Plasma Polymerized Allylamine—The Unique Cell-Attractive Nanolayer for Dental Implant Materials
Abstract
:1. Introduction
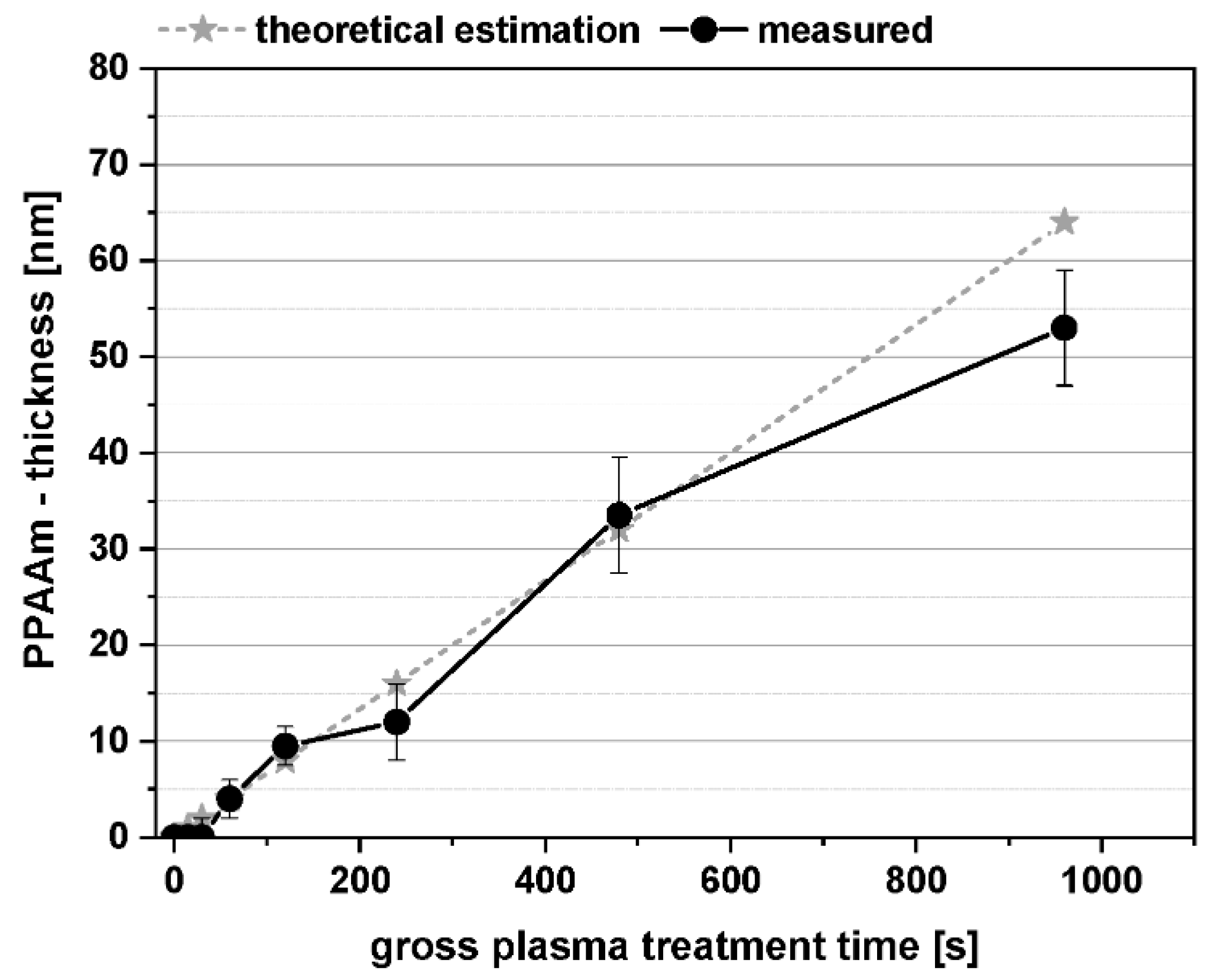
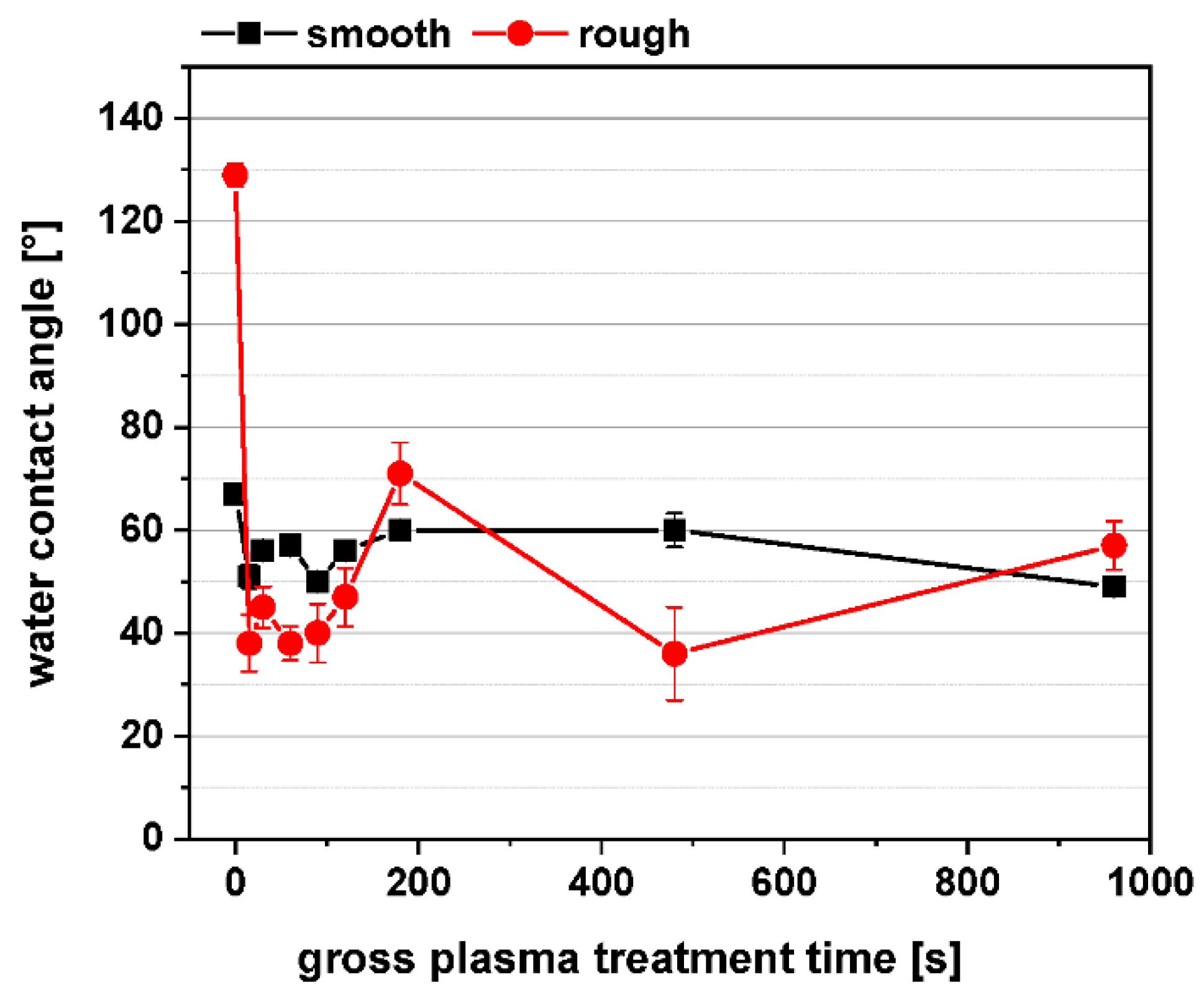
1.1. PPAAm Layer Characteristics—An Overview
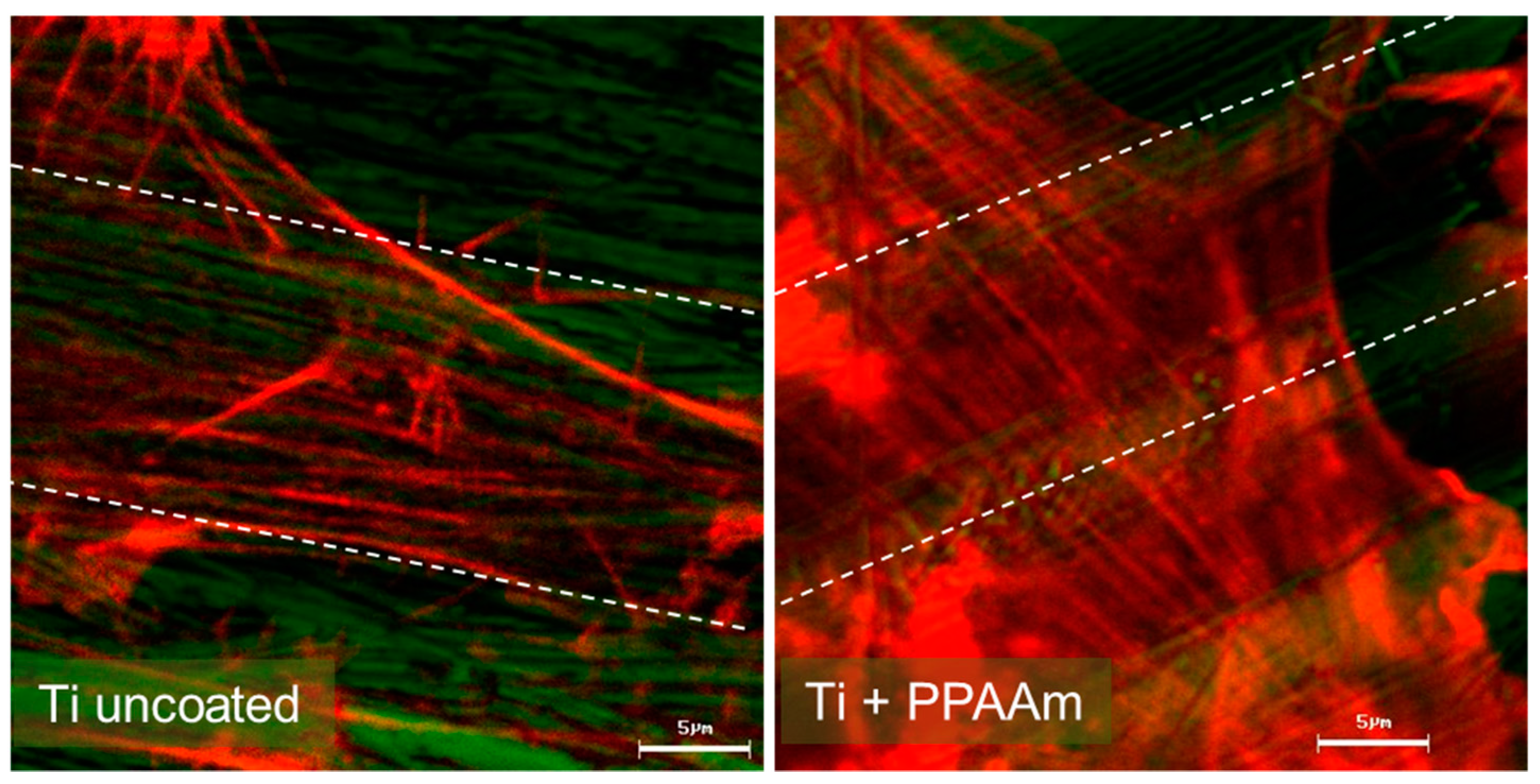
1.2. In Vitro Outcome
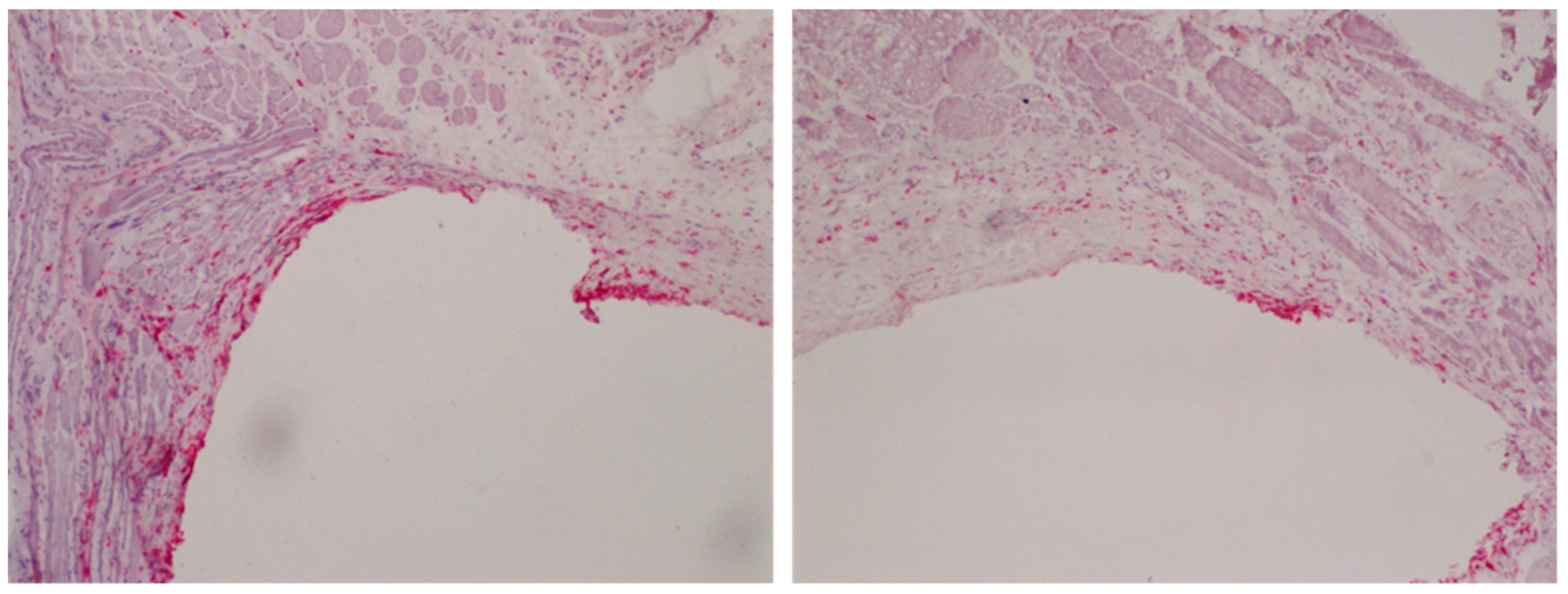
1.3. In Vivo Outcome
2. Materials and Methods
2.1. Substrates
2.2. Deposition of Plasma Polymerized Allylamine (PPAAm)
2.3. Surface Characterization
2.3.1. Grazing Incidence X-ray Diffraction (GIXRD), X-ray Photoelectron Spectroscopy (XPS), Fourier-Transform Infrared Spectroscopy FT-IR
2.3.2. Film Thickness, PPAAm Roughness
2.3.3. Surface Free Energy, Contact Angle, Zeta Potential
2.4. Cell Culture Experiments
2.4.1. Cell Morphology
2.4.2. Actin Cytoskeleton
2.4.3. Cell Adhesion
2.5. Statistics
3. Results and Discussion
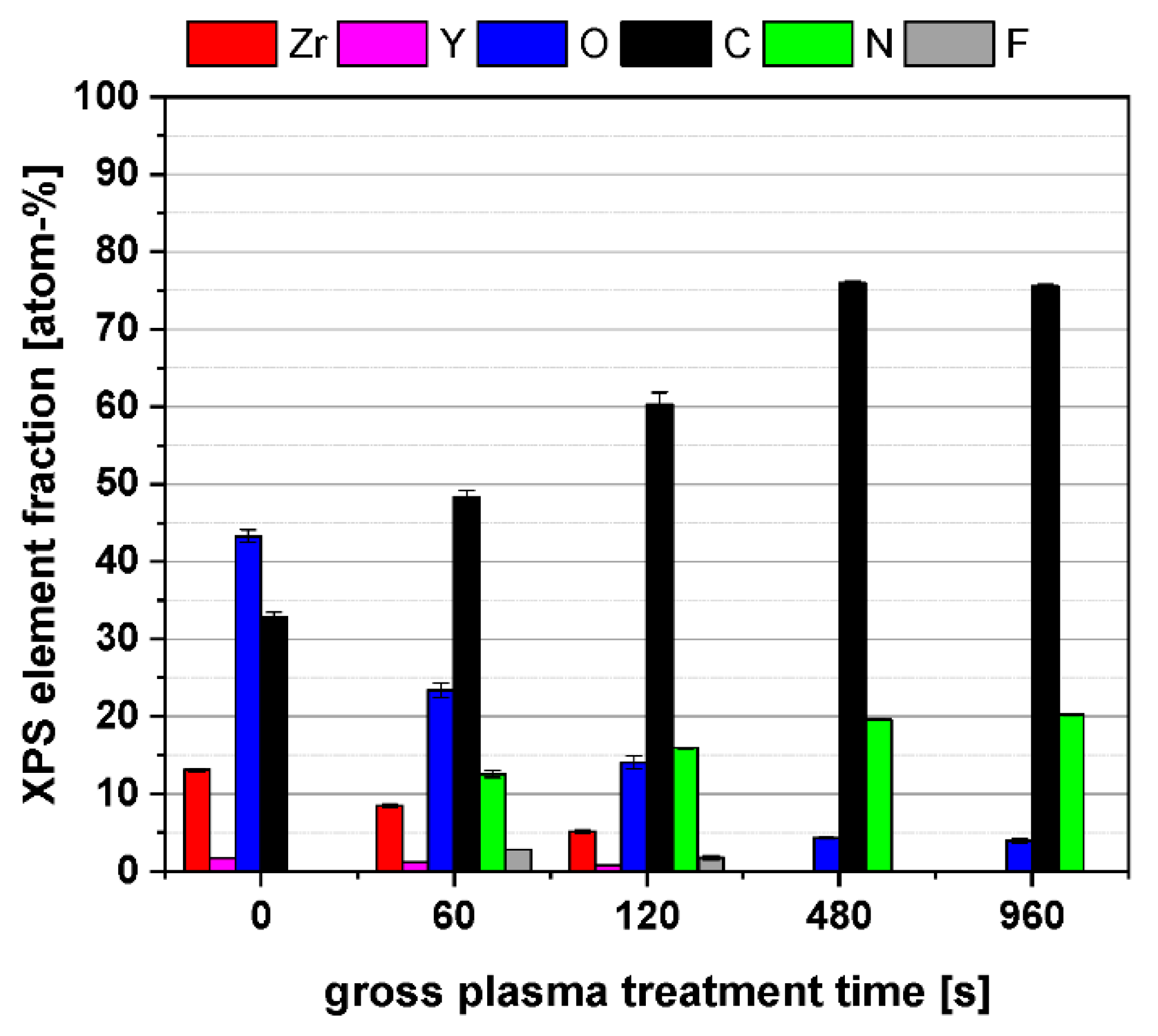
3.1. Physico-Chemical Surface Properties of PPAAm-Treated Y-TZP Ceramic
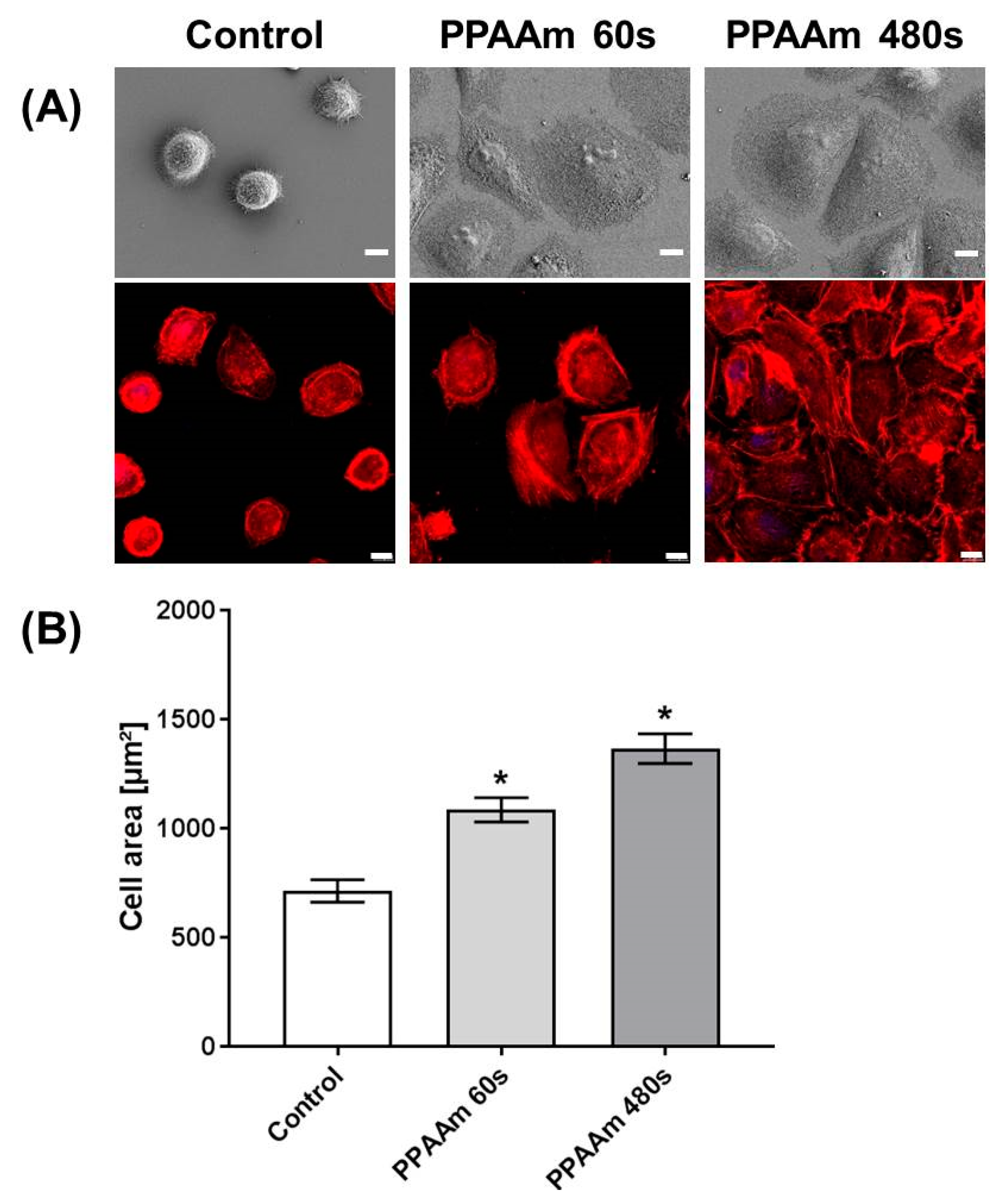
3.2. Cell Behavior on Titanium (Ti) and Ceramic Surfaces Bioactivated with Plasma Polymer (PPAAm)
4. Conclusions
5. Patent
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rebl, H. Osteoblast Physiology and Morphology on Cell Adhesive Plasma-Polymerized Allylamine and Ethylenediamine Layers. Ph.D. Thesis, University of Rostock, Rostock, Germany, 2014; p. 51. [Google Scholar]
- Nebe, J.B.; Luethen, F. Integrin- and hyaluronan-mediated cell adhesion on titanium—Hyaluronan-mediated adhesion. In Metallic Biomaterial Interfaces, 1st ed.; Breme, J., Kirkpatrick, C.J., Thull, R., Eds.; WILEY-VCH: Weinheim, Germany, 2008; pp. 179–182. ISBN 978-3-527-31860-5. [Google Scholar]
- Finke, B.; Lüthen, F.; Schröder, K.; Nebe, B.; Rychly, J.; Ohl, A. Amino functionalized titanium—A surface for improved osteoblast’s function. In Proceedings of the 18th International Symposium on Plasma Chemistry (ISPC-18), Kyoto, Japan, 26–31 August 2007; p. 692. [Google Scholar]
- Goreham, R.V.; Mierczynska, A.; Smith, L.E.; Sedev, R.; Vasilev, K. Small surface nanotopography encourages fibroblast and osteoblast cell adhesion. RSC Adv. 2013, 3, 10309. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Q.; Bachhuka, A.; Vasilev, K. Surafce modification by allylamine plasma polymerization promotes osteogenic differentiation of human adipose—Derived stem cells. ACS Appl. Mater. Interfaces 2014, 6, 9733. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.F.; Ibrahim, A.; Seifalian, A.M.; Butler, P.E.M.; Kalaskar, D.M.; Ferretti, P. Chemical group-dependent plasma polymerization preferentially directs adipose stem cell differentiation towards osteogenic or chondrogenic lineages. Acta Biomater. 2017, 50, 450. [Google Scholar] [CrossRef] [PubMed]
- Chaves, C.; Alshomer, F.; Palgrave, R.G.; Kalaskar, D.M. Plasma surface modification of polyhedral oligomeric silsequioxane-poly(carbonate-urea) urethane with allylamine enhances the response and osteogenic differentiation of adipose-derived stem cells. ACS Appl. Mater. Interfaces 2016, 8, 18701. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, M.; Sinha, U.; Visalakshan, R.M.; Cavallaro, A.; Vasilev, K. Preserving the reactivity of coatings plasma deposited from oxazoline precursors—An in depth study. Plasma Process Polym. 2018. [Google Scholar] [CrossRef]
- Michelmore, A.; Martinek, P.; Sah, V.; Short, R.D.; Vasilev, K. Surface morphology in the early stages of plasma polymer film growth from amine containing monomers. Plasma Process Polym. 2011, 8, 367–372. [Google Scholar] [CrossRef]
- Moerke, C.; Rebl, H.; Finke, B.; Dubs, M.; Nestler, P.; Airoudj, A.; Roucoules, V.; Schnabelrauch, M.; Koertge, A.; Anselme, K.; et al. Abrogated cell contact guidance on amino-functionalized micro-grooves. ACS Appl. Mater. Interfaces 2017, 9, 10461–10471. [Google Scholar] [CrossRef] [PubMed]
- Rebl, H.; Finke, B.; Schmidt, J.; Mohamad, H.S.; Ihrke, R.; Helm, C.A.; Nebe, J.B. Accelerated cell-surface interlocking on plasma polymer-modified porous ceramics. Mater. Sci. Eng. C 2016, 69, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Bergemann, C.; Cornelsen, M.; Quade, A.; Laube, T.; Schnabelrauch, M.; Rebl, H.; Weißmann, V.; Seitz, H.; Nebe, B. Continuous cellularization of calcium phosphate hybrid scaffolds induced by plasma polymer activation. Mater. Sci. Eng. C 2016, 59, 514–523. [Google Scholar] [CrossRef]
- Finke, B.; Rebl, H.; Hempel, F.; Schäfer, J.; Liefeith, K.; Weltmann, K.-D.; Nebe, J.B. Ageing of plasma-polymerised allylamine nanofilms and the maintenance of their cell adhesion capacity. Langmuir 2014, 30, 13914–13924. [Google Scholar] [CrossRef] [PubMed]
- Schnabelrauch, M.; Wyrwa, R.; Rebl, H.; Bergemann, C.; Finke, B.; Schlosser, M.; Walschus, U.; Lucke, S.; Weltmann, K.-D.; Nebe, J.B. Surface-coated polylactide fiber meshes as tissue engineering matrices with enhanced cell integration properties. Int. J. Polym. Sci. 2014, 2014, 439784. [Google Scholar] [CrossRef]
- Rebl, H.; Finke, B.; Lange, R.; Weltmann, K.-D.; Nebe, B. Impact of plasma chemistry versus titanium surface topography on osteoblast orientation. Acta Biomater. 2012, 8, 3840–3851. [Google Scholar] [CrossRef] [PubMed]
- Rebl, H.; Finke, B.; Rychly, J.; Schröder, K.; Nebe, J.B. Positively charged material surfaces generated by plasma polymerized allylamine enhance vinculin mobility in vital human osteoblasts. Adv. Eng. Mater. 2010, 12, 356–364. [Google Scholar] [CrossRef]
- Rebl, H.; Finke, B.; Schroeder, K.; Nebe, J.B. Time-dependent metabolic activity and adhesion of human osteoblast-like cells on sensor chips with a plasma polymer nanolayer. Int. J. Artif. Org. 2010, 33, 738–748. [Google Scholar] [CrossRef]
- Finke, B.; Luethen, F.; Schroeder, K.; Mueller, P.D.; Bergemann, C.; Frant, M.; Ohl, A.; Nebe, J.B. The effect of positively charged plasma polymerization on initial osteoblastic focal adhesion on titanium surfaces. Biomaterials 2007, 28, 4521–4534. [Google Scholar] [CrossRef] [PubMed]
- Kunz, F.; Rebl, H.; Quade, A.; Matschegewski, C.; Finke, B.; Nebe, J.B. Osteoblasts with impaired spreading capacity benefit from the positive charges of plasma polymerized allylamine. Eur. Cell Mater. 2015, 29, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Nebe, B.; Lüthen, F.; Finke, B.; Bergemann, C.; Schröder, K.; Rychly, J.; Liefeith, K.; Ohl, A. Improved initial osteoblast’s functions on amino-functionalized titanium surfaces. Biomol. Eng. 2007, 24, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, A.; Haenle, M.; Zietz, C.; Mittelmeier, W.; Neumann, H.G.; Heidenau, F.; Finke, B.; Bader, R. Mechanical characterization of anti-infectious, anti-allergic, and bioactive coatings on orthopedic implant surfaces. J. Mater. Sci. 2009, 44, 5544–5551. [Google Scholar] [CrossRef]
- DIN EN 582. Thermal Spraying; Determination of Tensile Adhesive Strength; German Version EN 582; DIN: Berlin, Germany, 1993. [Google Scholar]
- Akhavan, B.; Wise, S.G.; Bilek, M.M.M. Substrate regulated growth of plasma polymerized films on carbide-forming metals. Langmuir 2016, 32, 10835–10843. [Google Scholar] [CrossRef]
- Van Wachem, P.B.; Beugeling, T.; Feijen, J.; Bantjes, A.; Detmers, J.P.; van Aken, W.G. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials 1985, 6, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Gabler, C.; Zietz, C.; Göhler, R.; Fritsche, A.; Lindner, T.; Haenle, M.; Finke, B.; Meichsner, J.; Lenz, S.; Frerich, B.; et al. Evaluation of osseointegration of titanium alloyed implants modified by plasma polymerization. Int. J. Mol. Sci. 2014, 15, 2454–2464. [Google Scholar] [CrossRef] [PubMed]
- Moerke, C.; Staehlke, S.; Rebl, H.; Finke, B.; Nebe, J.B. Restricted cell functions on micropillars are alleviated by surface-nanocoating with amino groups. J. Cell Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Henrike Rebl, H.; Finke, B.; Mueller, P.; Gruening, M.; Nebe, J.B. Enhanced calcium ion mobilization in osteoblasts on amino group containing plasma polymer nanolayer. Cell Biosci. 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Koertge, A.; Nebe, B. Intracellular calcium dynamics dependent on defined microtopographical features of titanium. Biomaterials 2015, 46, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Triffitt, T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1–R9. [Google Scholar] [CrossRef]
- Hoene, A.; Walschus, U.; Patrzyk, M.; Finke, B.; Lucke, S.; Nebe, B.; Schroeder, K.; Ohl, A.; Schlosser, M. In vivo investigation of the inflammatory response against allylamine plasma polymer coated titanium implants in a rat model. Acta Biomater. 2010, 6, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Walschus, U.; Hoene, A.; Patrzyk, M.; Lucke, S.; Finke, B.; Polak, M.; Lukowski, G.; Bader, R.; Zietz, C.; Podbielski, A.; et al. A cell-adhesive plasma polymerized allylamine coating reduces the in vivo inflammatory response induced by Ti6Al4V plates modified with plasma immersion ion implantation of copper. J. Funct. Biomater. 2017, 8, 30. [Google Scholar] [CrossRef]
- Walschus, U.; Hoene, A.; Patrzyk, M.; Finke, B.; Polak, M.; Lucke, S.; Nebe, B.; Schroeder, K.; Podbielski, A.; Wilhelm, L.; et al. Serum profile of pro- and anti-inflammatory cytokines in rats following implantation of low-temperature plasma-modified titanium plates. J. Mater. Sci. Mater. Med. 2012, 23, 1299–1307. [Google Scholar] [CrossRef]
- Hoene, A.; Patrzyk, M.; Walschus, U.; Finke, B.; Lucke, S.; Nebe, B.; Schröder, K.; Schlosser, M. Systemic IFNγ predicts local implant macrophage response. J. Mater. Sci. Mater. Med. 2015, 23, 131. [Google Scholar] [CrossRef]
- Schröder, K.; Finke, B.; Ohl, A.; Lüthen, F.; Bergemann, C.; Nebe, B.; Rychly, J.; Walschus, U.; Schlosser, M.; Liefeith, K.; et al. Capability of differently charged plasma polymer coatings for control of tissue interactions with titanium surfaces. J. Adhes. Sci. Technol. 2010, 24, 1191–1205. [Google Scholar] [CrossRef]
- Everheart, D.E.; Reilly, C.N. Chemical derivatization in electron-spectroscopy for chemical analysis of surface functional groups introduced on low-density polyethylene film. Anal. Chem. 1981, 53, 665–676. [Google Scholar] [CrossRef]
- Greenler, R.G. Infrared study of adsorbed molecules on metal surfaces by reflection techniques. J. Chem. Phys. 1966, 44, 310–315. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte der Benetzungstheorie und ihre Anwendungen auf die Untersuchung und Veränderung der Oberflächeneigenschaften von Polymeren. Farbe Lack 1971, 77, 997–1005. [Google Scholar]
- Schröder, K.; Finke, B.; Jesswein, H.; Lüthen, F.; Diener, A.; Ihrke, R.; Ohl, A.; Weltmann, K.D.; Rychly, J.; Nebe, B. Similarities between plasma amino functionalized PEEK and titanium surfaces concerning enhancement of osteoblast cell adhesion. J. Adhes. Sci. Technol. 2010, 24, 905–923. [Google Scholar] [CrossRef]
- Staehlke, S.; Rebl, H.; Nebe, J.B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol. Int. 2018. [Google Scholar] [CrossRef]
- Mochales, C.; Maerten, A.; Rack, A.; Cloetens, P.; Mueller, W.D.; Zaslansky, P.; Fleck, C. Monoclinic phase transformations of zirconia-based dental prostheses, induced by clinically practised surface manipulations. Acta Biomater. 2011, 7, 2994–3002. [Google Scholar] [CrossRef]
- Wongkamhaeng, K.; Dawson, D.V.; Holloway, J.A.; Denry, I. Effect of Surface Modification on In-Depth Transformations and Flexural Strength of Zirconia Ceramics. J. Prosthodont. 2019, 28, e364–e375. [Google Scholar] [CrossRef]






| Materials | Chemistry | WCA (o) | Zeta Potential (Mv) | XPS (%) | Surface Energy (mN/m) | In Vitro Cell Behavior | Published |
|---|---|---|---|---|---|---|---|
| Silicon-titanium microgrooves, 20 × 2 µm (ZfM) | PPAAm; methyl-carboxyl- plasma polymer, COL-I, RGD; 480 s gross | 57° | +8.6 at pH 7.4 | N/C 31 O/C 4 | ‒ | Abrogated MG-63 cell contact guidance; randomly oriented actin stress fibers, PPAAm vs. all coatings | Moerke C et al., ACS Applied Material Interfaces, 2017 [10] |
| Ti-6Al-4V plasma chemical oxidation (PCO) | PPAAm; 60 s gross, 960 s gross | 57–60° | positively charged, by AFM | N/C 32 O/C 2 NH2/C 2.8 | polar 16 disperse 30 total 46 | Accelerated MG-63 cell-surface interlocking, actin formation around the pores of porous ceramics; 3-fold increase of cell area (30 min) | Rebl H et al., Materials Science and Engineering C, 2016 [11] |
| β-tricalcium phosphate hybrid scaffolds | PPAAm; 960 s gross | ‒ | ‒ | N/C 29 O/C 10 NH2/C 2.5 | ‒ | Continuous cellularization of hybrid 3D scaffolds (14 days); DNA concentration at the bottom 7.7-fold higher; MG-63 cell migration enhanced | Bergemann C et al., Materials Science and Engineering C, 2016 [12] |
| Ti-6Al-4V, cp, grade 2, polished | PPAAm; 960 s gross | 47° after prep. 40–50° after 360 days storage on air | +13.9 (2 days) +20.9 (20 days) +26.3 (200 days) at pH 6 | N/C 27 O/C 6 NH2/C 2.5 | polar 25 disperse 29 total 54 | Aging and γ-sterilization of PPAAm; maintenance of cell adhesion capacity up to 360 days 2.2-fold and 1.6-fold increased cell areas (30 min, 24 h, resp.); cell area still visibly larger after 360 days of storage in air (5, 10, 30, 60 min, 24 h) | Finke B et al., Langmuir, 2014 [13] |
| Electrospun poly(l-lactide-co-d/l-lactide) mesh | PPAAm; (preactivation in Ar/O2 plasma) 480 s gross | 5° | ‒ | N/C 28 O/C 5 NH2/C 2.5 | polar 40 disperse 28 total 68 | SV40-HUC-1 uroepithelial cells, Ca9-22 gingiva epithelial cells: enhanced cell integration in PLA fiber meshes; SV40-HUC-1 cell area: 1.26-fold, Ca9-22 cell area: 1.35-fold (both 30 min), spreading not influenced by γ-sterilization | Schnabelrauch M et al., International Journal of Polymer Science, 2014 [14] |
| Ti, cp, grade 2, P—polished, M—machined, CB—corund. blasted | PPAAm; PEG DA-COL-I, GDA-COL-I, 960 s gross | 47° P-PPAAm 56° M-PPAAm 41° CB-PPAAm | ‒ | ‒ | P-PPAAm total 55 M-PPAAm total 50 CB-PPAAm total 57 | Impact of plasma chemistry versus Ti surface topography: MG-63 cells literally melt into the groove structure; fewer elongated cells; 4.7-fold increased cell adhesion (5 min); 2.3-fold increased cell area (30 min) | Rebl H et al., Acta Biomaterialia, 2012 [15] |
| Borofloat glass | PPAAm; COL-I; 960 s gross | 50° | +14 at pH 6 | N/C 20 O/C 11 NH2/C 2–3 | ‒ | Enhanced vinculin mobility (1.5-fold, nm/min) in vital MG-63 cells; 1.5-fold increased vinculin contact length; 3.7-fold increase of cell area (30 min); all vs. COL-I | Rebl H et al., Advanced Engineering Materials, 2010 [16] |
| Bionas® 2500 metabolic sensor chips SC 1000 | PPAAm; 120 s gross | 45° | +14 at pH 6 | N/C 20 O/C 14 | polar 27 disperse 27 total 54 | MG-63 cell coverage of chips 2-fold, 72%; cell area increased 1.9-fold (4 h); vital cell adhesion significantly higher (0–24 h), e.g., 2.31-fold (2 h); acidification and oxygen consumption not influenced | Rebl H et al., International Journal of Artificial Organs, 2010 [17] |
| Ti, cp, grade 2 polished | PPAAm, PEG DA-COL-I, COL-I; 960 s gross | 48° | +7.7 at pH 6 | N/C 28 O/C 4 NH2/C 2.5 | ‒ | Hyaluronan-mediated MG-63 cell adhesion; cell adhesion increased 7-fold (15 min); accelerated formation of actin cytoskeleton and paxillin and vinculin (60 min), vs. Ti-P, comparable with collagen coatings | Finke B et al., Biomaterials, 2007 [18] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebe, J.B.; Rebl, H.; Schlosser, M.; Staehlke, S.; Gruening, M.; Weltmann, K.-D.; Walschus, U.; Finke, B. Plasma Polymerized Allylamine—The Unique Cell-Attractive Nanolayer for Dental Implant Materials. Polymers 2019, 11, 1004. https://doi.org/10.3390/polym11061004
Nebe JB, Rebl H, Schlosser M, Staehlke S, Gruening M, Weltmann K-D, Walschus U, Finke B. Plasma Polymerized Allylamine—The Unique Cell-Attractive Nanolayer for Dental Implant Materials. Polymers. 2019; 11(6):1004. https://doi.org/10.3390/polym11061004
Chicago/Turabian StyleNebe, J. Barbara, Henrike Rebl, Michael Schlosser, Susanne Staehlke, Martina Gruening, Klaus-Dieter Weltmann, Uwe Walschus, and Birgit Finke. 2019. "Plasma Polymerized Allylamine—The Unique Cell-Attractive Nanolayer for Dental Implant Materials" Polymers 11, no. 6: 1004. https://doi.org/10.3390/polym11061004







