Morphological Transitions of Photoresponsive Vesicles from Amphiphilic Polypeptoid Copolymers for Controlled Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
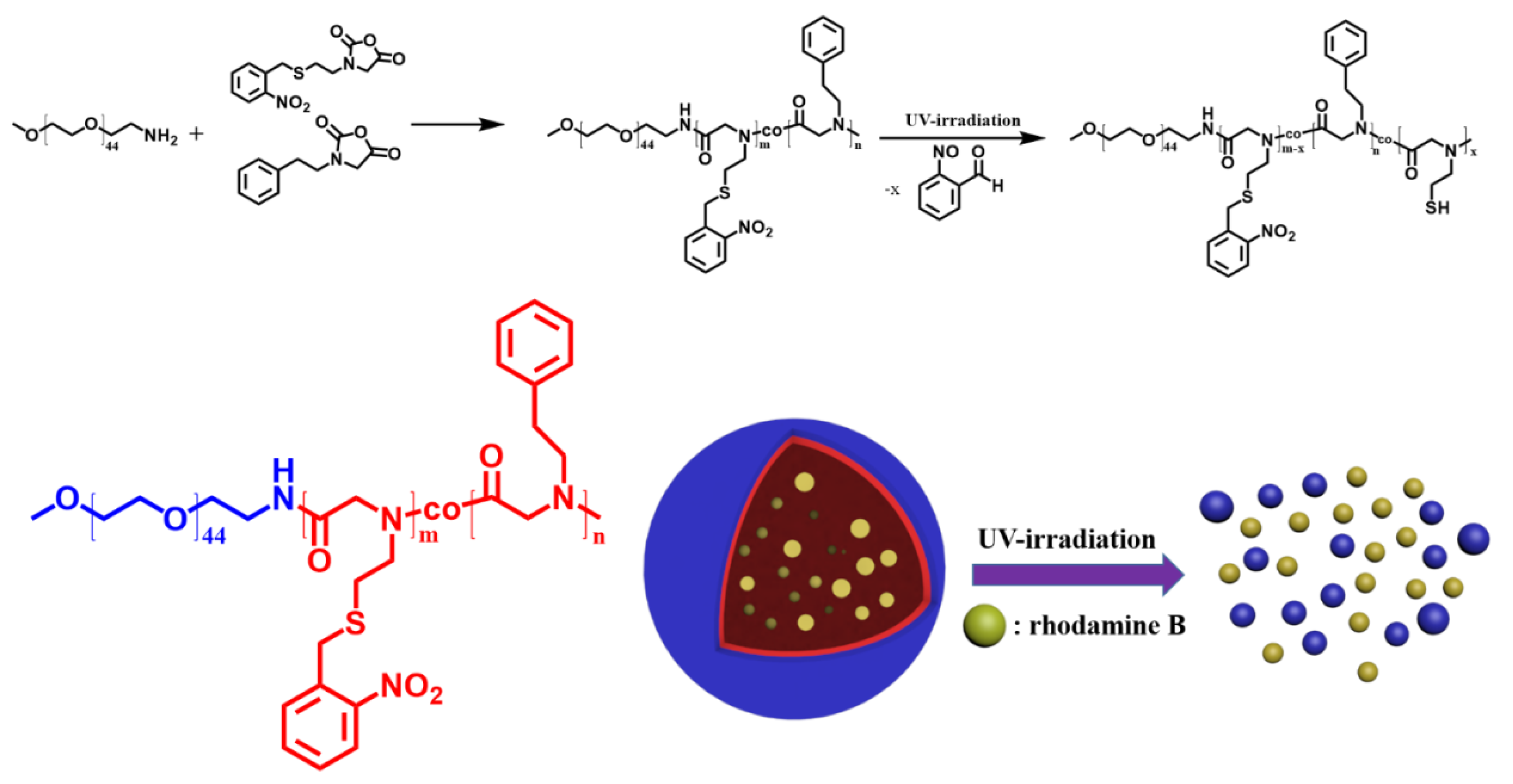
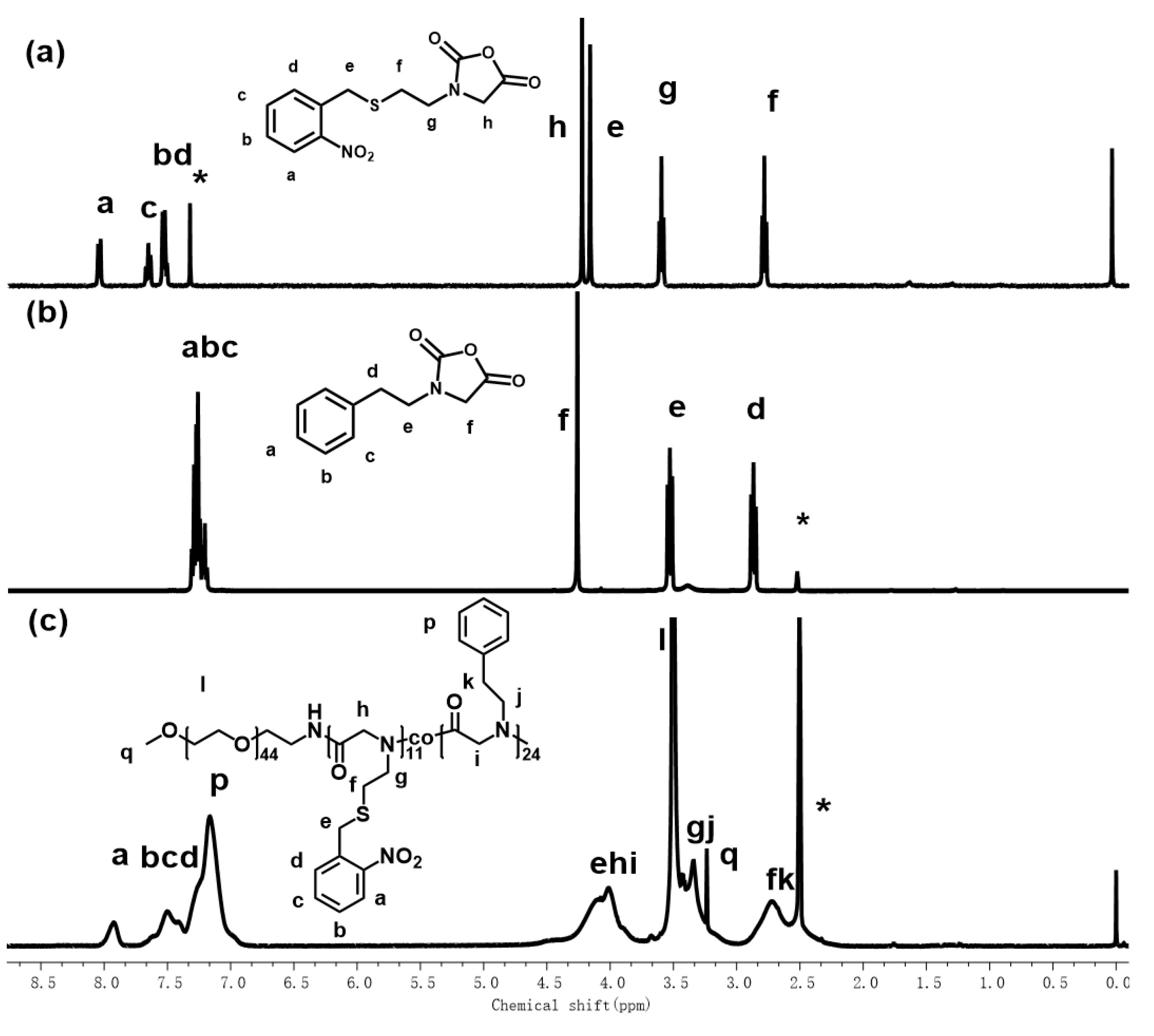
2.2. Synthesis of Poly(ethylene glycol)-b-poly(N-(S-(o-nitrobenzyl)-thioethyl) glycine)-co-poly(N-(2-phenylethyl) glycine) (PEG-b-PNSN-co-PNPE) Copolymer
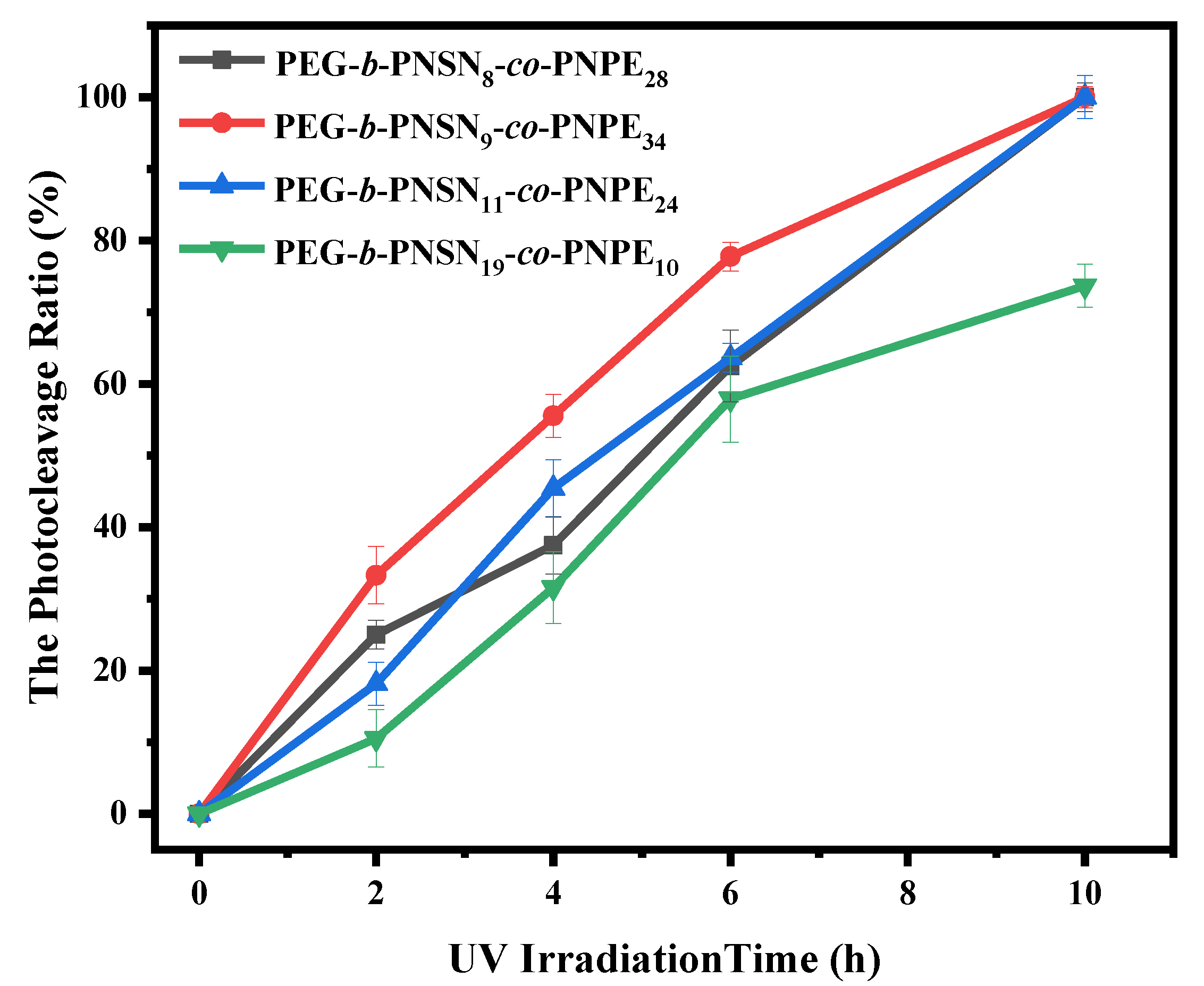
2.3. Cleavage of Copolymers with UV Irradiation
2.4. Preparation of Assemblies in Aqueous Solution
2.5. Preparation of Rhodamine B Loaded Vesicles in Aqueous Solution
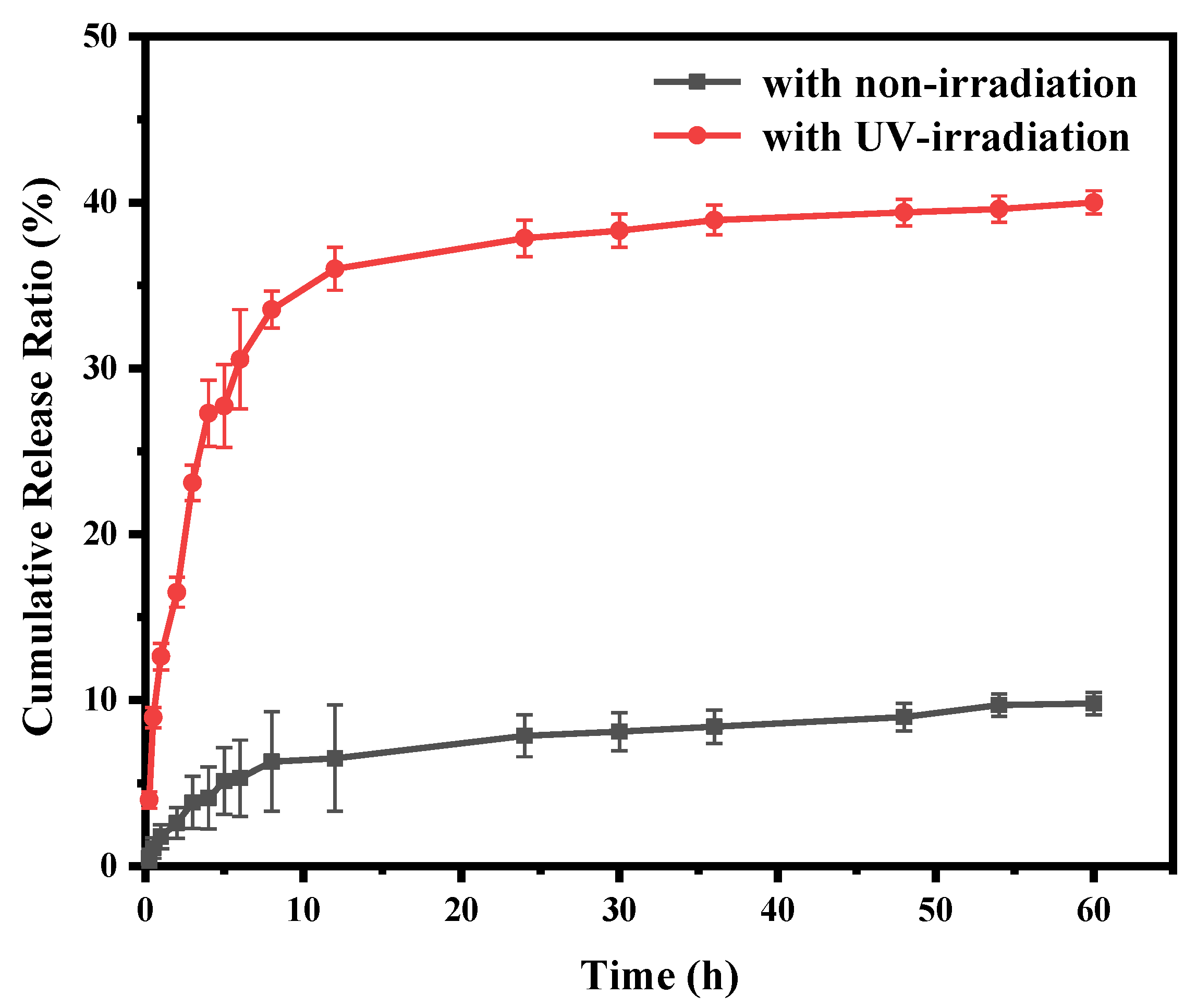
2.6. In Vitro Drug Release Profiles
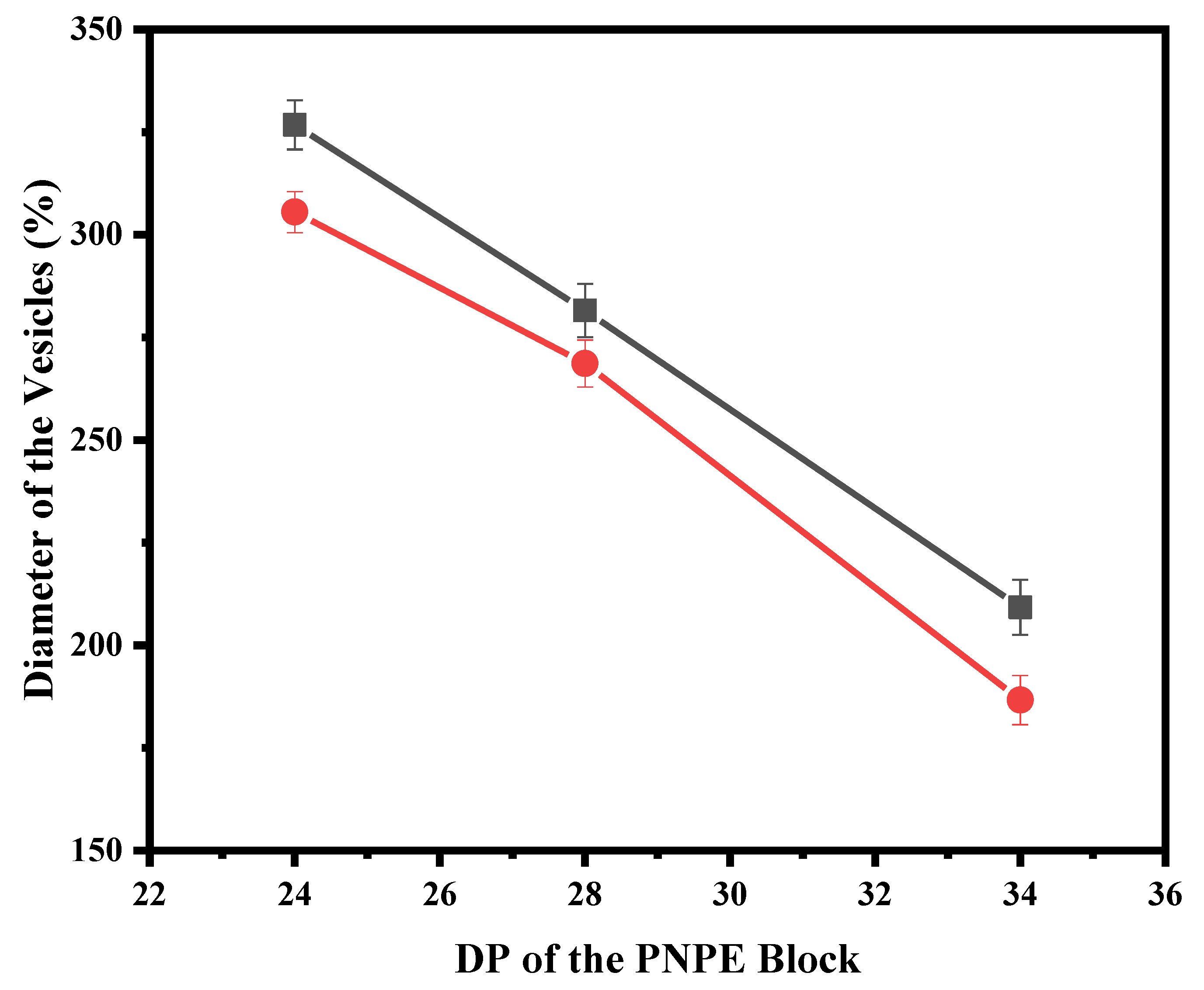
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.M.; Qian, Y.F.; Liu, T.; Zhang, G.Y.; Liu, S.Y. Light-Triggered Concomitant Enhancement of Magnetic Resonance Imaging Contrast Performance and Drug Release Rate of Functionalized Amphiphilic Diblock Copolymer Micelles. Biomacromolecules 2012, 13, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, L.; Guan, Y.; Su, Y.; Dong, C.M. Multi-responsive polypeptidosome: Characterization, morphology transformation, and triggered drug delivery. Macromol. Rapid Commun. 2014, 35, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Dong, C.M. Photoresponsive poly(S-(o-nitrobenzyl)-L-cysteine)-b-PEO from a L-cysteine N-carboxyanhydride monomer: Synthesis, self-assembly, and phototriggered drug release. Biomacromolecules 2012, 13, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.S.; Yang, L.X.; He, H.Y.; Hu, X.L.; Xie, Z.G.; Huang, Y.B.; Jing, X.B. Photo-cross-linked mPEG-poly(gamma-cinnamyl-L-glutamate) micelles as stable drug carriers. Polym. Chem. 2012, 3, 1300–1307. [Google Scholar] [CrossRef]
- Jain, A.K.; Gund, M.G.; Desai, D.C.; Borhade, N.; Senthilkumar, S.P.; Dhiman, M.; Mangu, N.K.; Mali, S.V.; Dubash, N.P.; Halder, S.; et al. Mutual prodrugs containing bio-cleavable and drug releasable disulfide linkers. Bioorg. Chem. 2013, 49, 40–48. [Google Scholar] [CrossRef]
- Liu, G.; Liu, N.; Zhou, L.; Su, Y.; Dong, C.-M. NIR-responsive polypeptide copolymer upconversion composite nanoparticles for triggered drug release and enhanced cytotoxicity. Polym. Chem. 2015, 6, 4030–4039. [Google Scholar] [CrossRef]
- Riber, C.F.; Smith, A.A.; Zelikin, A.N. Self-Immolative Linkers Literally Bridge Disulfide Chemistry and the Realm of Thiol-Free Drugs. Adv. Healthc. Mater. 2015, 4, 1887–1890. [Google Scholar] [CrossRef]
- Xuan, J.; Boissiere, O.; Zhao, Y.; Yan, B.; Tremblay, L.; Lacelle, S.; Xia, H.S.; Zhao, Y. Ultrasound-Responsive Block Copolymer Micelles Based on a New Amplification Mechanism. Langmuir 2012, 28, 16463–16468. [Google Scholar] [CrossRef]
- Il’ichev, Y.V.; Schworer, M.A.; Wirz, J. Photochemical reaction mechanisms of 2-nitrobenzyl compounds: Methyl ethers and caged ATP. J. Am. Chem. Soc. 2004, 126, 4581–4595. [Google Scholar] [CrossRef]
- Gaplovsky, M.; Il’ichev, Y.V.; Kamdzhilov, Y.; Kombarova, S.V.; Mac, M.; Schworer, M.A.; Wirz, J. Photochemical reaction mechanisms of 2-nitrobenzyl compounds: 2-nitrobenzyl alcohols form 2-nitroso hydrates by dual proton transfer. Photochem. Photobiol. Sci. 2005, 4, 33–42. [Google Scholar] [CrossRef]
- Huo, H.H.; Ma, X.Y.; Dong, Y.Q.; Qu, F.J. Light/temperature dual-responsive ABC miktoarm star terpolymer micelles for controlled release. Eur. Polym. J. 2017, 87, 331–343. [Google Scholar] [CrossRef]
- Zhu, K.N.; Liu, G.H.; Zhang, G.Y.; Hu, J.M.; Liu, S.Y. Engineering Cross-Linkable Plasmonic Vesicles for Synergistic Chemo-Photothermal Therapy Using Orthogonal Light Irradiation. Macromolecules 2018, 51, 8530–8538. [Google Scholar] [CrossRef]
- Li, P.; Dong, C.M. Phototriggered Ring-Opening Polymerization of a Photocaged l-Lysine N-Carboxyanhydride to Synthesize Hyperbranched and Linear Polypeptides. ACS Macro Lett. 2017, 6, 292–297. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, L.; Su, Y.; Dong, C.M. Plasmonic, Targeted, and Dual Drugs-Loaded Polypeptide Composite Nanoparticles for Synergistic Cocktail Chemotherapy with Photothermal Therapy. Biomacromolecules 2016, 17, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, J.; Dong, C.M. Photosensitive poly(o-nitrobenzyloxycarbonyl-l-lysine)-b-PEO polypeptide copolymers: Synthesis, multiple self-assembly behaviors, and the photo/pH-thermo-sensitive hydrogels. Polym. Chem. 2017, 8, 7033–7043. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, C.M. Quadruple thermo-photo-redox-responsive random copolypeptide nanogel and hydrogel. Chin. Chem. Lett. 2018, 29, 927–930. [Google Scholar] [CrossRef]
- Murphy, R.D.; Kimmins, S.; Hibbitts, A.J.; Heise, A. 3D-extrusion printing of stable constructs composed of photoresponsive polypeptide hydrogels. Polym. Chem. 2019, 10, 4675–4682. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Singh, S.P.; Bowman, C.N.; Anseth, K.S. Photodegradable, Photoadaptable Hydrogels via Radical-Mediated Disulfide Fragmentation Reaction. Macromolecules 2011, 44, 2444–2450. [Google Scholar] [CrossRef]
- Soorkia, S.; Dehon, C.; Kumar, S.S.; Pedrazzani, M.; Frantzen, E.; Lucas, B.; Barat, M.; Fayeton, J.A.; Jouvet, C. UV Photofragmentation Dynamics of Protonated Cystine: Disulfide Bond Rupture. J. Phys. Chem. Lett. 2014, 5, 1110–1116. [Google Scholar] [CrossRef]
- Zuckermann, R.N. Peptoid origins. Biopolymers 2011, 96, 545–555. [Google Scholar] [CrossRef]
- Sun, J.; Zuckermann, R.N. Peptoid polymers: A highly designable bioinspired material. ACS Nano 2013, 7, 4715–4732. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.A.; Xuan, S.T.; Li, A.; Simpson, J.M.; Sternhagen, G.L.; Yu, T.Y.; Darvish, O.A.; Jiang, N.S.; Zhang, D.H. Polypeptoid polymers: Synthesis, characterization, and properties. Biopolymers 2018, 109, 25. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Z. Peptoid applications in biomedicine and nanotechnology. Pept. Appl. Biomed. Biotechnol. Bioeng. 2018, 183–213. [Google Scholar] [CrossRef]
- Fu, X.; Tian, J.; Li, Z.; Sun, J.; Li, Z. Dual-responsive pegylated polypeptoids with tunable cloud point temperatures. Biopolymers 2018, 110, e23243. [Google Scholar] [CrossRef]
- Tian, J.; Sun, J.; Li, Z. Biomimetic pegylated polypeptoids with thermoresponsive properties. Polymer 2018, 138, 132–138. [Google Scholar] [CrossRef]
- Xing, C.; Shi, Z.; Tian, J.; Sun, J.; Li, Z. Charge-Determined LCST/UCST Behavior in Ionic Polypeptoids. Biomacromolecules 2018, 19, 2109–2116. [Google Scholar] [CrossRef]
- Ji, S.; Xu, L.; Fu, X.; Sun, J.; Li, Z. Light- and Metal Ion-Induced Self-Assembly and Reassembly Based on Block Copolymers Containing a Photoresponsive Polypeptide Segment. Macromolecules 2019, 52, 4686–4693. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Yang, X.; Ji, S.; Wei, Y.; Li, Z. Self-crosslinking assemblies with tunable nanostructures from photoresponsive polypeptoid-based block copolymers. Polym. Chem. 2020, 11, 337–343. [Google Scholar] [CrossRef]
- Wei, Y.; Tian, J.; Zhang, Z.; Zhu, C.; Sun, J.; Li, Z. Supramolecular Nanosheets Assembled from Poly(ethylene glycol)-b-poly(N-(2-phenylethyl)glycine) Diblock Copolymer Containing Crystallizable Hydrophobic Polypeptoid: Crystallization Driven Assembly Transition from Filaments to Nanosheets. Macromolecules 2019, 52, 1546–1556. [Google Scholar] [CrossRef] [Green Version]


| Samples | Feed Ratio a | Mn b (kDa) | Mn c (kDa) | Dispersity (Đ) c |
|---|---|---|---|---|
| PEG-b-PNSN8-co-PNPE28 | 1/10/30 | 8524 | 9833 | 1.25 |
| PEG-b-PNSN9-co-PNPE32 | 1/10/40 | 9420 | 12,500 | 1.17 |
| PEG-b-PNSN11-co-PNPE24 | 1/10/20 | 8636 | 11,410 | 1.14 |
| PEG-b-PNSN19-co-PNPE10 | 1/20/10 | 8398 | 9393 | 1.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, Z.; Sun, J. Morphological Transitions of Photoresponsive Vesicles from Amphiphilic Polypeptoid Copolymers for Controlled Release. Polymers 2020, 12, 798. https://doi.org/10.3390/polym12040798
Yang X, Wang Z, Sun J. Morphological Transitions of Photoresponsive Vesicles from Amphiphilic Polypeptoid Copolymers for Controlled Release. Polymers. 2020; 12(4):798. https://doi.org/10.3390/polym12040798
Chicago/Turabian StyleYang, Xu, Zhiwei Wang, and Jing Sun. 2020. "Morphological Transitions of Photoresponsive Vesicles from Amphiphilic Polypeptoid Copolymers for Controlled Release" Polymers 12, no. 4: 798. https://doi.org/10.3390/polym12040798






