | SA | XL | BG | 38,000 | 1.8 | 1.86:0.67 |
| 2 | 1 | 1 | ||||
| PSBS | SA | SB | BG | 20,000 | 1.4 | 4.24:1 |
| 2 | 1 | 1 | ||||
| PMBS | SA | MN | BG | 29,000 | 2.5 | 2.51:1 |
| 2 | 1 | 1 | ||||
| Material/Dose | Molar Composition [mol] | E_ 50% [MPa] | E_ 100% [MPa] | σr [MPa] | εr [%] | n [mol/m3] | ||
|---|---|---|---|---|---|---|---|---|
| - | SA | GL | BG | - | ||||
| PGBS_0 kGy | 2 | 1 | 1 | 1.34 +/−0.09 | 0.910 +/−0.04 | 1.34 +/−0.35 | 230 +/−92.66 | 120.45 +/−52.66 |
| PGBS_50 kGy | 2.77 +/−0.69 | 1.50 +/−0.35 | 1.62 +/−0.37 | 156 +/−47.44 | 142.31 +/−43.32 | |||
| PGBS_100 kGy | 2.44 +/−0.36 | 1.34 +/−0.15 | 1.55 +/−0.26 | 186 +/−59.41 | 139.20 +/−57.21 | |||
| PGBS_150 kGy | 1.95 +/−0.38 | 1.13 +/−0.16 | 1.22 +/−0.21 | 143 +/−30.86 | 110.42 +/−28.81 | |||
| - | SA | ER | BG | - | ||||
| PEBS_0 kGy | 2 | 1 | 1 | 0.799 +/−0.16 | 0.632 +/−0.09 | 1.06 +/−0.35 | 219 +/−85.12 | 189.58 +/−75.22 |
| PEBS _50 kGy | 2.84 +/−0.76 | 1.60 +/−0.33 | 1.75 +/−0.36 | 163 +/−40.65 | 249.23 +/−53.71 | |||
| PEBS_100 kGy | 2.06 +/−0.45 | 1.36 +/−0.15 | 1.38 +/−0.33 | 139 +/−47.89 | 215.32 +/−43.59 | |||
| PEBS_150 kGy | 2.33 +/−0.58 | 1.38 +/−0.20 | 1.56 +/−0.23 | 172 +/−43.60 | 231.42 +/−46.88 | |||
| - | SA | XL | BG | - | ||||
| PXBS_0 kGy | 2 | 1 | 1 | 0.345 +/−0.04 | 0.296 +/−0.04 | 0.931 +/−0.46 | 362 +/−68.8 | 287.42 +/−57.32 |
| PXBS_50 kGy | 0.492 +/−0.08 | 0.424 +/−0.07 | 1.41 +/−0.35 | 372 +/−52.3 | 310.58 +/−45.24 | |||
| PXBS_100 kGy | 0.456 +/−0.14 | 0.395 +/−0.11 | 1.47 +/−0.47 | 431 +/−81,13 | 321.44 +/−41.54 | |||
| PXBS_150 kGy | 0.345 +/−0.03 | 0.298 +/−0.03 | 1.29 +/−0.22 | 409 +/−32.91 | 299.32 +/−38.87 | |||
| - | SA | SB | BG | - | ||||
| PSBS_0 kGy | 2 | 1 | 1 | 0.536 +/−0.11 | 0.455 +/−0.03 | 1.32 +/−0.41 | 395 +/−37.08 | 430.15 +/−45.13 |
| PSBS_50 kGy | 0.826 +/−0.13 | 0.624 +/−0.07 | 1.49 +/−0.18 | 400 +/−17.50 | 437.11 +/−38.63 | |||
| PSBS_100 kGy | 0.234 +/−0.04 | 0.216 +/−0.03 | 0.77 +/−0.14 | 440 +/−57.21 | 425.38 +/−47.28 | |||
| PSBS _150 kGy | 0.311 +/−0.12 | 0.269 +/−0.09 | 0.89 +/−0.25 | 403 +/−33.90 | 387.31 +/−43.76 | |||
| - | SA | MN | BG | - | ||||
| PMBS_0 kGy | 2 | 1 | 1 | 0.329 +/−0.13 | 0.264 +/−0.08 | 0.593 +/−0.17 | 275 +/−44.70 | 221.32 +/−42.85 |
| PMBS_50 kGy | 0.394 +/−0.10 | 0.316 +/−0.06 | 0.557 +/−0.10 | 221 +/−23.87 | 241.48 +/−45.31 | |||
| PMBS_100 kGy | 0.384 +/−0.09 | 0.302 +/−0.06 | 0.629 +/−0.11 | 281 +/−30.51 | 254.32 +/−49.28 | |||
| PMBS_150 kGy | 0.347 +/−0.14 | 0.275 +/−0.08 | 0.489 +/−0.12 | 237 +/−29.77 | 189.41 +/−39.87 | |||
| Material/Dose | I HEATING | |||||
|---|---|---|---|---|---|---|
| Tg | ΔCp | Tm1 | ΔHm1 | Tm2 | ΔHm2 | |
| [°C] | [J/g°C] | [°C] | [J/g] | [°C] | [J/g] | |
| PGBS | - | |||||
| PGBS_0 kGy | −39.4 | 0.508 | 21.1 | 48.9 | 38.2 | 17.1 |
| PGBS_50 kGy | −36.1 | 0.319 | 18.9 | 35 | 37.2 | 18.7 |
| PGBS _100 kGy | −36.4 | 0.276 | 19.9 | 30.5 | 37.9 | 14.4 |
| PGBS _150 kGy | −37.5 | 0.216 | 18.6 | 31.2 | 34.2 | 12.1 |
| PEBS | - | |||||
| PEBS_0 kGy | −36.5 | 0.248 | 20.2 | 28.8 | 41.6 | 10.6 |
| PEBS_50 kGy | −35.9 | 0.341 | 16.4 | 24.6 | 39.6 | 22.2 |
| PEBS_100 kGy | −33.4 | 0.259 | 14.4 | 19.9 | 40.9 | 24.4 |
| PEBS_150 kGy | −37.4 | 0.267 | 14.9 | 26.9 | 37.4 | 10.5 |
| PXBS | - | |||||
| PXBS_0 kGy | −31.6 | 0.368 | 15.3 | 30.6 | 38.4 | 3.8 |
| PXBS_50 kGy | −30.9 | 0.387 | 16.6 | 21.5 | 37.4 | 6.7 |
| PXBS_100 kGy | −34.1 | 0.423 | 16.2 | 24.9 | 38.3 | 5.1 |
| PXBS_150 kGy | −33.5 | 0.39 | 15.6 | 23.9 | 38.3 | 6.5 |
| PSBS | - | |||||
| PSBS_0 kGy | −29.2 | 0.562 | 14.4 | 1.2 | 39.7 | 12.7 |
| PSBS_50 kGy | −29.7 | 0.646 | 13.9 | 0.485 | 38.6 | 10.6 |
| PSBS_100 kGy | −29.7 | 0.633 | 13.9 | 1.1 | 38.3 | 10.5 |
| PSBS_150 kGy | −29.2 | 0.6 | 14.3 | 0.543 | 38.7 | 9.5 |
| PMBS | - | |||||
| PMBS_0 kGy | −30.1 | 0.69 | − | − | 39.8 | 0.56 |
| PMBS_50 kGy | −30.9 | 0.726 | − | − | 38.4 | 2.3 |
| PMBS _100 kGy | −30.2 | 0.627 | − | − | 37 | 6,8 |
| PMBS_150 kGy | −29.7 | 0.64 | − | − | 38.7 | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piątek-Hnat, M.; Bomba, K.; Pęksiński, J.; Kozłowska, A.; Sośnicki, J.G.; Idzik, T.J. Effect of E-Beam Irradiation on Thermal and Mechanical Properties of Ester Elastomers Containing Multifunctional Alcohols. Polymers 2020, 12, 1043. https://doi.org/10.3390/polym12051043
Piątek-Hnat M, Bomba K, Pęksiński J, Kozłowska A, Sośnicki JG, Idzik TJ. Effect of E-Beam Irradiation on Thermal and Mechanical Properties of Ester Elastomers Containing Multifunctional Alcohols. Polymers. 2020; 12(5):1043. https://doi.org/10.3390/polym12051043
Chicago/Turabian StylePiątek-Hnat, Marta, Kuba Bomba, Jakub Pęksiński, Agnieszka Kozłowska, Jacek G. Sośnicki, and Tomasz J. Idzik. 2020. "Effect of E-Beam Irradiation on Thermal and Mechanical Properties of Ester Elastomers Containing Multifunctional Alcohols" Polymers 12, no. 5: 1043. https://doi.org/10.3390/polym12051043