Effect of Atmospheric Pressure Plasma Treatment on Adhesive Bonding of Carbon Fiber Reinforced Polymer
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
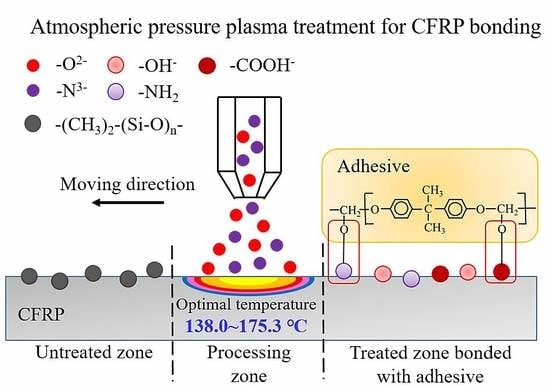
2.2. Plasma Treatment
2.3. Joint Fabrication
2.4. Quasi-Static Lap-Shear Tensile Test
2.5. Temperature Measurements
2.6. Scanning Electron Microscope (SEM) Observation
2.7. X-ray Photo Spectroscopy (XPS) Tests
2.8. Fourier Transform Infrared Spectroscopy (FTIR) Tests
2.9. Contact Angle Measurements
2.10. Differential Scanning Calorimetry (DSC) Tests
2.11. Thermogravimetric Analysis (TGA)
3. Results
3.1. Lap-Shear Tensile Testing Results
3.2. Temperature Measurement Results
3.3. Surface Morphologies of CFRP Substrates
3.4. Surface Chemistries of CFRP Substrates
3.5. Wettability of CFRP Substrates
3.6. Thermal Damage Temperature of CFRP Substrates
4. Discussion
5. Conclusions
- Atmospheric pressure plasma treatment increases the lap-shear strength of adhesive-bonded CFRP joints by 267% (from 8.6 MPa to 31.6 MPa) when a nozzle speed of 5 mm/s and a nozzle distance of 18 mm were applied, which is attributed to both the removal of surface contaminants (e.g., release agent) and the generation of polar groups (e.g., –OH, –COOH, and –NH2). Further study is required to quantify the contribution of each aspect to the improved CFRP joint strength.
- During the plasma treatment process, the surface temperature of CFRP substrate should be controlled between 138 °C and 175.3 °C. When the temperature is below 138 °C, favorable surface modification by plasma treatment is not obvious. A temperature higher than 175.3 °C causes the thermal damage of CFRP substrate.
- The surface temperature of CFRP substrate during APPT is expressed as a function of nozzle distance and nozzle speed to guide the selection of plasma treatment process parameters.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, A.L.; Philipp, H.; Michael, S. Eco-efficiency assessment of manufacturing carbon fiber reinforced polymers (CFRP) in aerospace industry. Aerosp. Sci. Technol. 2018. [Google Scholar]
- Subhani, M.; Globa, A.; Al-Ameri, R.; Moloney, J. Flexural strengthening of LVL beam using CFRP. Constr. Build. Mater. 2017, 150, 480–489. [Google Scholar] [CrossRef]
- Khalil, Y.F. Eco-efficient lightweight carbon-fiber reinforced polymer for environmentally greener commercial aviation industry. Sustain. Prod. Consum. 2017, 12, 16–26. [Google Scholar] [CrossRef]
- Adams, R.D. Adhesive Bonding: Science, Technology and Applications; CRC Press: Boca Raton, FL, USA; Woodhead Pub: Sawston, UK, 2005. [Google Scholar]
- Wu, Y.; Lin, J.; Carlson, B.E.; Lu, P.; Balogh, M.P.; Irish, N.P.; Mei, Y. Effect of laser ablation surface treatment on performance of adhesive-bonded aluminum alloys. Surf. Coat. Technol. 2016, 304, 340–347. [Google Scholar] [CrossRef]
- Davis, G.D. Contamination of surfaces: Origin, detection and effect on adhesion. Surf. Interface Anal. 1993, 20, 368–372. [Google Scholar] [CrossRef]
- Wingfield, J.R.J. Treatment of composite surfaces for adhesive bonding. Int. J. Adhes. Adhes. 1993, 13, 151–156. [Google Scholar] [CrossRef]
- Jang, J.; Yang, H. The effect of surface treatment on the performance improvement of carbon fiber/polybenzoxazine composites. J. Mater. Sci. 2000, 35, 2297–2303. [Google Scholar] [CrossRef]
- Semitekolos, D.; Kainourgios, P.; Jones, C.; Rana, A.; Koumoulos, E.P.; Charitidis, C.A. Advanced carbon fibre composites via poly methacrylic acid surface treatment; surface analysis and mechanical properties investigation. Compos. Part B Eng. 2018, 155, 237–243. [Google Scholar] [CrossRef]
- Fischer, F.; Kreling, S.; Jäschke, P.; Frauenhofer, M.; Kracht, D.; Dilger, K. Laser surface pre-treatment of CFRP for adhesive bonding in consideration of the absorption behavior. J. Adhes. 2012, 88, 350–363. [Google Scholar] [CrossRef]
- Rossi, F.; Kylian, O.; Rauscher, H.; Hasiwa, M.; Gilliland, D. Low pressure plasma discharges for the sterilization and decontamination of surfaces. New J. Phys. 2009, 11, 115017. [Google Scholar] [CrossRef] [Green Version]
- Dhayal, M.; Alexander, M.R.; Bradley, J.W. The surface chemistry resulting from low-pressure plasma treatment of polystyrene: The effect of residual vessel bound oxygen. Appl. Surf. Sci. 2006, 252, 7957–7963. [Google Scholar] [CrossRef]
- Kusano, Y. Atmospheric pressure plasma processing for polymer adhesion: A review. J. Adhes. 2014, 90, 755–777. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.G. Characteristics of plasma surface treated composite adhesive joints at high environmental temperature. Compos. Struct. 2002, 57, 37–46. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.G. Adhesion characteristics of plasma surface treated carbon/epoxy composite. J. Adhes. Sci. Technol. 2003, 17, 1017–1037. [Google Scholar] [CrossRef]
- Williams, T.S.; Yu, H.; Yeh, P.C.; Yang, J.M.; Hicks, R.F. Atmospheric pressure plasma effects on the adhesive bonding properties of stainless steel and epoxy composites. J. Compos. Mater. 2014, 48, 219–233. [Google Scholar] [CrossRef]
- Zaldivar, R.J.; Nokes, J.; Steckel, G.L.; Kim, H.I.; Morgan, B.A. The Effect of atmospheric plasma treatment on the chemistry, morphology and resultant bonding behavior of a pan-based carbon fiber-reinforced epoxy composite. J. Compos. Mater. 2010, 44, 137–156. [Google Scholar] [CrossRef]
- Schäfer, J.; Hofmann, T.; Holtmannspötter, J.; Frauenhofer, M.; von Czarnecki, J.; Gudladt, H.-J. Atmospheric-pressure plasma treatment of polyamide 6 composites for bonding with polyurethane. J. Adhes. Sci. Technol. 2015, 29, 13. [Google Scholar] [CrossRef]
- Comyn, J.; Mascia, L.; Xiao, G.; Parker, B.M. Plasma-treatment of polyetheretherketone (PEEK) for adhesive bonding. Int. J. Adhes. Adhes. 1996, 16, 97–104. [Google Scholar] [CrossRef]
- Wang, S.; Min, J.; Lin, J.; Sun, C.; Yang, S. Effect of atmospheric pressure plasma treatment on the lap-shear strength of adhesive-bonded sheet molding compound joints. Automot. Innov. 2018, 1, 237–246. [Google Scholar] [CrossRef]
- Standard Test Method for Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading (Metal-to-Metal); ASTM D1002-2001; American Society for Testing Materials: West Conshohocken, PA, USA, 2001.
- Wang, X.; Lin, J.; Min, J.; et al. Effect of atmospheric pressure plasma treatment on strength of adhesive-bonded aluminum AA5052. J. Adhes. 2018, 94, 1–22. [Google Scholar] [CrossRef]
- Weber, R.; Hafner, M.; Michalowski, A.; Mucha, P.; Graf, T. Analysis of thermal damage in laser processing of CFRP. In Proceedings of the International Congress on Applications of Lasers and Electro-Optics, Orlando, FL, USA, 23–27 October 2011. [Google Scholar]
- Sun, C.; Min, J.; Lin, J.; Wan, H.; Yang, S.; Wang, S. The effect of laser ablation treatment on the chemistry, morphology and bonding strength of CFRP joints. Int. J. Adhes. Adhes. 2018, 84, 325–334. [Google Scholar] [CrossRef]
- Hartwig, A.; Vitr, G.; Dieckhoff, S.; Hennemann, O.-D. Surface treatment of an epoxy resin by CO2 laser irradiation. Angew. Makromol. Chem. Macromol. Chem. Phys. 1996, 238, 177–189. [Google Scholar] [CrossRef]
- Xingwen, S.; Shenghua, M. Study on silicone oil release agent of PU elastomer mould. Polyurethane Ind. 2005, 5762, 65–71. [Google Scholar]
- Bónová, L.; Zahoranová, A.; Kováčik, D.; Zahoran, M.; Mičušík, M.; Černák, M. Atmospheric pressure plasma treatment of flat aluminum surface. Appl. Surf. Sci. 2015, 331, 79–86. [Google Scholar] [CrossRef]
- Rhee, K.Y.; Choi, N.S.; Park, S.J. Effect of plasma treatment of aluminum on the bonding characteristics of aluminum-CFRP composite joints. J. Adhes. Sci. Technol. 2002, 16, 14. [Google Scholar] [CrossRef]
- Rojewska, M.; Bartkowiak, A.; Strzemiecka, B.; Jamrozik, A.; Voelkel, A.; Prochaska, K. Surface properties and surface free energy of cellulosic etc. mucoadhesive polymers. Carbohydr. Polym. 2017, 171, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Zheng, R.; Lin, J.; Wang, P.C.; Zhu, C.; Wu, Y. Effect of adhesive characteristics on static strength of adhesive-bonded aluminum alloys. Int. J. Adhes. Adhes. 2015, 57, 85–94. [Google Scholar] [CrossRef]
- Boutar, Y.; Naïmi, S.; Mezlini, S.; Sik Ali, M.B. Effect of surface treatment on the shear strength of aluminium adhesive single-lap joints for automotive applications. Int. J. Adhes. Adhes. 2016, 67, 38–43. [Google Scholar] [CrossRef]
- Zimmermann, S.; Specht, U.; Spieß, L.; Romanus, H.; Krischok, S.; Himmerlich, M.; Ihde, J. Improved adhesion at titanium surfaces via laser-induced surface oxidation and roughening. Mater. Sci. Eng. A 2012, 558, 755–760. [Google Scholar] [CrossRef]
- Fedoseev, M.; Gurina, M.; Sdobnov, V.; Kondyurin, A. Study of the reaction of epoxides with carboxylic acids by IR and Raman spectrometry. J. Raman Spectrosc. 1996, 27, 413–418. [Google Scholar] [CrossRef]
- Blank, W.J.; He, Z.A.; Picci, M. Catalysis of the epoxy-carboxyl reaction. J. Coat. Technol. 2002, 74, 33–41. [Google Scholar] [CrossRef]


















| Material | Fiber Diameter (μm) | Density (g/cm3) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|---|
| Carbon fiber | 7 | 1.76 | 3530 | 1.5 |
| Material | Density (g/cm3) | Specific Heat (J/(kg·°C)) | Curing Temperature (°C) | Vaporization Temperature (°C) |
|---|---|---|---|---|
| Epoxy resin | 1.20 | 1000 | 120 | 250 |
| Adhesive | Elastic Modulus (GPa) | Tensile Strength (MPa) | Shear Strength (MPa) | Elongation (%) |
|---|---|---|---|---|
| 3M DP460 | 2.7 | 37 | 32 | 4 |
| Fracture Modes | Substrate Failure | Cohesive Failure | Adhesive Failure |
|---|---|---|---|
| Schematic diagrams |  |  |  |
| (mm) | (mm/s) | ||
|---|---|---|---|
| 1 | 5 | 10 | |
| 10 |  Substrate failure |  Substrate failure |  Substrate failure |
| 14 |  Substrate failure |  Substrate failure |  Substrate failure |
| 18 |  Substrate failure |  Cohesive failure |  Adhesive failure |
| 22 |  Adhesive failure |  Adhesive failure |  Adhesive failure |
| 26 |  Adhesive failure |  Adhesive failure |  Adhesive failure |
| Chemical Element (wt %) | C | O | N | Si | |
|---|---|---|---|---|---|
| CFRP substrates | As-received | 74.61 | 17.69 | 3.92 | 3.41 |
| P5-18 | 63.72 | 28.88 | 5.68 | 1.02 | |
| P10-18 | 67.94 | 25.12 | 4.16 | 1.66 | |
| P5-26 | 72.99 | 18.93 | 3.93 | 2.89 | |
| Functional Groups (wt %) | C=O/O–C= | C–O/C–N | C–C/C–H | |
|---|---|---|---|---|
| CFRP substrates | As-received | 3.8 | 15.2 | 81.0 |
| P5-18 | 8.3 | 36.4 | 55.3 | |
| Testing Liquids | Distilled Water | Diiodomethane | Ethylene Glycol | |
|---|---|---|---|---|
| CFRP substrates | As-received | 108.6° ± 2.1° | 67.1° ± 3.2° | 89.7° ± 2.7° |
| P5-18 | 32.4° ± 3.3° | 54.2° ± 1.6° | 43.1° ± 4.1° | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Min, J.; Lin, J.; Wan, H. Effect of Atmospheric Pressure Plasma Treatment on Adhesive Bonding of Carbon Fiber Reinforced Polymer. Polymers 2019, 11, 139. https://doi.org/10.3390/polym11010139
Sun C, Min J, Lin J, Wan H. Effect of Atmospheric Pressure Plasma Treatment on Adhesive Bonding of Carbon Fiber Reinforced Polymer. Polymers. 2019; 11(1):139. https://doi.org/10.3390/polym11010139
Chicago/Turabian StyleSun, Chengcheng, Junying Min, Jianping Lin, and Hailang Wan. 2019. "Effect of Atmospheric Pressure Plasma Treatment on Adhesive Bonding of Carbon Fiber Reinforced Polymer" Polymers 11, no. 1: 139. https://doi.org/10.3390/polym11010139