Influence of Oxidation Degree on the Physicochemical Properties of Oxidized Inulin
Abstract
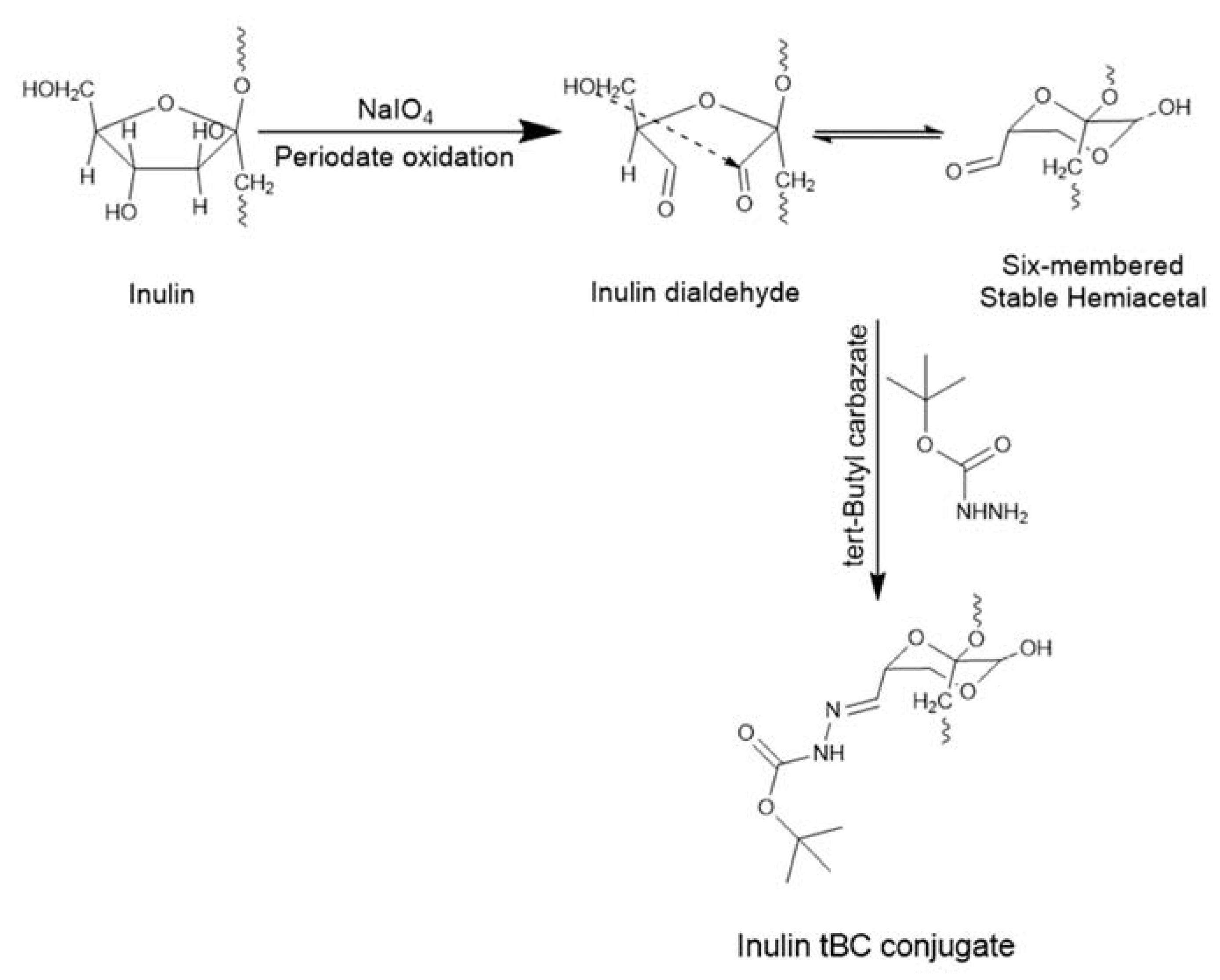
:1. Introduction
2. Materials and Methods
2.1. Oxidation of Inulin to Inulin Aldehyde Derivative
2.2. Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy)
2.3. Determination of Aldehyde Content Using TNBS Assay (Carbazate-TNBS Assay)
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. TGA
2.6. UV Spectrophotometry and Solubility
2.7. Scanning Electron Microscopy (SEM)
2.8. X-ray Diffraction (XRD)
2.9. Differential Scanning Calorimetric (DSC) Analysis
3. Results
3.1. Synthesis and Degree of Oxidation
3.2. FTIR
3.3. Scanning Electron Microscope (SEM) Analysis
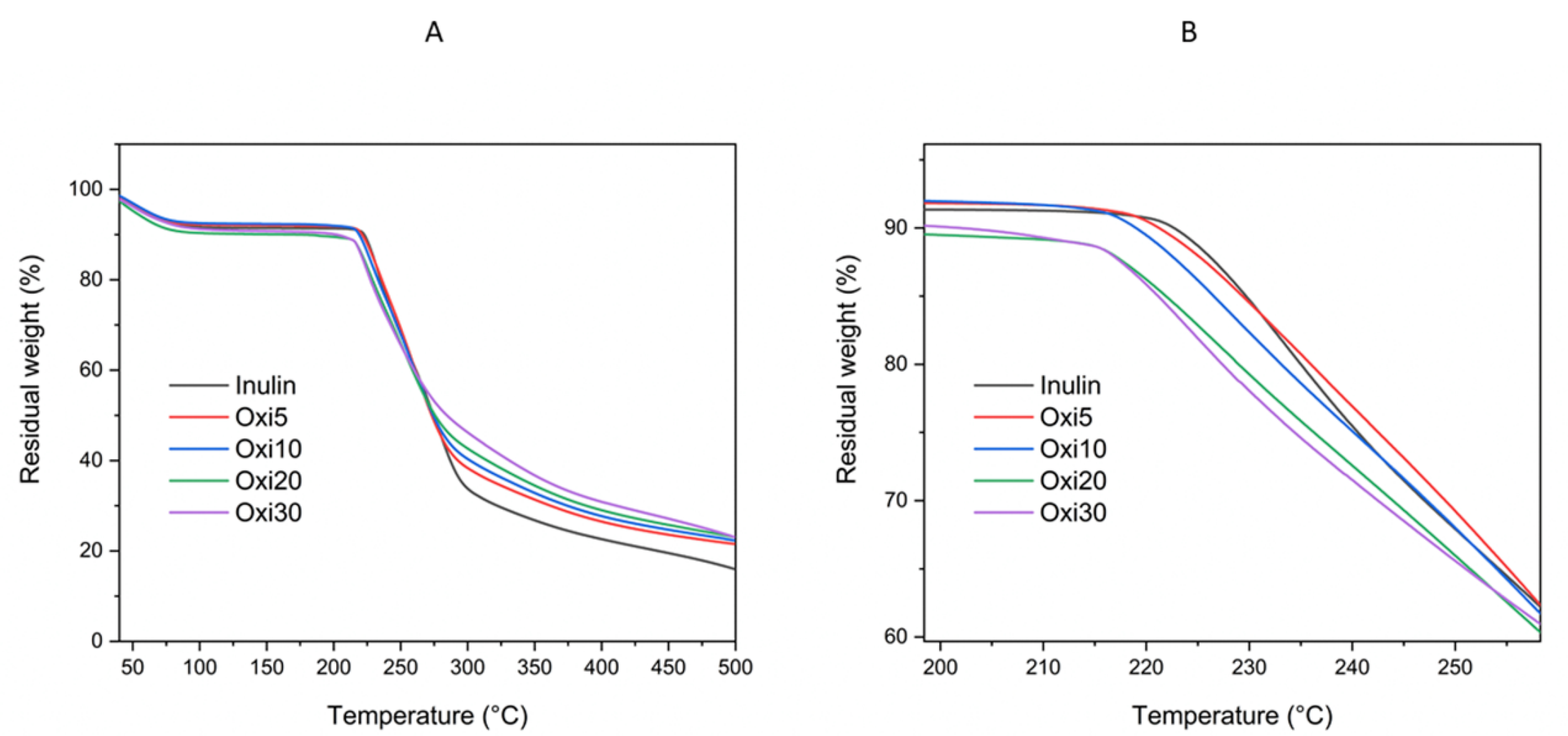
3.4. TGA/DTG
3.5. DSC
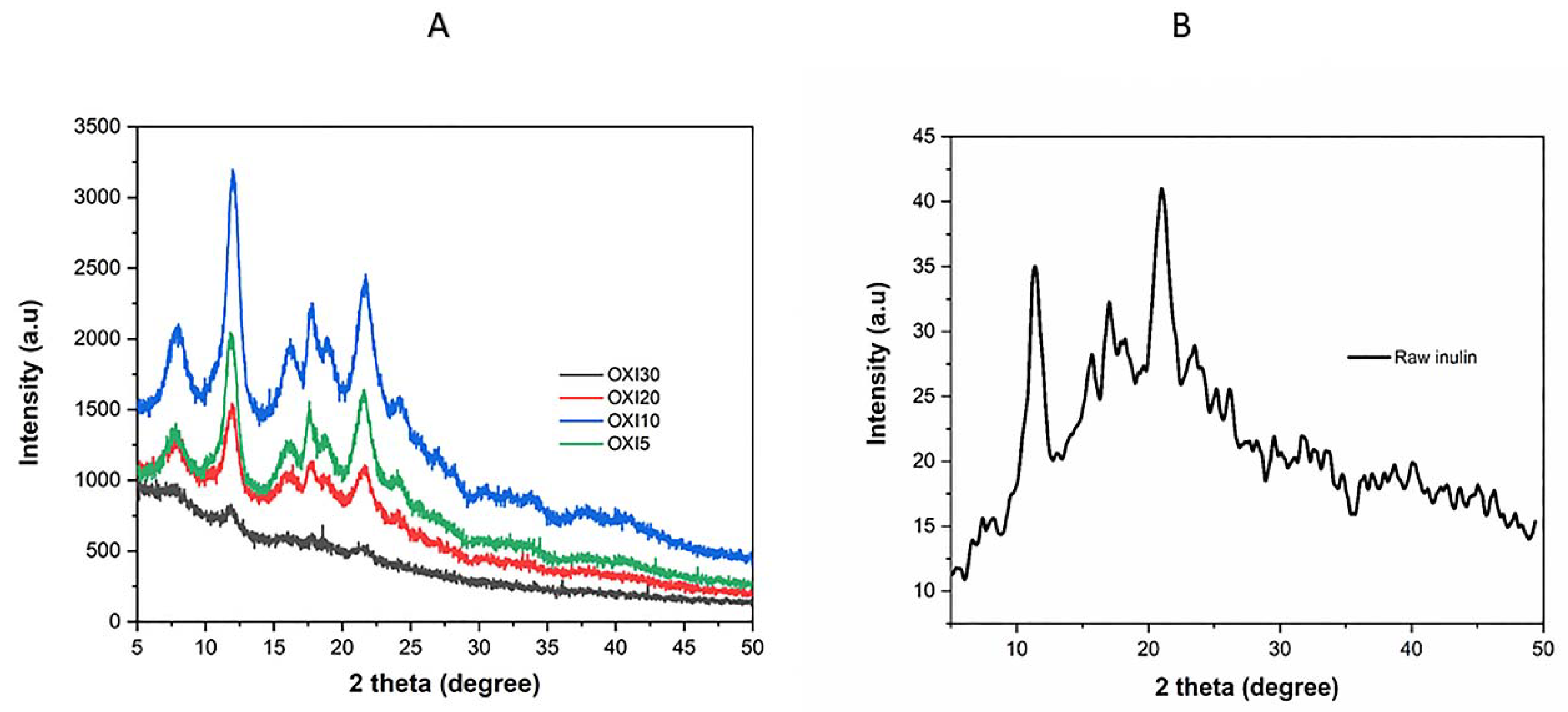
3.6. XRD
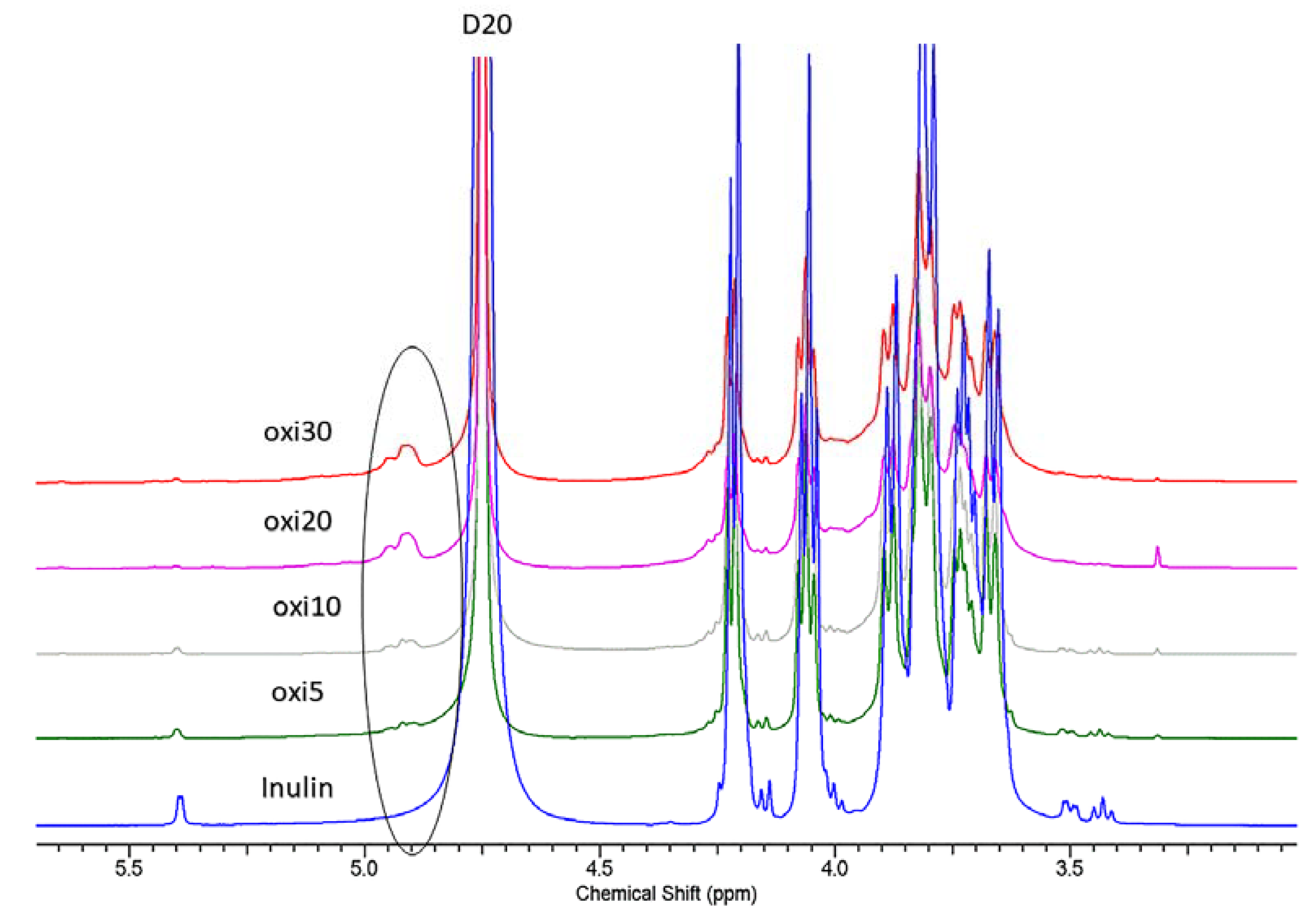
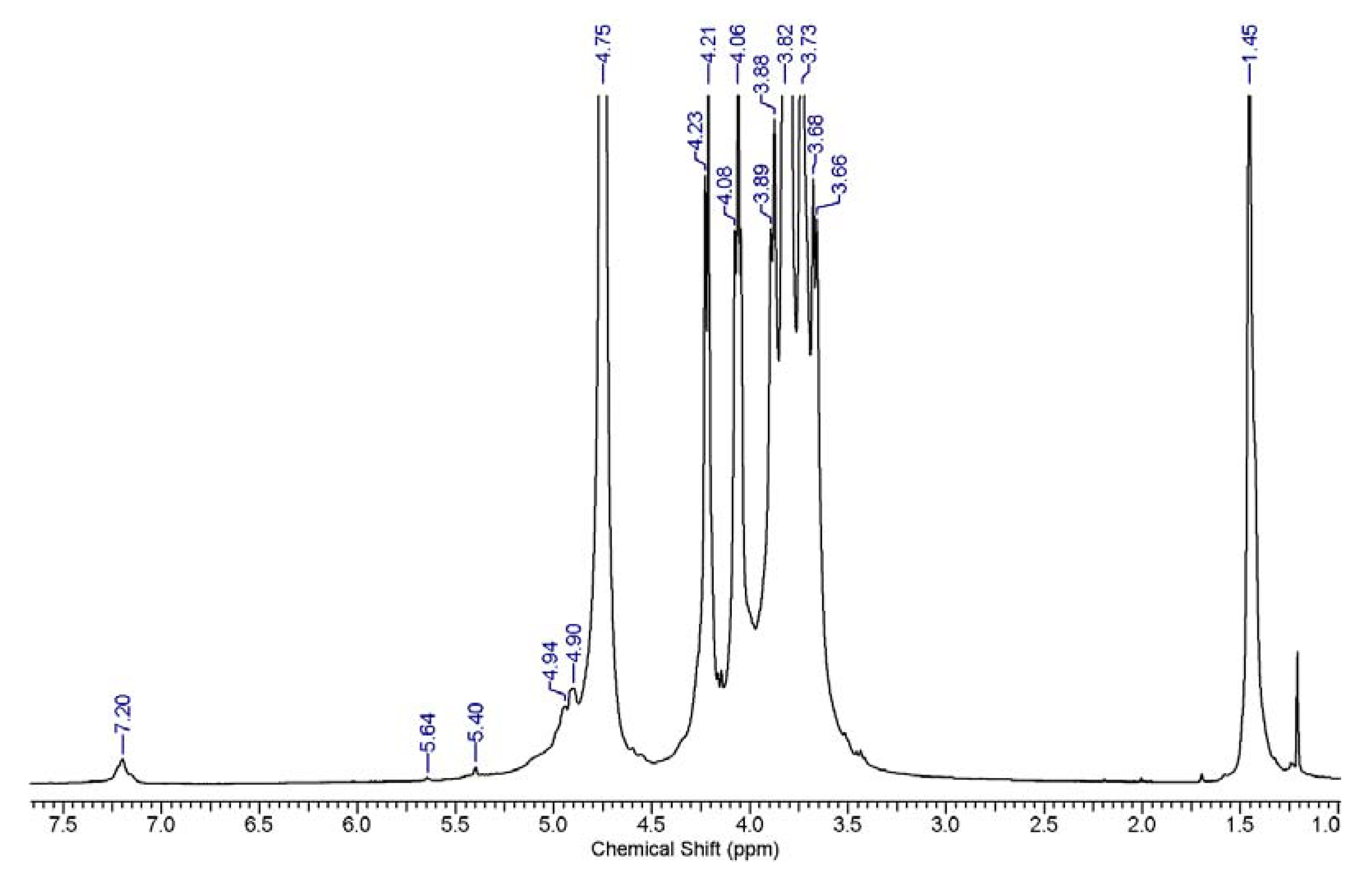
3.7. 1H and 13C NMR Analysis
3.8. Solubility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carabin, I.G.; Flamm, W.G. Evaluation of safety of inulin and oligofructose as dietary fiber. Regul. Toxicol. Pharmacol. 1999, 30, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Van Arkel, J.; Sevenier, R.; Hakkert, J.C.; Bouwmeester, H.J.; Koops, A.J.; van der Meer, I.M. Tailor-made fructan synthesis in plants: A review. Carbohydr. Polym. 2013, 93, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mutanda, T.; Mokoena, M.P.; Olaniran, A.O.; Wilhelmi, B.S.; Whiteley, C.G. Microbial enzymatic production and applications of short-chain fructooligosaccharides and inulooligosaccharides: Recent advances and current perspectives. J. Ind. Microbiol. Biotechnol. 2014, 41, 893–906. [Google Scholar] [CrossRef]
- Roberfroid, M.B.; Van Loo, J.A.; Gibson, G.R. The bifidogenic nature of chicory inulin and its hydrolysis products. J. Nutr. 1998, 128, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Gupta, A.K. Applications of inulin and oligofructose in health and nutrition. J. Biosci. 2002, 27, 703–714. [Google Scholar] [CrossRef]
- Keenan, D.F.; Resconi, V.C.; Kerry, J.P.; Hamill, R.M. Modelling the influence of inulin as a fat substitute in comminuted meat products on their physico-chemical characteristics and eating quality using a mixture design approach. Meat Sci. 2014, 96, 1384–1394. [Google Scholar] [CrossRef]
- Rodriguez Furlan, L.T.; Padilla, A.P.; Campderros, M.E. Development of reduced fat minced meats using inulin and bovine plasma proteins as fat replacers. Meat Sci. 2014, 96, 762–768. [Google Scholar] [CrossRef]
- Kocer, D.; Hicsasmaz, Z.; Bayindirli, A.; Katnas, S. Bubble and pore formation of the high-ratio cake formulation with polydextrose as a sugar- and fat-replacer. J. Food Eng. 2007, 78, 953–964. [Google Scholar] [CrossRef]
- Rodríguez-García, J.; Salvador, A.; Hernando, I. Replacing Fat and Sugar with Inulin in Cakes: Bubble Size Distribution, Physical and Sensory Properties. Food Bioprocess Technol. 2014, 7, 964–974. [Google Scholar] [CrossRef]
- Mittal, S.; Bajwa, U. Effect of fat and sugar substitution on the quality characteristics of low calorie milk drinks. J. Food Sci. Technol. 2012, 49, 704–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintor, A.; Severiano-Perez, P.; Totosaus, A. Optimization of fat-reduced ice cream formulation employing inulin as fat replacer via response surface methodology. Food Sci. Technol. Int. 2014, 20, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, J.; Coussement, P.; De Leenheer, L.; Hoebregs, H.; Smits, G. On the presence of Inulin and Oligofructose as natural ingredients in the western diet. Crit. Rev. Food Sci. Nutr. 1995, 35, 525–552. [Google Scholar] [CrossRef] [PubMed]
- Coussement, P.A. Inulin and oligofructose: Safe intakes and legal status. J. Nutr. 1999, 129, 1412s–1417s. [Google Scholar] [CrossRef]
- Rodriguez Furlan, L.T.; Perez Padilla, A.; Campderros, M.E. Improvement of gluten-free bread properties by the incorporation of bovine plasma proteins and different saccharides into the matrix. Food Chem. 2015, 170, 257–264. [Google Scholar] [CrossRef]
- Rezaei, R.; Khomeiri, M.; Aalami, M.; Kashaninejad, M. Effect of inulin on the physicochemical properties, flow behavior and probiotic survival of frozen yogurt. J. Food Sci. Technol. 2014, 51, 2809–2814. [Google Scholar] [CrossRef] [Green Version]
- Laguna, L.; Primo-Martin, C.; Salvador, A.; Sanz, T. Inulin and erythritol as sucrose replacers in short-dough cookies: Sensory, fracture, and acoustic properties. J. Food Sci. 2013, 78, S777–S784. [Google Scholar] [CrossRef]
- Kelly, G. Inulin-type prebiotics--a review: Part 1. Altern. Med. Rev. A J. Clin. Ther. 2008, 13, 315–329. [Google Scholar]
- Matusek, A.; Merész, P.; Le, T.K.D.; Örsi, F. Effect of temperature and pH on the degradation of fructo-oligosaccharides. Eur. Food Res. Technol. 2009, 228, 355–365. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Introducing inulin-type fructans. Br. J. Nutr. 2005, 93, S13–S25. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Chaito, C.; Judprasong, K.; Puwastien, P. Inulin content of fortified food products in Thailand. Food Chem 2016, 193, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Azizi, M.H.; Ghasemlou, M.; Vaziri, M. Application of inulin in cheese as prebiotic, fat replacer and texturizer: A review. Carbohydr. Polym. 2015, 119, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.; Ginic-Markovic, M.; Cooper, P.; Petrovsky, N. Inulin—A versatile polysaccharide with multiple pharmaceutical and food chemical uses. J. Excip. Food Chem. 2010, 1, 27–50. [Google Scholar]
- Pitarresi, G.; Triolo, D.; Giorgi, M.; Fiorica, C.; Calascibetta, F.; Giammona, G. Inulin-based hydrogel for oral delivery of flutamide: Preparation, characterization, and in vivo release studies. Macromol Biosci 2012, 12, 770–778. [Google Scholar] [CrossRef]
- Mandracchia, D.; Tripodo, G.; Latrofa, A.; Dorati, R. Amphiphilic inulin-D-alpha-tocopherol succinate (INVITE) bioconjugates for biomedical applications. Carbohydr. Polym. 2014, 103, 46–54. [Google Scholar] [CrossRef]
- Mandracchia, D.; Tripodo, G.; Trapani, A.; Ruggieri, S.; Annese, T.; Chlapanidas, T.; Trapani, G.; Ribatti, D. Inulin based micelles loaded with curcumin or celecoxib with effective anti-angiogenic activity. Eur. J. Pharm. Sci. 2016, 93, 141–146. [Google Scholar] [CrossRef]
- Licciardi, M.; Li Volsi, A.; Sardo, C.; Mauro, N.; Cavallaro, G.; Giammona, G. Inulin-Ethylenediamine Coated SPIONs Magnetoplexes: A Promising Tool for Improving siRNA Delivery. Pharm. Res. 2015, 32, 3674–3687. [Google Scholar] [CrossRef]
- Jain, A.K.; Sood, V.; Bora, M.; Vasita, R.; Katti, D.S. Electrosprayed inulin microparticles for microbiota triggered targeting of colon. Carbohydr. Polym. 2014, 112, 225–234. [Google Scholar] [CrossRef]
- Poulain, N.; Dez, I.; Perrio, C.; Lasne, M.C.; Prud’homme, M.P.; Nakache, E. Microspheres based on inulin for the controlled release of serine protease inhibitors: Preparation, characterization and in vitro release. J. Control. Release 2003, 92, 27–38. [Google Scholar] [CrossRef]
- Van den Mooter, G.; Vervoort, L.; Kinget, R. Characterization of methacrylated inulin hydrogels designed for colon targeting: In vitro release of BSA. Pharm. Res. 2003, 20, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, G.; Gao, M.; Liu, X.; Ji, B.; Hua, R.; Zhou, Y.; Yang, Y. RGD-peptide conjugated inulin-ibuprofen nanoparticles for targeted delivery of Epirubicin. Colloids Surf. B Biointerfaces 2016, 144, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.D.; Petrovsky, N. Delta inulin: A novel, immunologically active, stable packing structure comprising beta-D-[2 -> 1] poly(fructo-furanosyl) alpha-D-glucose polymers. Glycobiology 2011, 21, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Amer, H.; Nypelö, T.; Sulaeva, I.; Bacher, M.; Henniges, U.; Potthast, A.; Rosenau, T. Synthesis and Characterization of Periodate-Oxidized Polysaccharides: Dialdehyde Xylan (DAX). Biomacromolecules 2016, 17, 2972–2980. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, W.; Xiao, J.; Zhao, X.; Zhu, Y.; Wu, Y. Preparation and characterization of dialdehyde starch by one-step acid hydrolysis and oxidation. Int. J. Biol. Macromol. 2017, 103, 1257–1264. [Google Scholar] [CrossRef]
- Pandit, A.H.; Mazumdar, N.; Ahmad, S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2019, 137, 853–869. [Google Scholar] [CrossRef]
- Charhouf, I.; Bennamara, A.; Abdelmjid, A.; Chenite, A.; Zhu, J.; Berrada, M. Characterization of a Dialdehyde Chitosan Generated by Periodate Oxidation. Int. J. Sci. Basic Appl. Res. (IJSBAR) 2014, 16, 336–348. [Google Scholar]
- Maia, J.; Carvalho, R.A.; Coelho, J.F.J.; Simões, P.N.; Gil, M.H. Insight on the periodate oxidation of dextran and its structural vicissitudes. Polymer 2011, 52, 258–265. [Google Scholar] [CrossRef]
- Guo, J.; Ge, L.; Li, X.; Mu, C.; Li, D. Periodate oxidation of xanthan gum and its crosslinking effects on gelatin-based edible films. Food Hydrocoll. 2014, 39, 243–250. [Google Scholar] [CrossRef]
- Schacht, E.; Ruys, L.; Vermeersch, J.; Remon, J.P.; Duncan, R. Use of polysaccharides as drug carriers. Dextran and inulin derivatives of procainamide. Ann. N. Y. Acad. Sci. 1985, 446, 199–212. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Fouladian, P.; Parikh, A.; Barclay, T.G.; Song, Y.; Garg, S. Preparation and Characterization of Oxidized Inulin Hydrogel for Controlled Drug Delivery. Pharmaceutics 2019, 11, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schacht, E.; Buys, L.; Vermeersch, J.; Remon, J.P. Polymer-drug combinations: Synthesis and characterization of modified polysaccharides containing procainamide moieties. J. Control. Release 1984, 1, 33–46. [Google Scholar] [CrossRef]
- Stevens, C.V.; Meriggi, A.; Booten, K. Chemical modification of inulin, a valuable renewable resource, and its industrial applications. Biomacromolecules 2001, 2, 1–16. [Google Scholar] [CrossRef]
- Ishak, M.F.; Painter, T. Kinetic evidence for hemiacetal formation during the oxidation of dextran in aqueous periodate. Carbohydr. Res. 1978, 64, 189–197. [Google Scholar] [CrossRef]
- Tabandeh, M.R.; Aminlari, M. Synthesis, physicochemical and immunological properties of oxidized inulin-L-asparaginase bioconjugate. J. Biotechnol. 2009, 141, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.; Ginic-Markovic, M.; Johnston, M.R.; Cooper, P.D.; Petrovsky, N. Analysis of the hydrolysis of inulin using real time (1)H NMR spectroscopy. Carbohydr. Res. 2012, 352, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Bouhadir, K.H.; Hausman, D.S.; Mooney, D.J. Synthesis of cross-linked poly(aldehyde guluronate) hydrogels. Polymer 1999, 40, 3575–3584. [Google Scholar] [CrossRef]
- Jia, X.; Burdick, J.A.; Kobler, J.; Clifton, R.J.; Rosowski, J.J.; Zeitels, S.M.; Langer, R. Synthesis and Characterization of in Situ Cross-Linkable Hyaluronic Acid-Based Hydrogels with Potential Application for Vocal Fold Regeneration. Macromolecules 2004, 37, 3239–3248. [Google Scholar] [CrossRef]
- Schacht, E.; Vermeersch, J.; Vandoorne, F.; Vercauteren, R.; Remon, J.P. Synthesis and characterization of some modified polysaccharides containing drug moieties. J. Control. Release 1985, 2, 245–256. [Google Scholar] [CrossRef]
- Fares, M.M.; Salem, M.S.; Khanfar, M. Inulin and poly(acrylic acid) grafted inulin for dissolution enhancement and preliminary controlled release of poorly water-soluble Irbesartan drug. Int. J. Pharm. 2011, 410, 206–211. [Google Scholar] [CrossRef]
- Barclay, T.G.; Rajapaksha, H.; Thilagam, A.; Qian, G.; Ginic-Markovic, M.; Cooper, P.D.; Gerson, A.; Petrovsky, N. Physical characterization and in silico modeling of inulin polymer conformation during vaccine adjuvant particle formation. Carbohydr. Polym. 2016, 143, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plappert, S.F.; Quraishi, S.; Pircher, N.; Mikkonen, K.S.; Veigel, S.; Klinger, K.M.; Potthast, A.; Rosenau, T.; Liebner, F.W. Transparent, Flexible, and Strong 2,3-Dialdehyde Cellulose Films with High Oxygen Barrier Properties. Biomacromolecules 2018, 19, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.-f.; Kan, J.; Wen, X.-y.; Jin, C.-h. Synthesis, characterization and in vitro anti-diabetic activity of catechin grafted inulin. Int. J. Biol. Macromol. 2014, 64, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Afinjuomo, F.; Barclay, T.G.; Song, Y.; Parikh, A.; Petrovsky, N.; Garg, S. Synthesis and characterization of a novel inulin hydrogel crosslinked with pyromellitic dianhydride. React. Funct. Polym. 2019, 134, 104–111. [Google Scholar] [CrossRef]
- Gong, H.; Liu, M.; Zhang, B.; Cui, D.; Gao, C.; Ni, B.; Chen, J. Synthesis of oxidized guar gum by dry method and its application in reactive dye printing. Int. J. Biol. Macromol. 2011, 49, 1083–1091. [Google Scholar] [CrossRef]
- Dan, A.; Ghosh, S.; Moulik, S.P. Physicochemical studies on the biopolymer inulin: A critical evaluation of its self-aggregation, aggregate-morphology, interaction with water, and thermal stability. Biopolymers 2009, 91, 687–699. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Barclay, T.G.; Parikh, A.; Song, Y.; Chung, R.; Wang, L.; Liu, L.; Hayball, J.D.; Petrovsky, N.; Garg, S. Design and Characterization of Inulin Conjugate for Improved Intracellular and Targeted Delivery of Pyrazinoic Acid to Monocytes. Pharmaceutics 2019, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-R.; Wang, X.-L.; Zhao, G.-M.; Wang, Y.-Z. Preparation and properties of oxidized starch with high degree of oxidation. Carbohydr. Polym. 2012, 87, 2554–2562. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A.J. Thermal stability of cellulose and their nanoparticles: Effect of incremental increases in carboxyl and aldehyde groups. Carbohydr. Polym. 2014, 114, 339–343. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Wang, Y.; Gao, W. Study on physico-chemical properties of dialdehyde yam starch with different aldehyde group contents. Thermochim. Acta 2011, 512, 196–201. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Luo, K.H.; Bridgwater, A.V.; Fang, M.X. Kinetic study on thermal decomposition of woods in oxidative environment. Fuel 2009, 88, 1024–1030. [Google Scholar] [CrossRef] [Green Version]
- Cooper, P.D.; Barclay, T.G.; Ginic-Markovic, M.; Petrovsky, N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology 2013, 23, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Ronkart, S.; Paquot, M.; Blecker, C.; Fougnies, C.; Doran, L.; Lambrechts, J.-C.; Norberg, B.; Deroanne, C. Impact of the Crystallinity on the Physical Properties of Inulin during Water Sorption. Food Biophys. 2009, 4, 49–58. [Google Scholar] [CrossRef]
- Blecker, C.; Chevalier, J.-P.; Fougnies, C.; Van Herck, J.-C.; Deroanne, C.; Paquot, M. Characterisation of different inulin samples by DSC: Influence of polymerisation degree on melting temperature. J. Therm. Anal. Calorim. 2003, 71, 215–224. [Google Scholar] [CrossRef]
- Ronkart, S.; Deroanne, C.; Paquot, M.; Fougnies, C.; Lambrechts, J.-C.; Blecker, C. Characterization of the Physical State of Spray-Dried Inulin. Food Biophys. 2007, 2, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Ronkart, S.N.; Paquot, M.; Fougnies, C.; Deroanne, C.; Blecker, C.S. Effect of water uptake on amorphous inulin properties. Food Hydrocoll. 2009, 23, 922–927. [Google Scholar] [CrossRef]
- Li, H.; Wu, B.; Mu, C.; Lin, W. Concomitant degradation in periodate oxidation of carboxymethyl cellulose. Carbohydr. Polym. 2011, 84, 881–886. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Wegrzynowska-Drzymalska, K.; Chelminiak-Dudkiewicz, D.; Kowalonek, J.; Kaczmarek, H. Photochemical Reactions in Dialdehyde Starch. Molecules (Baselswitzerland) 2018, 23, 3358. [Google Scholar] [CrossRef] [Green Version]
- Kim, U.-J.; Kuga, S.; Wada, M.; Okano, T.; Kondo, T. Periodate Oxidation of Crystalline Cellulose. Biomacromolecules 2000, 1, 488–492. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Cao, L.; Ji, J.; Gao, J. Three Inulin-Type Fructans from Codonopsis pilosula (Franch.) Nannf. Roots and Their Prebiotic Activity on Bifidobacterium longum. Molecules 2018, 23, 3123. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Zhang, S.; Ju, B. Preparation of water-soluble oxidized starch with high carbonyl content by sodium hypochlorite. Starch-Stärke 2014, 66, 115–123. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Lin, X.; Xiao, H.; Miao, Q.; Huang, L.; Chen, L.; Wu, H. TEMPO-Oxidized Cellulose with High Degree of Oxidation. Polymers 2017, 9, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Sample | Theoretical DO | DO from TNBS Assay |
|---|---|---|
| Oxi5 | 5 | 2.8 + 0.36 |
| Oxi10 | 10 | 5.1 + 1.06 |
| Oxi20 | 20 | 11.2 + 1.45 |
| Oxi30 | 30 | 19.4 + 1.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afinjuomo, F.; Fouladian, P.; Barclay, T.G.; Song, Y.; Petrovsky, N.; Garg, S. Influence of Oxidation Degree on the Physicochemical Properties of Oxidized Inulin. Polymers 2020, 12, 1025. https://doi.org/10.3390/polym12051025
Afinjuomo F, Fouladian P, Barclay TG, Song Y, Petrovsky N, Garg S. Influence of Oxidation Degree on the Physicochemical Properties of Oxidized Inulin. Polymers. 2020; 12(5):1025. https://doi.org/10.3390/polym12051025
Chicago/Turabian StyleAfinjuomo, Franklin, Paris Fouladian, Thomas G. Barclay, Yunmei Song, Nikolai Petrovsky, and Sanjay Garg. 2020. "Influence of Oxidation Degree on the Physicochemical Properties of Oxidized Inulin" Polymers 12, no. 5: 1025. https://doi.org/10.3390/polym12051025






