Hybrid Bilayer PLA/Chitosan Nanofibrous Scaffolds Doped with ZnO, Fe3O4, and Au Nanoparticles with Bioactive Properties for Skin Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
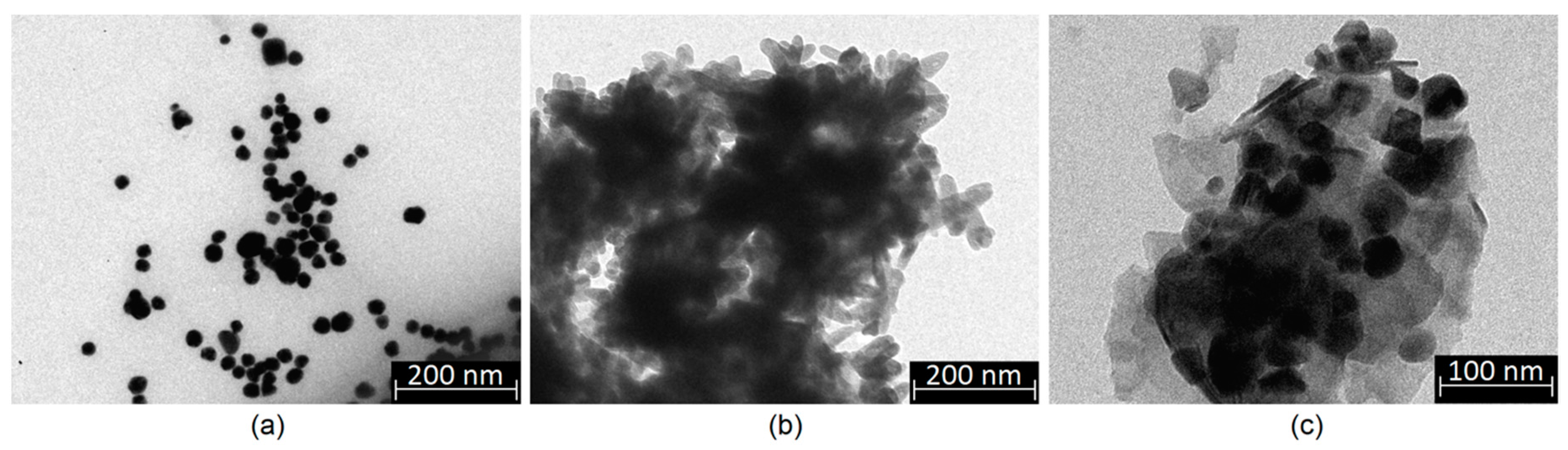
2.2.1. Nanoparticles Preparation
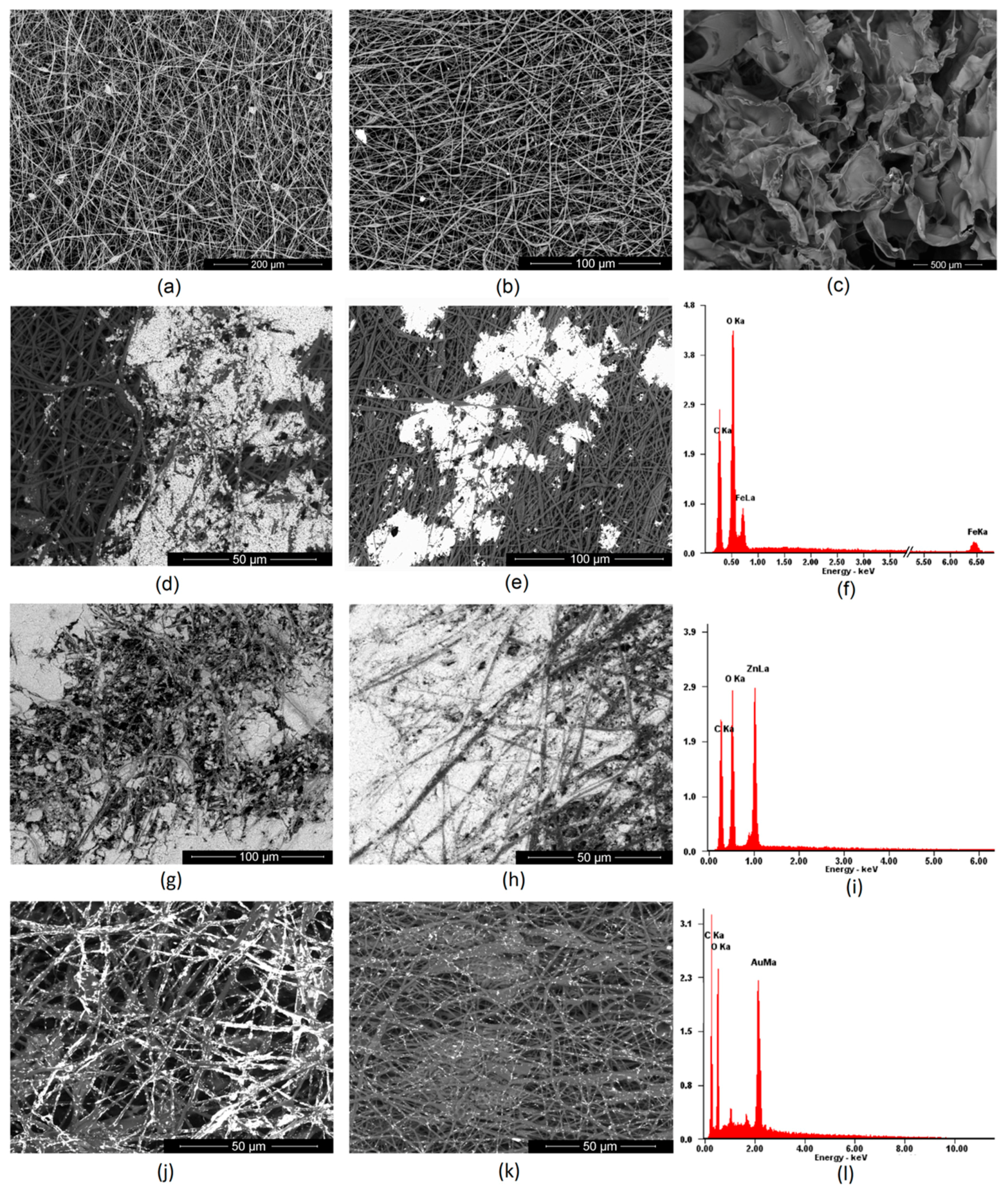
2.2.2. Nanofibers Preparation
2.2.3. Chitosan Acylation
2.2.4. Hybrid Scaffolds Preparation
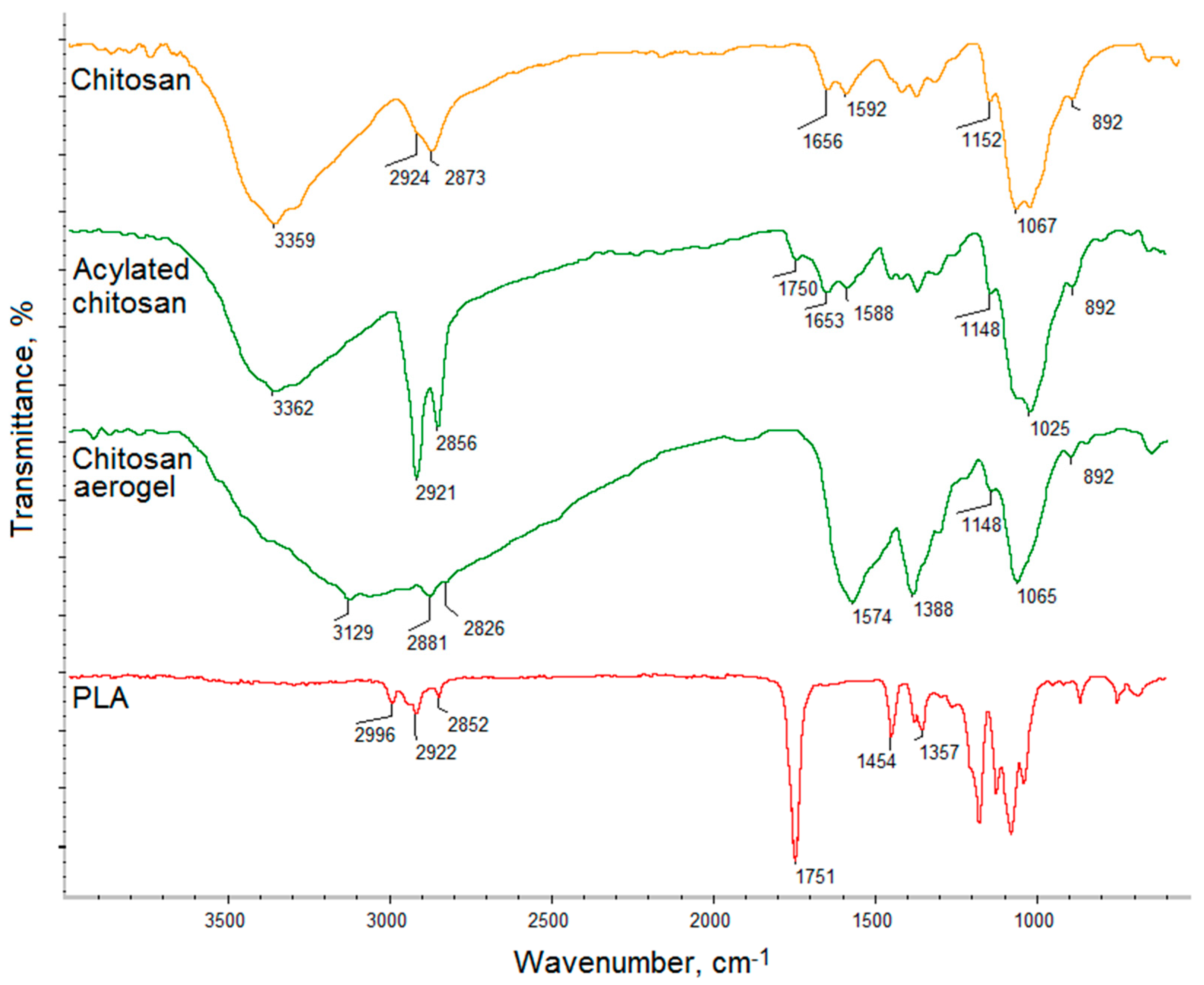
2.2.5. FT-IR Analysis
2.2.6. Morphology Study
2.2.7. Conductivity Study
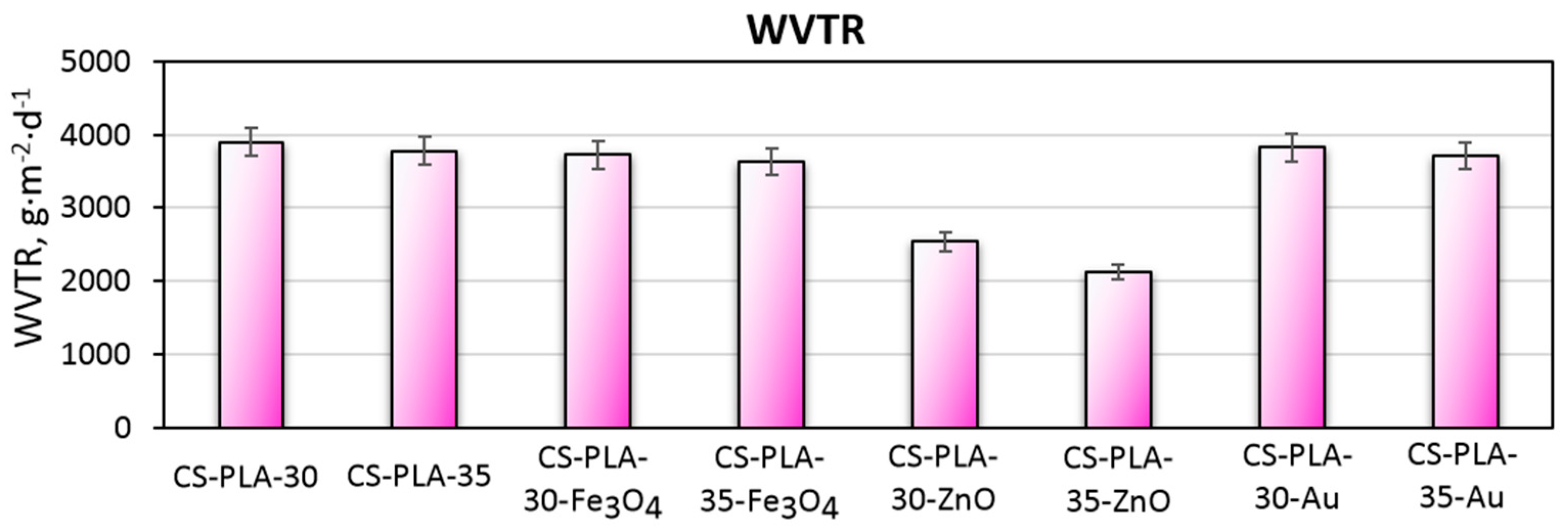
2.2.8. Water Vapor Permeability
2.2.9. Mechanical Study
2.2.10. Biodegradation
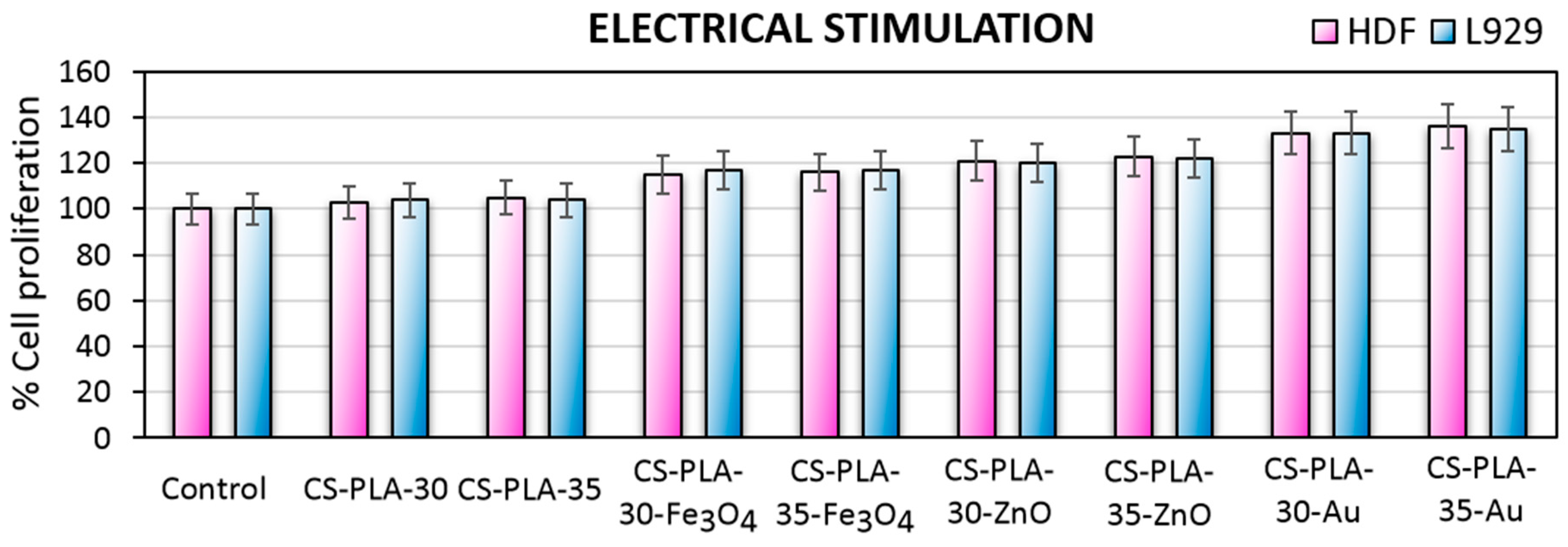
2.2.11. Cytotoxicity and Electrical Stimulation of Cell Proliferation Study
2.2.12. Phenotype Study
3. Results and Discussion
3.1. FT-IR Analysis
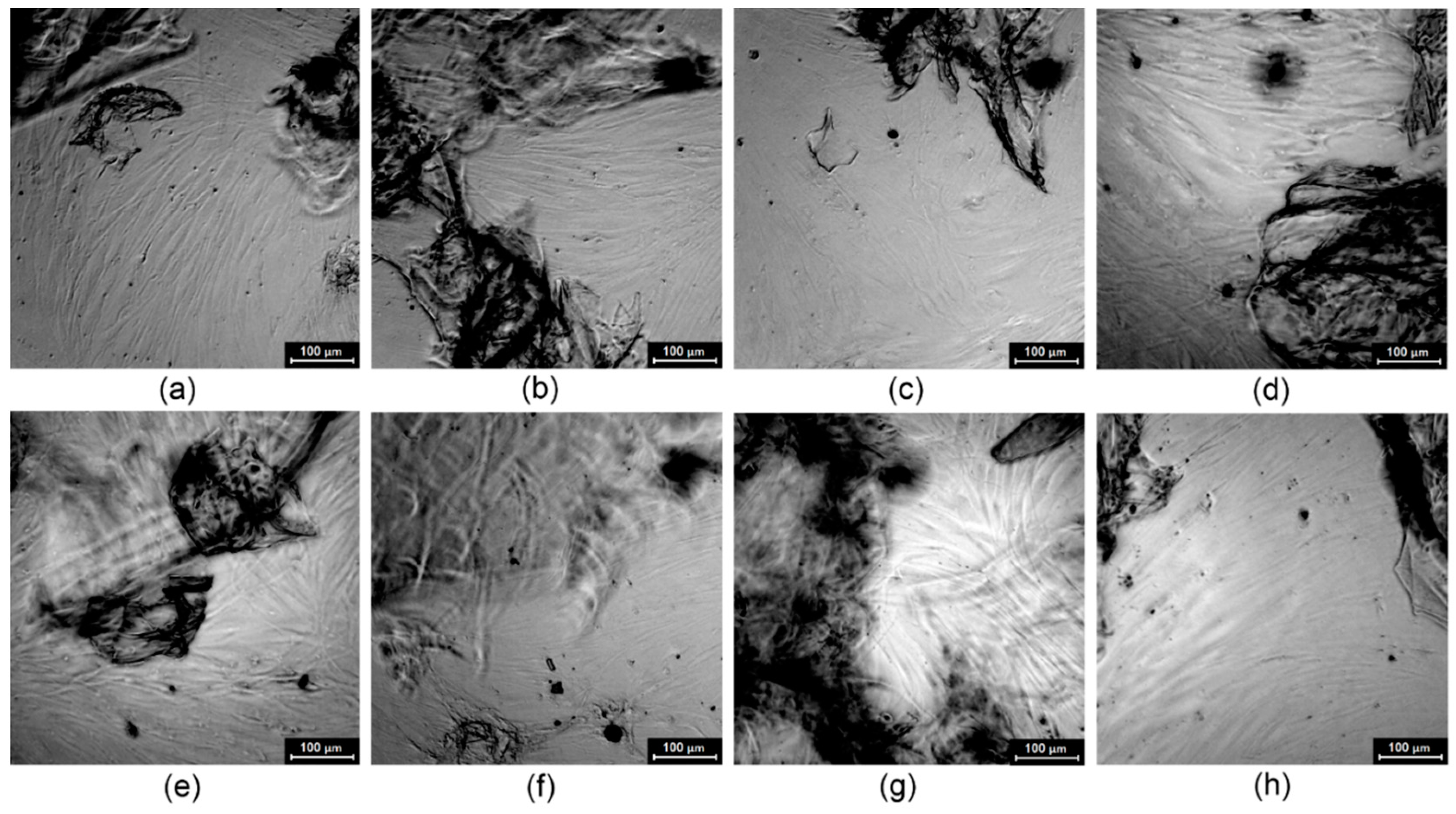
3.2. Morphology Analysis
3.3. Electrical Properties of the Conductive Layer
3.4. Water Vapor Permeability of the Scaffolds
3.5. Mechanical Properties Study
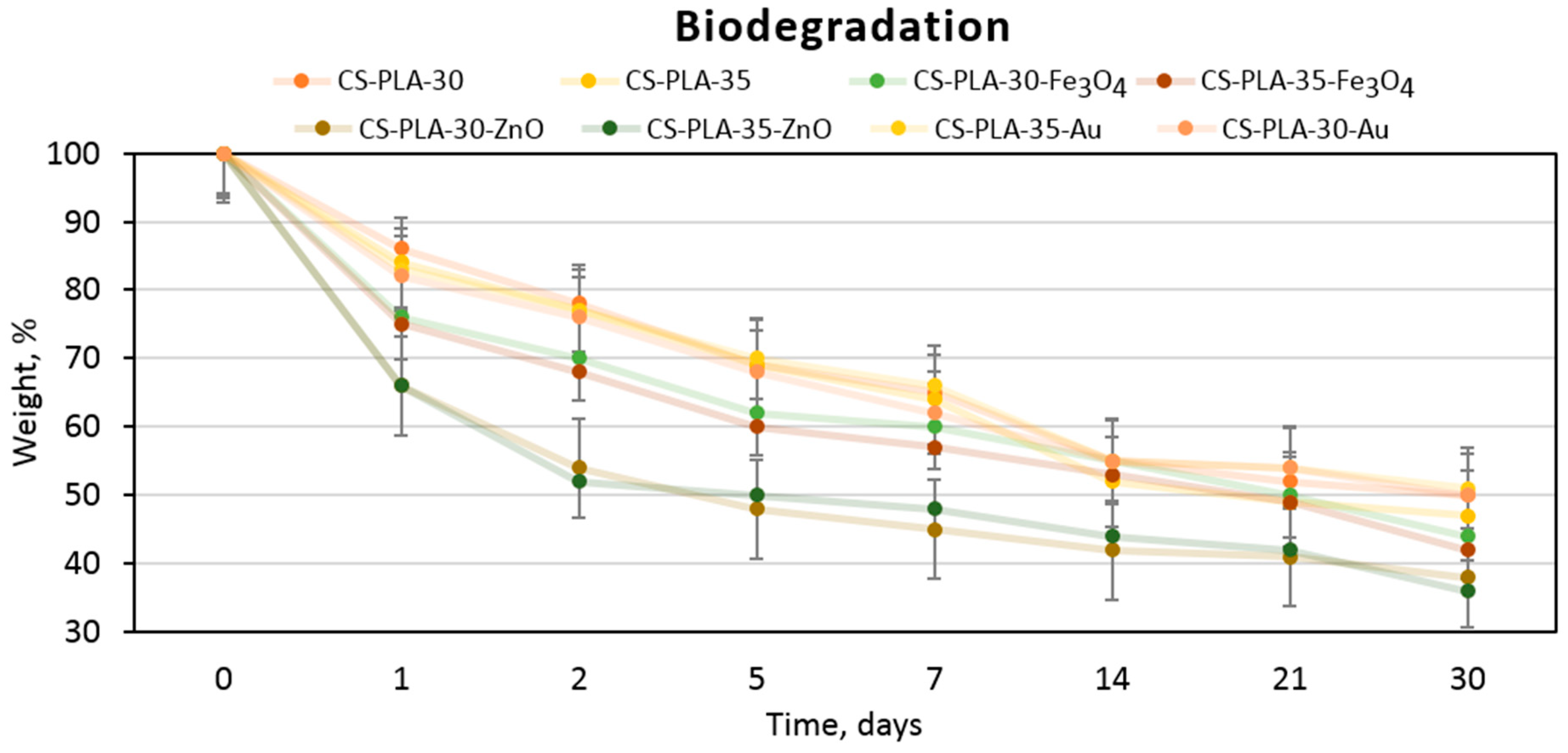
3.6. Biodegradation Study
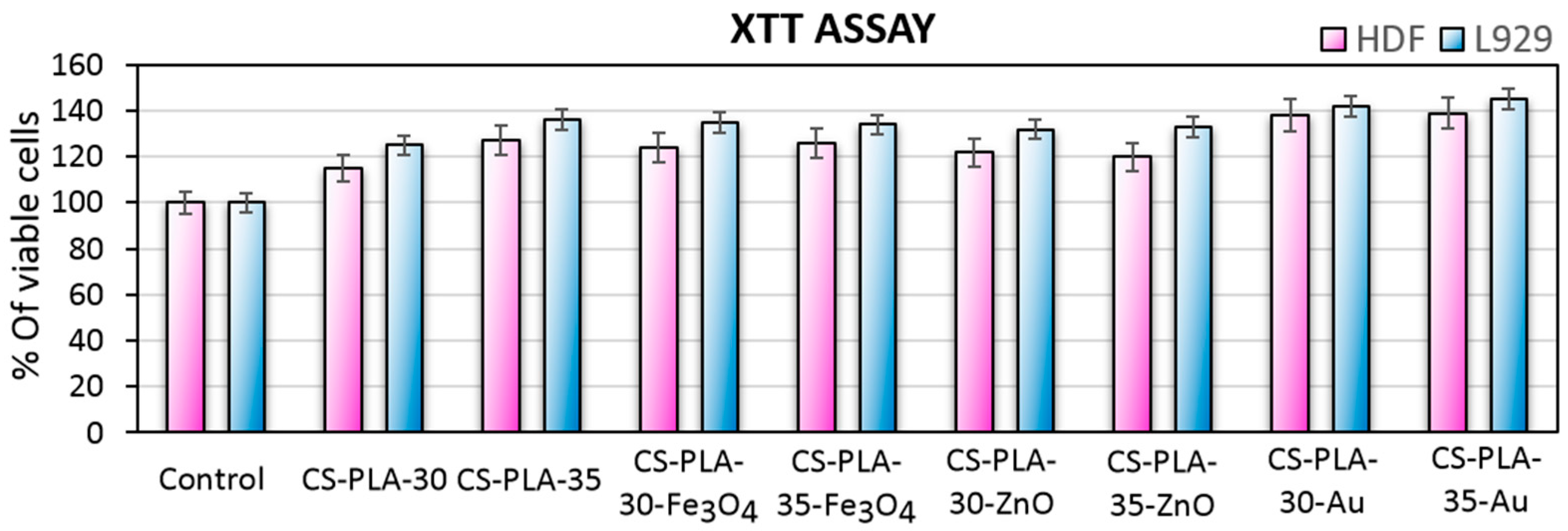
3.7. Cytotoxicity Study
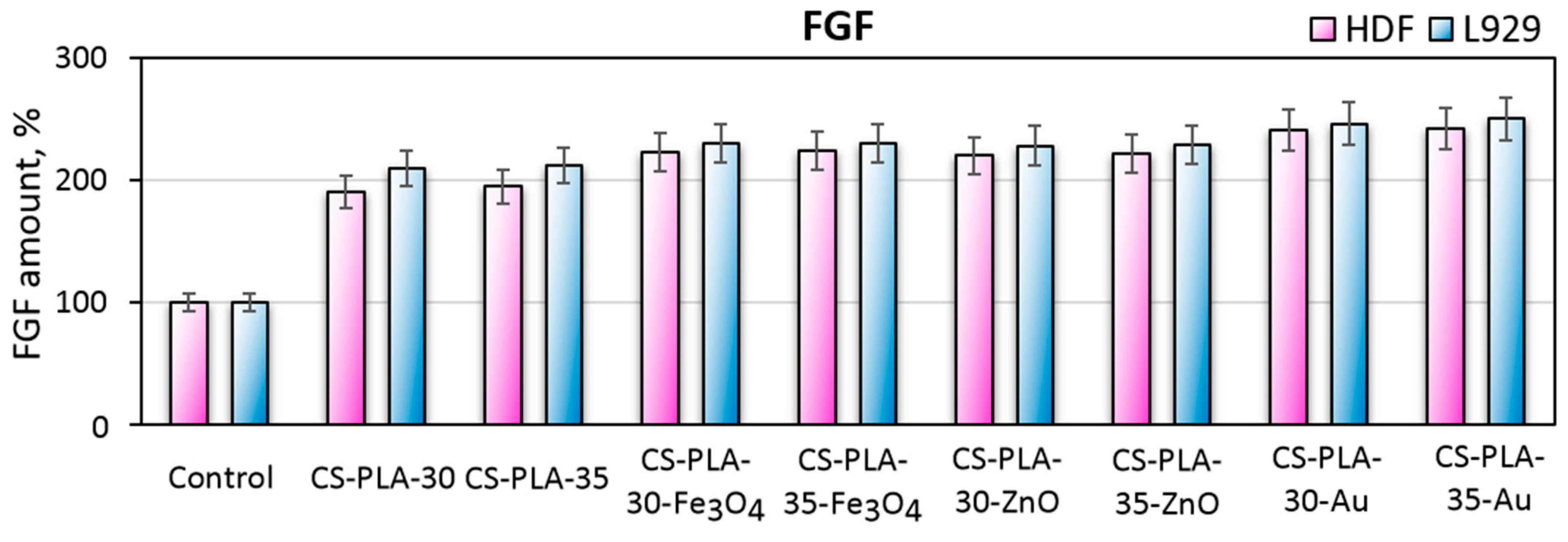
3.8. Fibroblast Specific Protein Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Burn, K.C. Wound healing and treatment: Review and advancements. Crit Care 2015, 19, 243. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The Role of the Extracellular Matrix Components in Cutaneous Wound Healing. BioMed Res. Int. 2014, 2014, 747584. [Google Scholar] [CrossRef] [Green Version]
- Palo, M.; Rönkönharju, S.; Tiirik, K.; Viidik, L.; Sandler, N.; Kogermann, K. Bi-Layered Polymer Carriers with Surface Modification by Electrospinning for Potential Wound Care Applications. Pharmaceutics 2019, 11, 678. [Google Scholar] [CrossRef] [Green Version]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Cassan, D.; Becker, A.; Glasmacher, B.; Roger, Y.; Hoffmann, A.; Gengenbach, T.R.; Easton, C.D.; Hänsch, R.; Menzel, H. Blending chitosan-g-poly(caprolactone) with poly(caprolactone) by electrospinning to produce functional fiber mats for tissue engineering applications. J. Appl. Polym. Sci. 2019, 94, 48650. [Google Scholar] [CrossRef]
- Yusof, M.R.; Shamsudin, R.; Zakaria, S.; Hamid, M.A.A.; Yalcinkaya, F.; Abdullah, Y.; Yacob, N. Fabrication and Characterization of Carboxymethyl Starch/Poly(l-Lactide) Acid/β-Tricalcium Phosphate Composite Nanofibers via Electrospinning. Polymers 2019, 11, 1468. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.V.; Asadian, M.; Onyshchenko, I.; Declercq, H.; Morent, R.; De Geyter, N. Biocompatibility of Cyclopropylamine-Based Plasma Polymers Deposited at Sub-Atmospheric Pressure on Poly (ε-caprolactone) Nanofiber Meshes. Nanomaterials 2019, 9, 1215. [Google Scholar] [CrossRef] [Green Version]
- Cavanaugh, M.; Silantyeva, E.; Koh, G.P.; Malekzadeh, E.; Lanzinger, W.D.; Willits, R.K.; Becker, M.L. RGD-Modified Nanofibers Enhance Outcomes in Rats after Sciatic Nerve Injury. J. Funct. Biomater. 2019, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.S.; Bhaduri, S. Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics 2019, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Niiyama, E.; Uto, K.; Lee, C.M.; Sakura, K.; Ebara, M. Alternating Magnetic Field-Triggered Switchable Nanofiber Mesh for Cancer Thermo-Chemotherapy. Polymers 2018, 10, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, S.; Iijima, K.; Sasaki, K.; Hashizume, M.; Kawabe, M.; Otsuka, H. Cartilage Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells in Three-Dimensional Silica Nonwoven Fabrics. Appl. Sci. 2018, 8, 1398. [Google Scholar] [CrossRef] [Green Version]
- Szentivanyi, A.L.; Zernetsch, H.; Menzel, H.; Glasmacher, B. A review of developments in electrospinning technology: New opportunities for the design of artificial tissue structures. Int. J. Artif. Organs 2011, 34, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Shahidi, F.; Abuzaytoun, R. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005, 49, 93–135. [Google Scholar]
- Kumar, P.T.; Lakshmanan, V.K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 45, 2618–2629. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcanerand, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cheng, F.; Gao, J.; Wang, L. Antibacterial wound dressing from chitosan/polyethylene oxide nanofibers mats embedded with silver nanoparticles. J. Biomater. Appl. 2015, 29, 1086–1095. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Chompoosor, A.; Saha, K.; Ghosh, P.S.; Macarthy, D.J.; Miranda, O.R.; Zhu, Z.J.; Arcaro, K.F.; Rotello, V.M. The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage by cationic gold nanoparticles. Small 2010, 6, 2246–2249. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Chen, S.; Zhong, L.; Wang, B.; Wang, H.; Hong, F. Zn2+-loaded TOBC nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing. Int. J. Biol. Macromol. 2019, 143, 235–242. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Kim, I.S.; Ogasawarad, H.; Ni, Q.Q. Characterizations and application of CA/ZnO/AgNP composite nanofibers for sustained antibacterial properties. Mater. Sci. Eng. C 2019, 105, 110077. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, T.; Sheng, B.; Zhou, T.; Zhang, Q.; Wu, F.; Zhou, N.; Shen, J.; Zhang, M.; Sun, Y. Functionalization of polyvinyl alcohol composite film wrapped in am-ZnO@CuO@Au nanoparticles for antibacterial application and wound healing. Appl. Mater. Today 2019, 17, 36–44. [Google Scholar] [CrossRef]
- Cai, N.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring mechanical and antibacterial properties of chitosan/gelatin nanofiber membranes with Fe3O4 nanoparticles for potential wound dressing application. Appl. Surf. Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 composite membrane improves proliferation and survival of L929 fibroblast cells: Application in wound dressing and skin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Piątkowski, M.; Janus, Ł.; Bogdał, D.; Matysek, D.; Cablik, V. 3D scaffolds prepared from acylated chitosan applicable in skin regeneration—Synthesis and characterization. Int. J. Polym. Anal. Charact. 2019, 24, 75–86. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical Stimulation Promotes Wound Healing by Enhancing Dermal Fibroblast Activity and Promoting Myofibroblast Transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef] [Green Version]
- Sluzky, V.; Shahrokh, Z.; Stratton, P.; Eberlein, G.; Wang, Y.J. Chromatographic Methods for Quantitative Analysis of Native, Denatured, and Aggregated Basic Fibroblast Growth Factor in Solution Formulations. Pharm. Res. 1994, 11, 485–490. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin Nanofibrils in Poly(Lactic Acid) (PLA) Nanocomposites: Dispersion and Thermo-Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [Green Version]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Gao, J.; Hu, X.; Guo, H.; Wang, F.; Qia, Y.; Wang, L. Collagen/Polyethylene Oxide Nanofibrous Membranes with Improved Hemostasis and Cytocompatibility for Wound Dressing. J. Appl. Sci. 2018, 8, 1226. [Google Scholar] [CrossRef] [Green Version]
- Massoumi, B.; Abbasian, M.; Jahanban-Esfahlan, R.; Mohammad-Rezaei, R.; Khalilzadeh, B.; Samadian, H.; Rezaei, A.; Derakhshankhah, H.; Jaymand, M. A novel bio-inspired conductive, biocompatible, and adhesive terpolymer based on polyaniline, polydopamine, and polylactide as scaffolding biomaterial for tissue engineering application. Int. J. Biol. Macromol. 2019, in press. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Rezaeian, I.; Bakhshandeh, B.; Heshmatian, B.; Ganjali, M.R. Bio-Conductive Scaffold Based on Agarose-Polyaniline for Tissue Engineering. J. Skin Stem Cell 2017, 4, e67394. [Google Scholar] [CrossRef]
- Talikowska, M.; Fu, X.; Lisak, G. Application of conducting polymers to wound care and skin tissue engineering: A review. Biosens. Bioelectron. 2019, 135, 50–63. [Google Scholar] [CrossRef]
- Sun, Y.S. Electrical Stimulation for Wound-Healing: Simulation on the Effect of Electrode Configurations. BioMed Res. Int. 2017, 2017, 5289041. [Google Scholar] [CrossRef] [Green Version]
- Tang, P.; Han, L.; Li, P.; Jia, Z.; Wang, K.; Zhang, H.; Tan, H.; Guo, T.; Lu, X. Mussel-Inspired Electroactive and Antioxidative Scaffolds with Incorporation of Polydopamine-Reduced Graphene Oxide for Enhancing Skin Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 7703–7714. [Google Scholar] [CrossRef]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef] [Green Version]
- Lima, L.L.; Taketa, T.B.; Beppu, M.M.; De Oliveira Sousa, I.M.; Foglio, M.A.; Moraes, A.M. Coated electrospun bioactive wound dressings: Mechanical properties and ability to control lesion microenvironment. Mater. Sci. Eng. C 2019, 100, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Bao, X.; Huang, G.; Xu, F.; Zhang, X. Differential Effects of Directional Cyclic Stretching on the Functionalities of Engineered Cardiac Tissues. ACS Appl. Bio Mater. 2019, 2, 3508–3519. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Zhao, G.; Qing, H.; Huang, G.; Genin, G.M.; Lu, T.J.; Luo, Z.; Xu, F.; Zhang, X. Reduced graphene oxide functionalized nanofibrous silk fibroin matrices for engineering excitable tissues. NPG Asia Mater. 2018, 10, 982–994. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, X.; Lu, T.J.; Xu, F. Recent Advances in Electrospun Nanofibrous Scaffolds for Cardiac Tissue Engineering. Adv. Funct. Mater. 2015, 25, 5726–5738. [Google Scholar] [CrossRef]






| Sample | Acetylated CS, g | NPs Type, % | PLA, % | Solvent | Potential, kV |
|---|---|---|---|---|---|
| CS-PLA-30 | 0.5 | - | 10 | acetone | 30 |
| CS-PLA-35 | - | 35 | |||
| CS-PLA-30-Fe3O4 | Fe3O4, 1.0 | 30 | |||
| CS-PLA-35-Fe3O4 | Fe3O4, 1.0 | 35 | |||
| CS-PLA-30-ZnO | ZnO, 1.0 | 30 | |||
| CS-PLA-35-ZnO | ZnO, 1.0 | 35 | |||
| CS-PLA-30-Au | Au, 1.0 | 30 | |||
| CS-PLA-35-Au | Au, 1.0 | 35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Bogdał, D.; Matýsek, D. Hybrid Bilayer PLA/Chitosan Nanofibrous Scaffolds Doped with ZnO, Fe3O4, and Au Nanoparticles with Bioactive Properties for Skin Tissue Engineering. Polymers 2020, 12, 159. https://doi.org/10.3390/polym12010159
Radwan-Pragłowska J, Janus Ł, Piątkowski M, Bogdał D, Matýsek D. Hybrid Bilayer PLA/Chitosan Nanofibrous Scaffolds Doped with ZnO, Fe3O4, and Au Nanoparticles with Bioactive Properties for Skin Tissue Engineering. Polymers. 2020; 12(1):159. https://doi.org/10.3390/polym12010159
Chicago/Turabian StyleRadwan-Pragłowska, Julia, Łukasz Janus, Marek Piątkowski, Dariusz Bogdał, and Dalibor Matýsek. 2020. "Hybrid Bilayer PLA/Chitosan Nanofibrous Scaffolds Doped with ZnO, Fe3O4, and Au Nanoparticles with Bioactive Properties for Skin Tissue Engineering" Polymers 12, no. 1: 159. https://doi.org/10.3390/polym12010159






