Enhanced In-Vitro Hemozoin Polymerization by Optimized Process using Histidine-Rich Protein II (HRPII)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Biological Experiments
2.2.1. Expression and Production of the HRP-II
2.2.2. Enzyme Purification
2.3. Heme to the Synthesis of Hemozoin
2.3.1. Polymerization of the Heme
2.3.2. Estimation of the Hemozoin
2.4. Physico-Chemical Measurements
3. Results and Discussion
3.1. Comparison of the Conductivity of Various Porphyrin Complexes and the Heme
3.2. Production of the HRP-II and its Application to the Hemozoin Polymerization
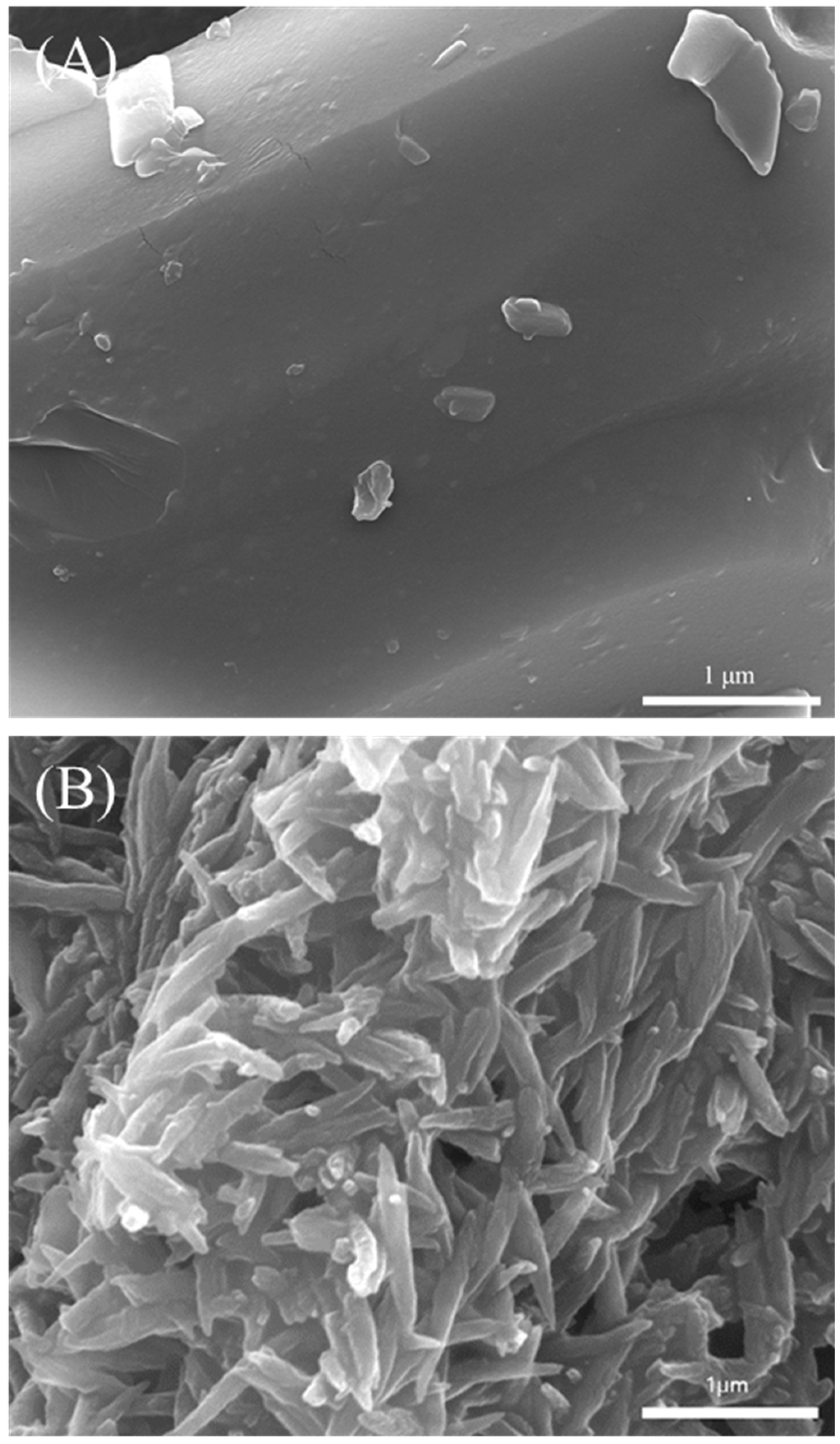
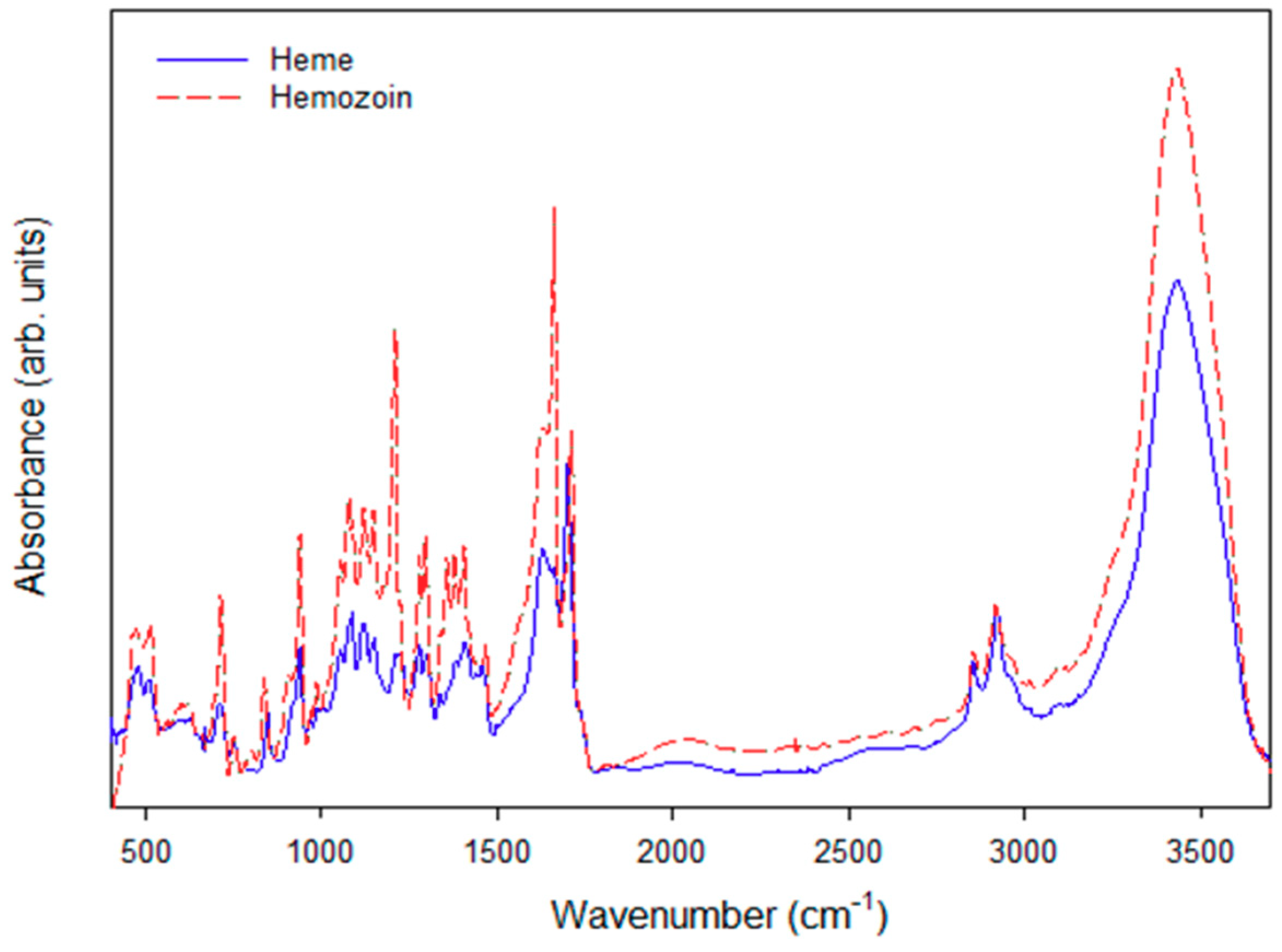
3.3. Qualitative Characterization of the Polymerized Hemozoin
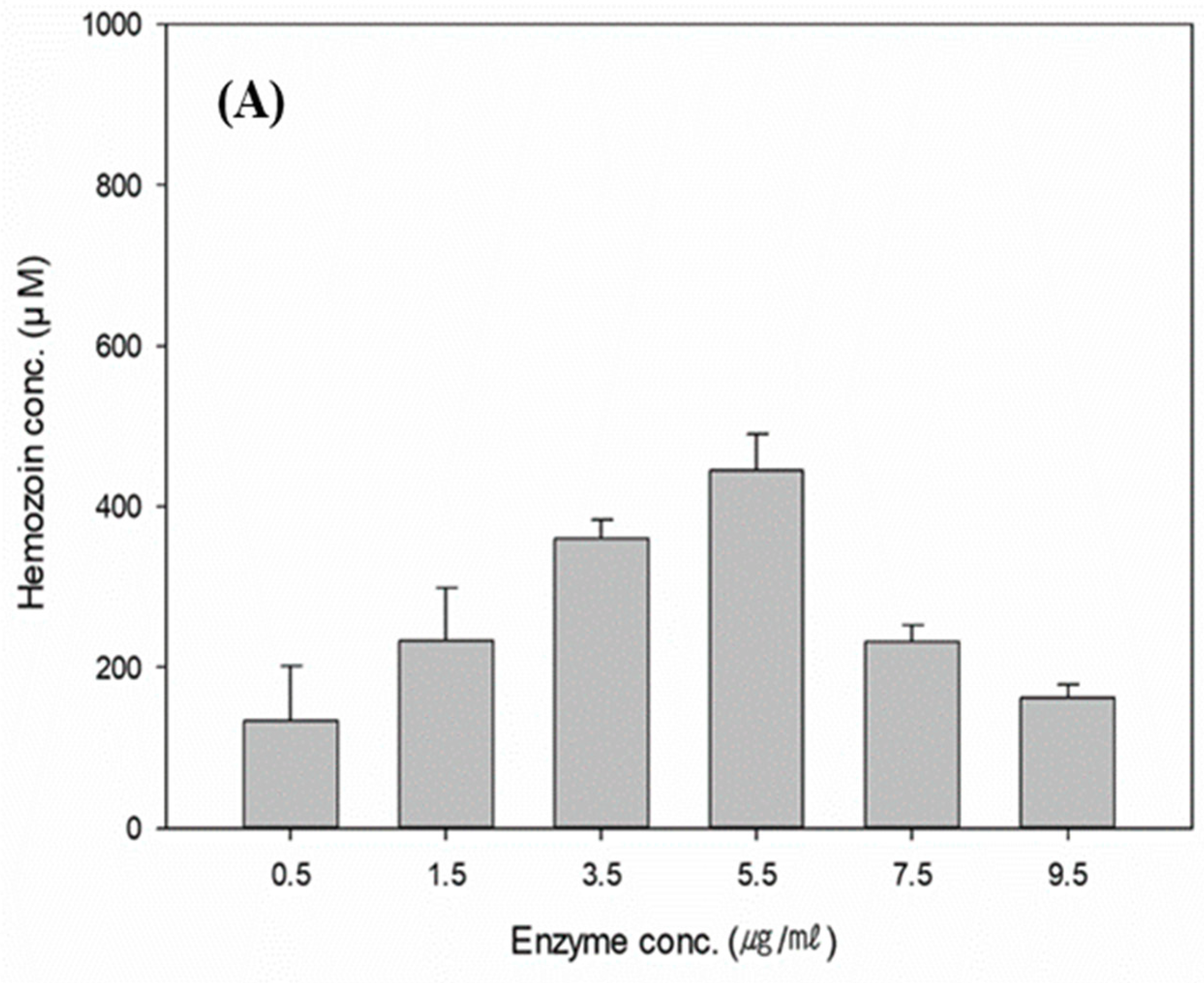
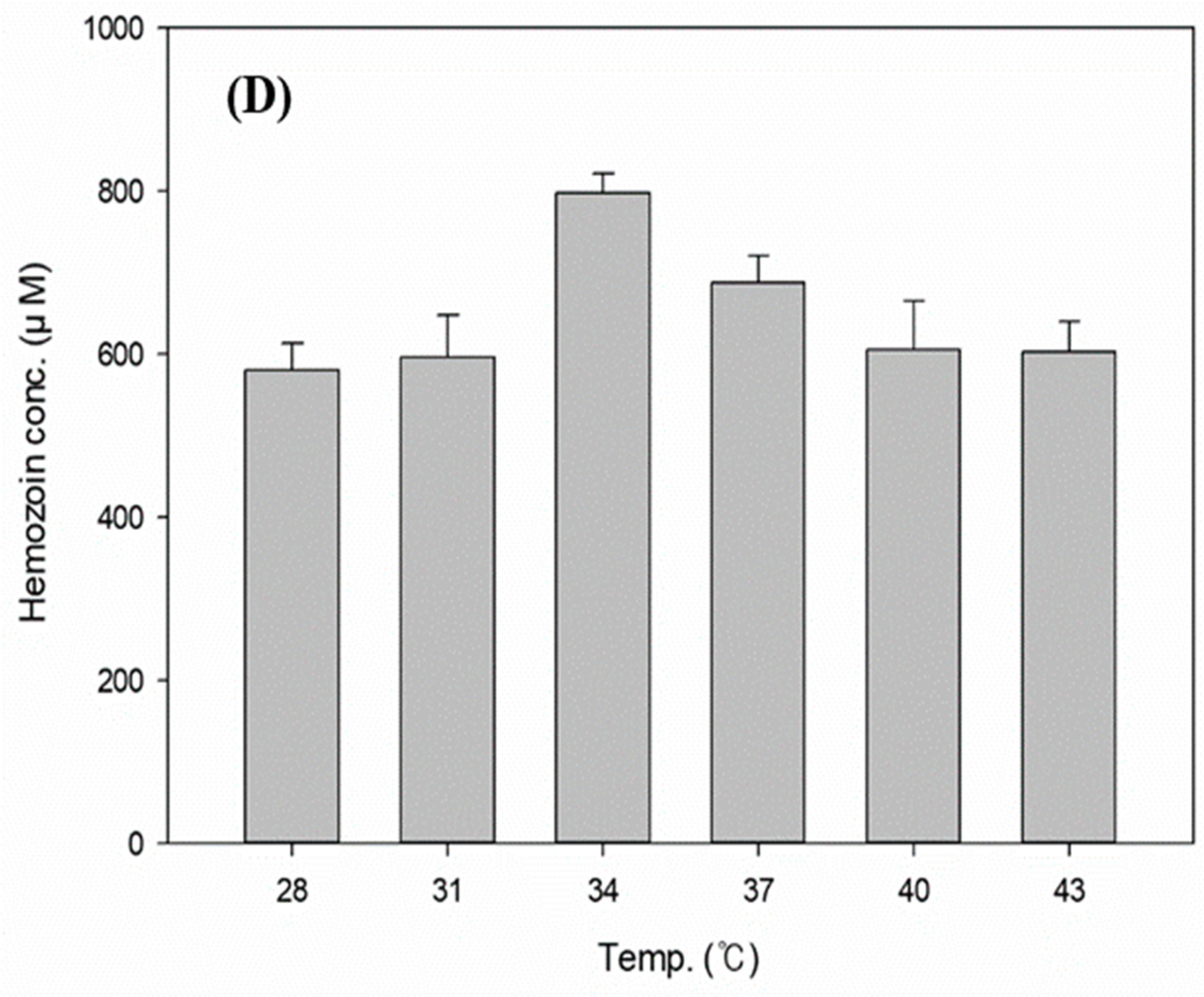
3.4. Effects of the Reaction Conditions on Hemozoin Polymerization
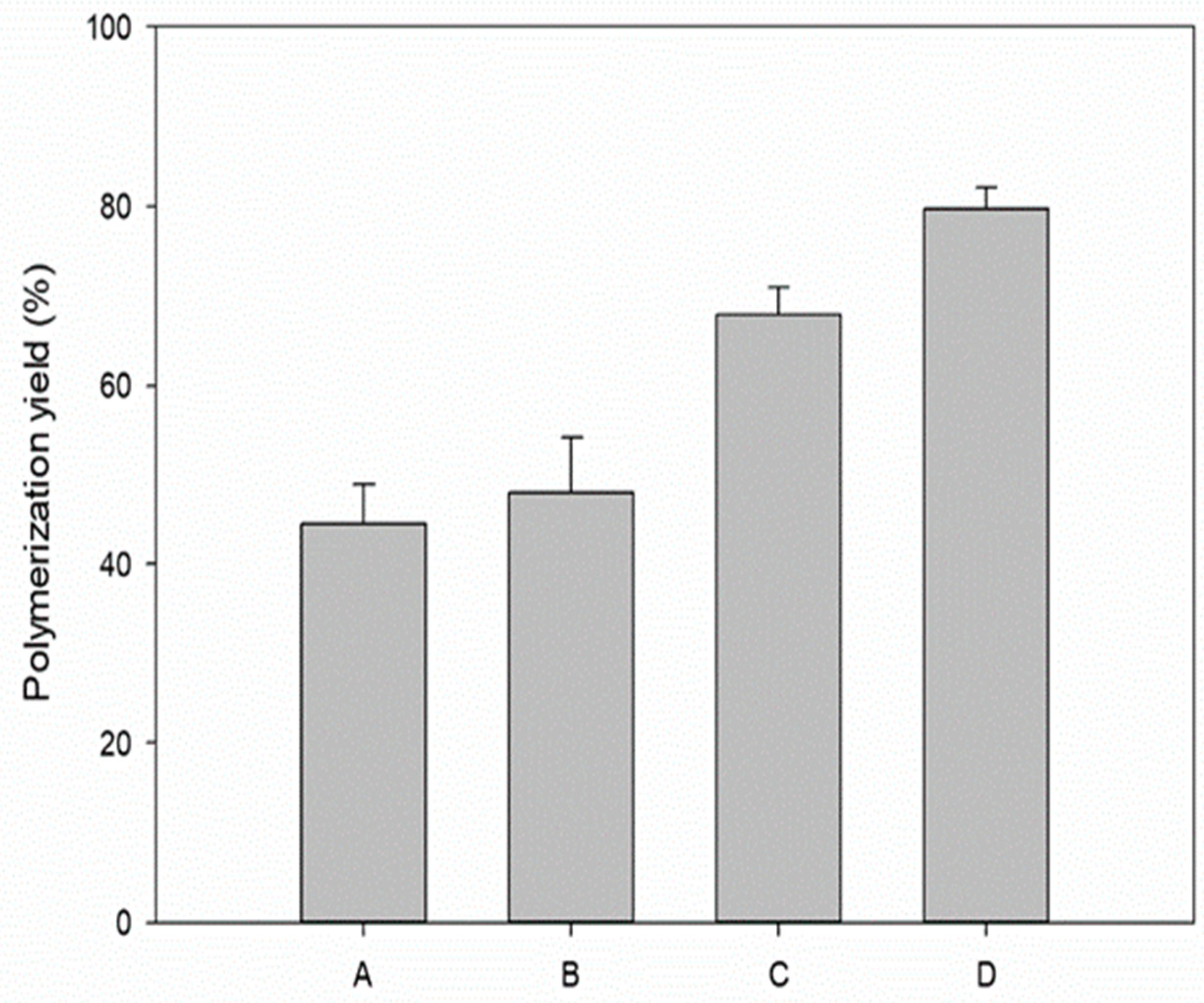
3.5. Comparison of the Polymerization Yields under the Determined Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Das, T.K.; Prusty, S. Review on Conducting Polymers and Their Applications. Polym. Technol. Eng. 2012, 51, 1487–1500. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Kim, D.S.; Yoo, H.Y.; Park, C.; Kim, S.W. Biodiesel production by lipases co-immobilized on the functionalized activated carbon. Bioresour. Technol. Rep. 2019, 7, 100248. [Google Scholar] [CrossRef]
- Lee, S.L.; Chang, C.-J. Recent Developments about Conductive Polymer Based Composite Photocatalysts. Polymers 2019, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, J.; Li, J.; Kang, Q.; Shen, D.; Chen, L. Quantum dots based imprinting fluorescent nanosensor for the selective and sensitive detection of phycocyanin: A general imprinting strategy toward proteins. Sen. Actuators B Chem. 2018, 255, 268–274. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Huang, Y.; Kormakov, S.; He, X.; Gao, X.; Zheng, X.; Liu, Y.; Sun, J.; Wu, D. Conductive Polymer Composites from Renewable Resources: An Overview of Preparation, Properties, and Applications. Polymers 2019, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Al Mousawi, A.; Poriel, C.; Dumur, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.P.; Lalevée, J. Zinc Tetraphenylporphyrin as High Performance Visible Light Photoinitiator of Cationic Photosensitive Resins for LED Projector 3D Printing Applications. Macromolecules 2017, 50, 746–753. [Google Scholar] [CrossRef]
- Ko, Y.J.; Joo, Y.-C.; Hyeon, J.E.; Lee, E.; Lee, M.-E.; Seok, J.; Kim, S.W.; Park, C.; Han, S.O. Biosynthesis of organic photosensitizer Zn-porphyrin by diphtheria toxin repressor (DtxR)-mediated global upregulation of engineered heme biosynthesis pathway in Corynebacterium glutamicum. Sci. Rep. 2018, 8, 14460. [Google Scholar] [CrossRef]
- Frühbeißer, S.; Mariani, G.; Gröhn, F. Porphyrin Diacid-Polyelectrolyte Assemblies: Effective Photocatalysts in Solution. Polymers 2016, 8, 180. [Google Scholar] [CrossRef]
- Collman, J.P.; McDevitt, J.T.; Yee, G.T.; Leidner, C.R.; McCullough, L.G.; Little, W.A.; Torrance, J.B. Conductive polymers derived from iron, ruthenium, and osmium metalloporphyrins: The shish-kebab approach. Proc. Natl. Acad. Sci. USA 1986, 83, 4581–4585. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yu, J.; Kang, Q.; Shen, D.; Li, J.; Chen, L. Molecular imprinting ratiometric fluorescence sensor for highly selective and sensitive detection of phycocyanin. Biosens. Bioelectron. 2016, 77, 624–630. [Google Scholar] [CrossRef] [PubMed]
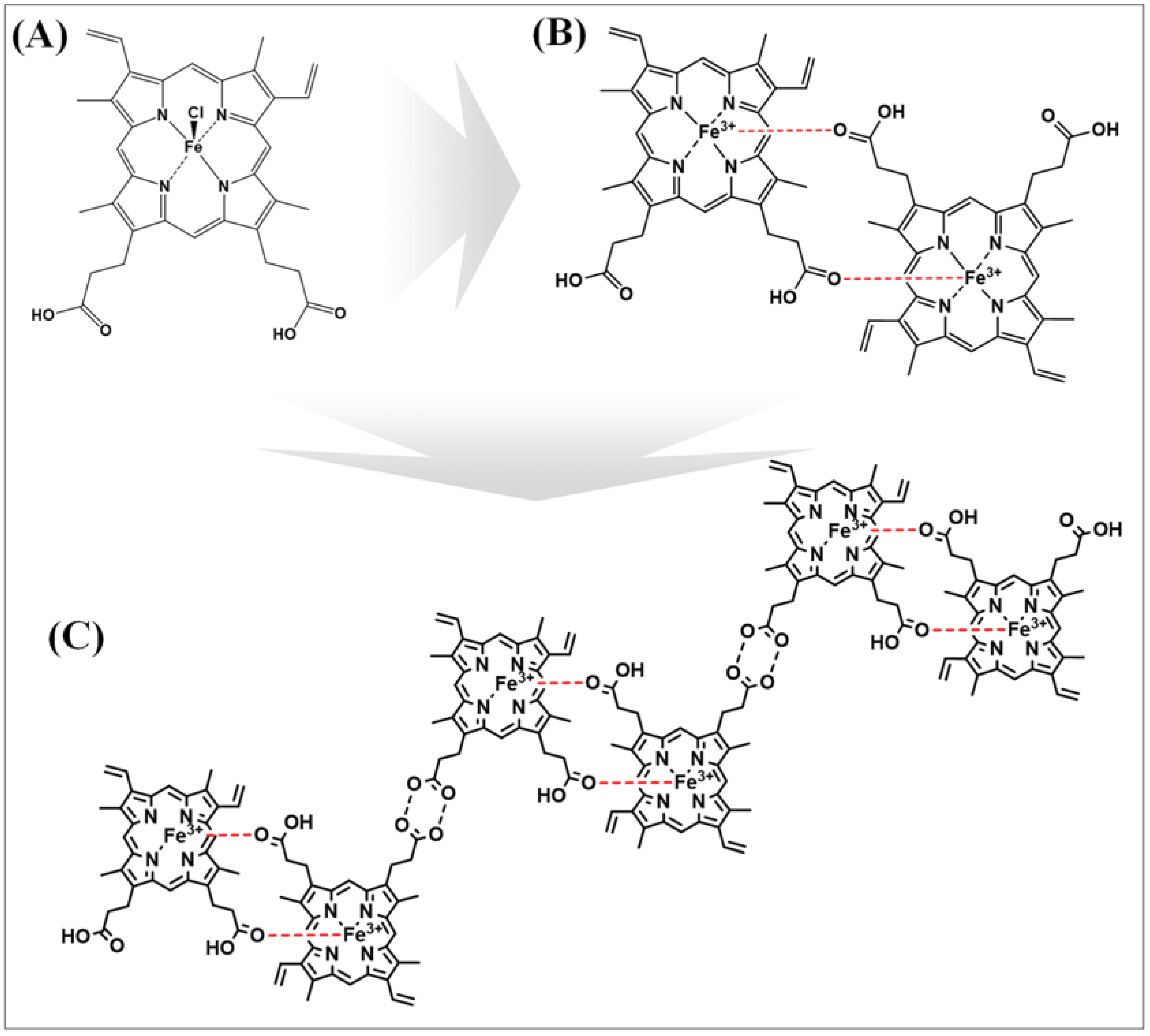
- Egan, T.J. Haemozoin formation. Mol. Biochem. Parasitol. 2008, 157, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Hempelmann, E. Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitor. Parasitol. Res. 2007, 100, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.V.; Babbarwal, V.K.; Okoyeh, J.N.; Joshi, R.M.; Puri, S.K.; Singh, R.L.; Chauhan, V.S. Hemozoin formation in malaria: A two-step process involving histidine-rich proteins and lipids. Biochem. Biophys. Res. Commun. 2003, 308, 736–743. [Google Scholar] [CrossRef]
- Tripathi, A.K.; I Khan, S.; A Walker, L.; Tekwani, B.L. Spectrophotometric determination of de novo hemozoin/β-hematin formation in an in vitro assay. Anal. Biochem. 2004, 325, 85–91. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Kycia, S.W.; Gomez, A.; Kosar, A.J.; Bohle, D.S.; Hempelmann, E.; Menezes, D.; Vannier-Santos, M.A.; Oliveira, P.L.; Ferreira, S.T. Structural and morphological characterization of hemozoin produced by Schistosoma mansoni and Rhodnius prolixus. FEBS Lett. 2005, 579, 6010–6016. [Google Scholar] [CrossRef]
- Tekwani, B.L.; Walker, L.A. Targeting the hemozoin synthesis pathway for new antimalarial drug discovery: Technologies for in vitro β-hematin formation assay. Comb. Chem. High Throughput Screen. 2005, 8, 63–79. [Google Scholar] [CrossRef]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef]
- Bennett, J.E.; Malinski, T. Conductive polymeric porphyrin films: Application in the electrocatalytic oxidation of hydrazine. Chem. Mater. 1991, 3, 490–495. [Google Scholar] [CrossRef]
- Ballarin, B.; Masiero, S.; Seebar, R.; Tonelli, D. Modification of electrodes with porphyrin-functionalised conductive polymer. J. Electroanal. Chem. 1988, 449, 173–180. [Google Scholar] [CrossRef]
- Geng, J.; Jung, H.-T. Porphyrin Functionalized Graphene Sheets in Aqueous Suspensions: From the Preparation of Graphene Sheets to Highly Conductive Graphene Films. J. Phys. Chem. C 2010, 114, 8227–8234. [Google Scholar] [CrossRef]
- Hyeon, J.E.; Jeong, D.W.; Ko, Y.J.; Kim, S.W.; Park, C.; Han, S.O. Biomimetic magnetoelectric nanocrystals synthesized by polymerization of heme as advanced nanomaterials for biosensing application. Biosens. Bioelectron. 2018, 114, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Musto, A.; Navarra, A.; Togna, A.R.; Cammisotto, V.; Gargiulo, V.; Alfè, M.; Di Capua, R.; Fiorito, S.; Parisi, S.; Pezzella, A. Supplementing π-systems: Eumelanin and graphene-like integration towards highly conductive materials for the mammalian cell culture bio-interface. J. Mater. Chem. B 2015, 3, 5070–5079. [Google Scholar]
- Scholl, P.F.; Kumar, N.; Kongkasuriyachai, D.; Demirev, P.A.; Feldman, A.B.; Lin, J.S.; Sullivan, D.J. Rapid detection of malaria infection in vivo by laser desorption mass spectrometry. Am. J. Trop. Med. Hyg. 2004, 71, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Maltha, J.; Guiraud, I.; Lompo, P.; Kaboré, B.; Gillet, P.; Van Geet, C.; Tinto, H.; Jacobs, J. Accuracy of PfHRP2 versus Pf-pLDH antigen detection by malaria rapid diagnostic tests in hospitalized children in a seasonal hyperendemic malaria transmission area in Burkina Faso. Malar. J. 2014, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Chandra, S.; Malhotra, P.; Chauhan, V.S. Heme binding and polymerization by Plasmodium falciparum histidine rich protein II: Influence of pH on activity and conformation. FEBS Lett. 1999, 459, 267–271. [Google Scholar] [CrossRef]
- Kim, C.K.; Choi, H.S.; Lee, S.J.; Lee, J.H.; Lee, J.H.; Yoo, H.Y.; Han, S.O.; Kim, S.W. Production of xylanase from a novel engineered Pichia pastoris and application to enzymatic hydrolysis process for biorefinery. Process. Biochem. 2018, 65, 130–135. [Google Scholar] [CrossRef]
- Thapa, L.P.; Lee, S.J.; Yoo, H.Y.; Choi, H.S.; Park, C.; Kim, S.W. Development of glycerol-utilizing Escherichia coli strain for the production of bioethanol. Enzym. Microb. Technol. 2013, 53, 206–215. [Google Scholar] [CrossRef]
- Choi, H.S.; Thapa, L.P.; Lee, S.J.; Cho, J.; Park, C.; Kim, D.S.; Kim, S.B.; Kim, S.W. Production and characterization of cellobiose dehydrogenase from Phanerochaete chrysosporium KCCM 60256 and its application for an enzymatic fuel cell. Korean J. Chem. Eng. 2016, 33, 3434–3441. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Pradeep, G.C.; Lee, S.K.; Park, D.H.; Cho, S.S.; Choi, Y.H.; Yoo, J.C.; Kim, S.W. Understanding β-mannanase from Streptomyces sp. CS147 and its potential application in lignocellulose based biorefining. Biotechnol. J. 2015, 10, 1894–1902. [Google Scholar] [CrossRef]
- Regmi, S.; Yoo, H.Y.; Choi, Y.H.; Choi, Y.S.; Yoo, J.C.; Kim, S.W. Prospects for Bio-Industrial Application of an Extremely Alkaline Mannanase from Bacillus subtilis subsp. inaquosorum CSB31. Biotechnol. J. 2017, 12, 1700113. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Jin, J.-H.; Kim, S.W. Effect of gel electrolytes on the performance of a minimized flexible micro-supercapacitor based on graphene/PEDOT composite using pen lithography. J. Ind. Eng. Chem. 2019, 71, 184–190. [Google Scholar] [CrossRef]
- Kobayashi, N.; Nevin, W.A.; Mizunuma, S.; Awaji, H.; Yamaguchi, M. Ring-expanded porphyrins as an approach towards highly conductive molecular semiconductors. Chem. Phys. Lett. 1993, 205, 51–54. [Google Scholar] [CrossRef]
- Ohno, H. Electron transfer reaction of heme-proteins in ion conductive polymer matrix. Electrochimica Acta 1998, 43, 1581–1587. [Google Scholar] [CrossRef]
- Kepp, K.P. Heme: From quantum spin crossover to oxygen manager of life. Coord. Chem. Rev. 2017, 344, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-W.; Wang, J. Structure and function of heme proteins in non-native states: A mini-review. J. Inorg. Biochem. 2013, 129, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Slater, A.F.; Swiggard, W.J.; Orton, B.R.; Flitter, W.D.; Goldberg, D.E.; Cerami, A.; Henderson, G.B. An iron-carboxylate bond links the heme units of malaria pigment. Proc. Natl. Acad. Sci. USA 1991, 88, 325–329. [Google Scholar] [CrossRef]
- Tempera, C.; Franco, R.; Caro, C.; André, V.; Eaton, P.; Burke, P.; Hänscheid, T. Characterization and optimization of the haemozoin-like crystal (HLC) assay to determine Hz inhibiting effects of anti-malarial compounds. Malar. J. 2015, 14, 373. [Google Scholar] [CrossRef]
- Pandey, A.V.; Joshi, R.; Tekwani, B.L.; Singh, R.L.; Chauhan, V.S. Synthetic peptides corresponding to a repetitive sequence of malarial histidine rich protein bind haem and inhibit haemozoin formation in vitro. Mol. Biochem. Parasitol. 1997, 90, 281–287. [Google Scholar] [CrossRef]
- Arai, K.; Arai, M.; Iwasaki, S.; Saito, S. Agitation effect on the rate of soapless emulsion polymerization of methyl methacrylate in water. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 1203–1215. [Google Scholar] [CrossRef]


| Conductive Polymer | Chemical Formula | Conductivity (μS/cm) | Ref. |
|---|---|---|---|
| Metalloporphyrins (Fe) | C44H30N4Fe | 2.8 × 10−5 | [10] |
| Metalloporphyrins (Ru) | C44H30N4Ru | 1.0 × 10−5 | [10] |
| Metalloporphyrins (Os) | C44H30N4Os | 8.0 × 10−5 | [10] |
| Tetraphenylporphyrin (Zn) | C44H30N4Zn | <1.0 × 10−5 | [33] |
| Tetrabenzoporphyrin (Zn) | C36H22N4Zn | 4.0 × 10−4 | [33] |
| Heme | C34H32ClFeN4O4 | 4.0 × 10−3 | This study |
| Hemozoin | –[C34H32ClFeN4O4]– | 6.2 × 10−3 | This study |
| Purification Step | Total Protein (μg) | Total Activity (U) | Specific Activity (U/mg) | Yield (%) | Purification (folds) |
|---|---|---|---|---|---|
| Crude broth | 17.8 | 0.40 | 22.5 | 100.0 | 1.0 |
| Ni–NTA affinity column | 5.5 | 0.32 | 57.0 | 79.1 | 2.5 |
| Element | Carbon (C) (%) | Oxygen (O) (%) | Iron (Fe) (%) |
|---|---|---|---|
| Heme | 71.14 | 7.35 | 4.27 |
| Hemozoin (polymerized by HRP-II) | 75.63 | 9.56 | 9.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Kim, H.R.; Lee, J.H.; Lee, S.K.; Chun, Y.; Han, S.O.; Yoo, H.Y.; Park, C.; Kim, S.W. Enhanced In-Vitro Hemozoin Polymerization by Optimized Process using Histidine-Rich Protein II (HRPII). Polymers 2019, 11, 1162. https://doi.org/10.3390/polym11071162
Lee JH, Kim HR, Lee JH, Lee SK, Chun Y, Han SO, Yoo HY, Park C, Kim SW. Enhanced In-Vitro Hemozoin Polymerization by Optimized Process using Histidine-Rich Protein II (HRPII). Polymers. 2019; 11(7):1162. https://doi.org/10.3390/polym11071162
Chicago/Turabian StyleLee, Ju Hun, Hyeong Ryeol Kim, Ja Hyun Lee, Soo Kweon Lee, Youngsang Chun, Sung Ok Han, Hah Young Yoo, Chulhwan Park, and Seung Wook Kim. 2019. "Enhanced In-Vitro Hemozoin Polymerization by Optimized Process using Histidine-Rich Protein II (HRPII)" Polymers 11, no. 7: 1162. https://doi.org/10.3390/polym11071162








