Electrospin-Coating of Paper: A Natural Extracellular Matrix Inspired Design of Scaffold
Abstract
:1. Introduction
2. Materials and Methods
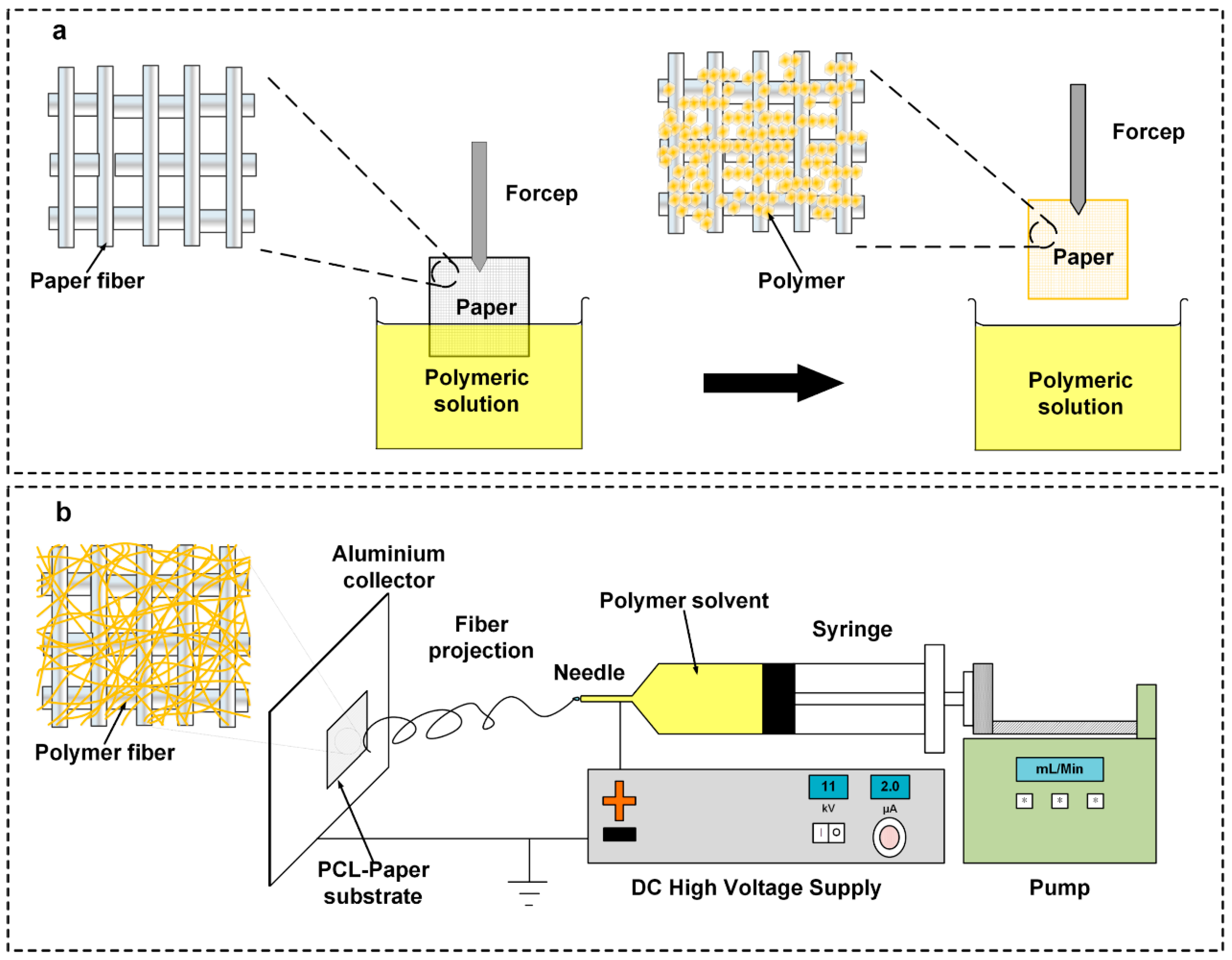
2.1. Dip-Coating of Filter Paper (DFP)
2.2. Electrospin-Coating of Paper (ES-PCL/FP)
2.3. Electrospun PCL (ES-PCL)
2.4. Porosity Measurement
2.5. Characterization of Scaffold Mechanical Properties
2.6. Field Emission Scanning Electron Microscopy (FESEM)
2.7. Cell Culture
2.8. Characterization of Cell Viability and Morphology
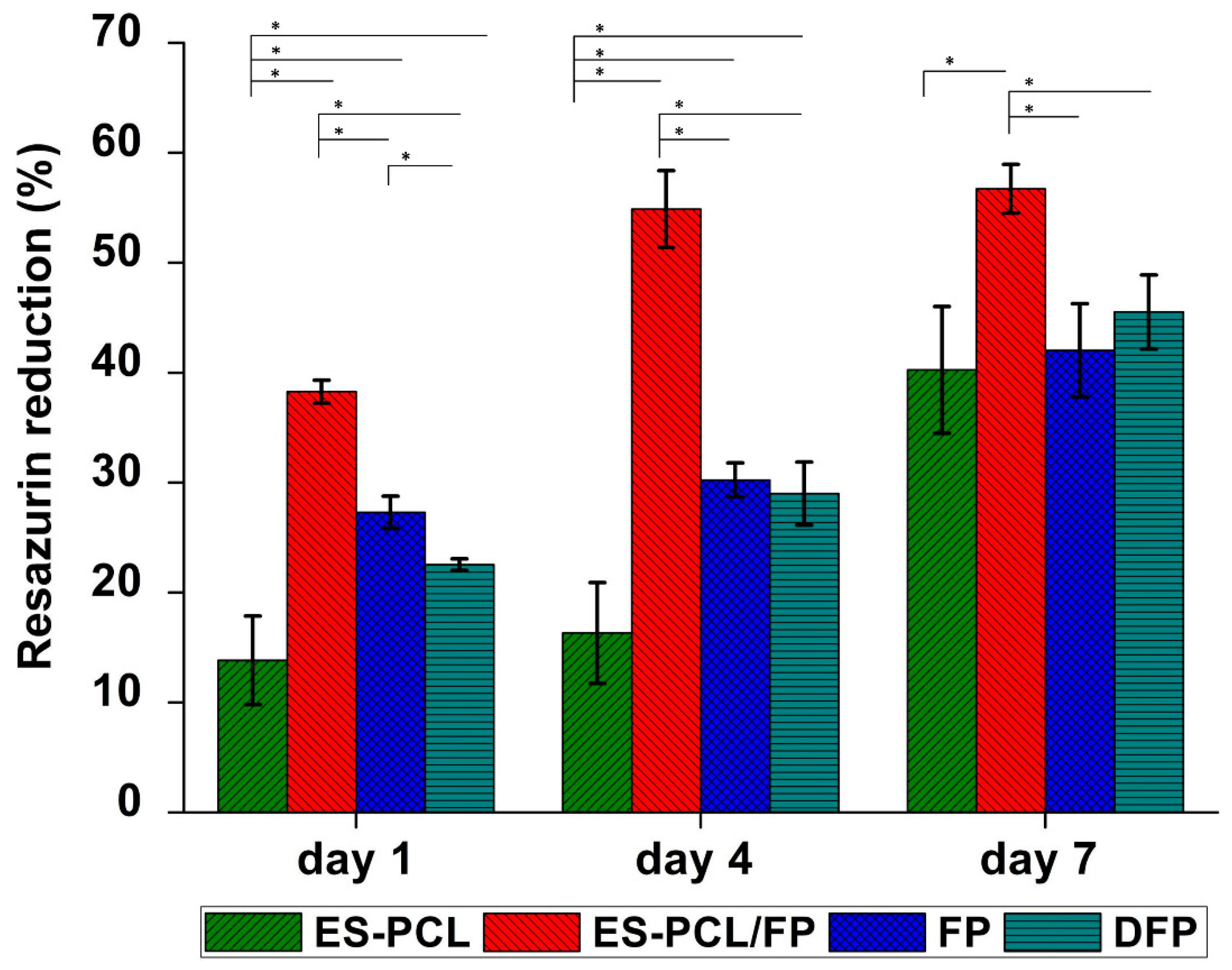
2.8.1. Resazurin Reduction Assay
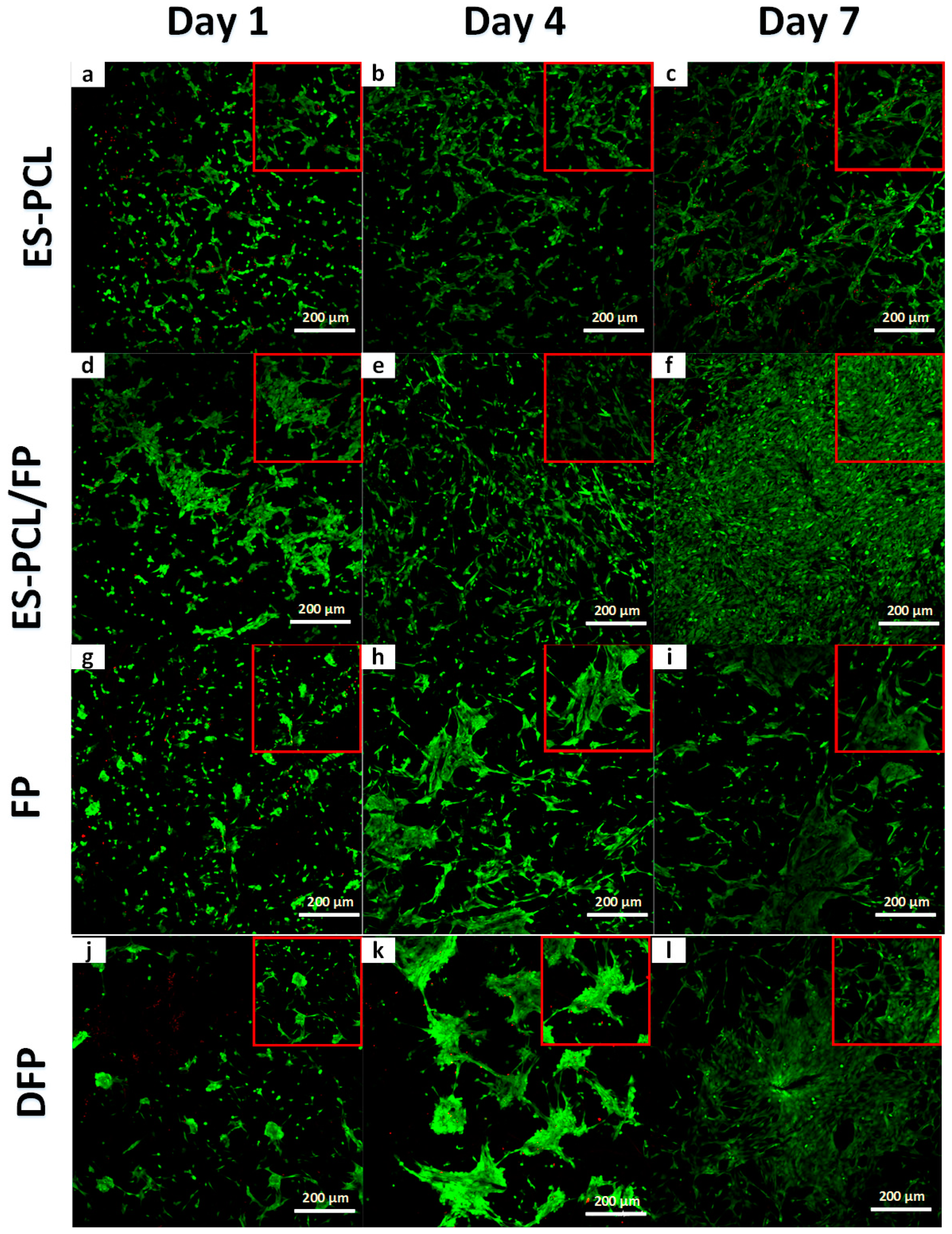
2.8.2. Live/Dead Confocal
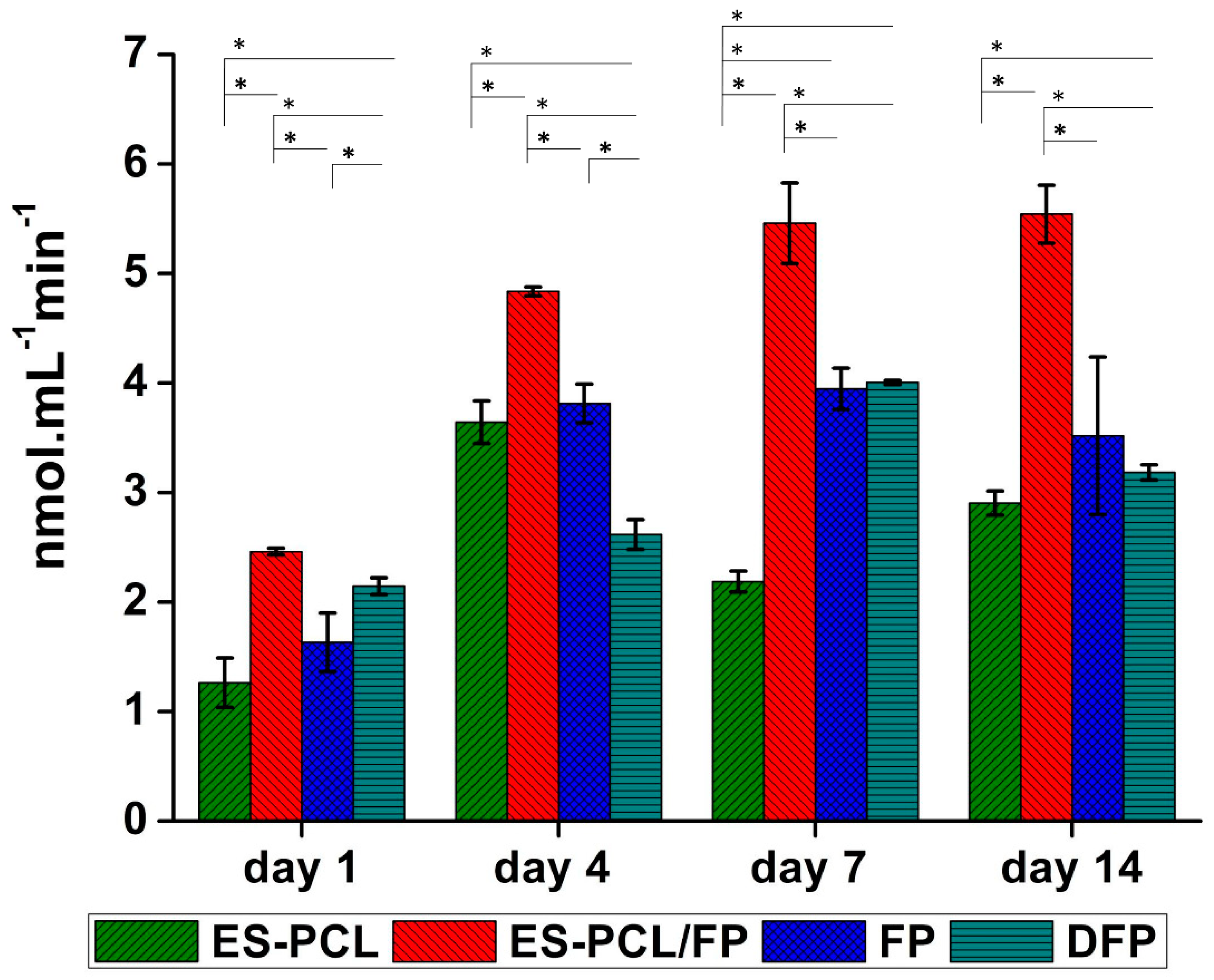
2.8.3. Alkaline Phosphatase (ALP) Assay
2.8.4. Cell Fixation for FESEM
2.9. Statistical Analysis
3. Results and Discussion
3.1. Scaffold Morphological Analysis
3.2. Cell-Scaffold Interactions
3.3. Cell Proliferation Assay
3.4. Live/Dead Assay
3.5. Alkaline Phosphatase (ALP) Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Qing, H.B.; Li, Z.D.; Han, Y.L.; Lin, M.; Yang, H.; Li, A.; Lu, T.J.; Li, F.; Xu, F. Paper: A promising material for human-friendly functional wearable electronics. Mater. Sci. Eng. R-Rep. 2017, 112, 1–22. [Google Scholar] [CrossRef]
- Cheng, M.L.; Lin, C.C.; Su, H.L.; Chen, P.Y.; Sun, Y.M. Processing and characterization of electrospun poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) nanofibrous membranes. Polymer 2008, 49, 546–553. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. Engl. 2007, 46, 1318–1320. [Google Scholar] [CrossRef]
- Hong, B.; Xue, P.; Wu, Y.; Bao, J.; Chuah, Y.J.; Kang, Y. A concentration gradient generator on a paper-based microfluidic chip coupled with cell culture microarray for high-throughput drug screening. Biomed. Microdevices 2016, 18, 21. [Google Scholar] [CrossRef]
- Derda, R.; Laromaine, A.; Mammoto, A.; Tang, S.K.; Mammoto, T.; Ingber, D.E.; Whitesides, G.M. Paper-supported 3D cell culture for tissue-based bioassays. Proc. Natl. Acad. Sci. USA 2009, 106, 18457–18462. [Google Scholar] [CrossRef]
- Derda, R.; Tang, S.K.; Laromaine, A.; Mosadegh, B.; Hong, E.; Mwangi, M.; Mammoto, A.; Ingber, D.E.; Whitesides, G.M. Multizone paper platform for 3D cell cultures. PLoS ONE 2011, 6, e18940. [Google Scholar] [CrossRef]
- Mosadegh, B.; Dabiri, B.E.; Lockett, M.R.; Derda, R.; Campbell, P.; Parker, K.K.; Whitesides, G.M. Three-dimensional paper-based model for cardiac ischemia. Adv. Healthc. Mater. 2014, 3, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Yu, S.J.; Yang, K.; Jin, Y.; Cho, A.N.; Kim, J.; Lee, B.; Yang, H.S.; Im, S.G.; Cho, S.W. Paper-based bioactive scaffolds for stem cell-mediated bone tissue engineering. Biomaterials 2014, 35, 9811–9823. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Gao, B.; Yong, K.W.; Li, Y.H.; Shi, M.; Zhao, X.; Li, Z.D.; Zhang, X.H.; Pingguan-Murphy, B.; Yang, H.; et al. Paper-based cell culture platform and its emerging biomedical applications. Mater. Today 2017, 20, 32–44. [Google Scholar] [CrossRef]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Diban, N.; Stamatialis, D.F. Functional Polymer Scaffolds for Blood Vessel Tissue Engineering. Adv. Polym. Med. 2011, 309–310, 93–99. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Suwantong, O. Biomedical applications of electrospun polycaprolactone fiber mats. Polym. Adv. Technol. 2016, 27, 1264–1273. [Google Scholar] [CrossRef]
- Qi, A.; Hoo, S.P.; Friend, J.; Yeo, L.; Yue, Z.; Chan, P.P. Hydroxypropyl cellulose methacrylate as a photo-patternable and biodegradable hybrid paper substrate for cell culture and other bioapplications. Adv. Healthc. Mater. 2014, 3, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Sepulveda, G.; Aguiar, T.Q.; Gama, F.M.; Domingues, L. Modification of paper properties using carbohydrate-binding module 3 from the Clostridium thermocellum CipA scaffolding protein produced in Pichia pastoris: Elucidation of the glycosylation effect. Cellulose 2015, 22, 2755–2765. [Google Scholar] [CrossRef]
- Levy, I.; Nussinovitch, A.; Shpigel, E.; Shoseyov, O. Recombinant cellulose crosslinking protein: A novel paper-modification biomaterial. Cellulose 2002, 9, 91–98. [Google Scholar] [CrossRef]
- Juvonen, H.; Maattanen, A.; Lauren, P.; Ihalainen, P.; Urtti, A.; Yliperttula, M.; Peltonen, J. Biocompatibility of printed paper-based arrays for 2-D cell cultures. Acta Biomater. 2013, 9, 6704–6710. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Ray, R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 2001, 55, 141–150. [Google Scholar] [CrossRef]
- Ong, J.L.; Guda, T. Translating Biomaterials for Bone Graft: Bench-top to Clinical Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Azari, P.; Hosseini, S.; Murphy, B.P.; Martinez-Chapa, S.O. Electrospun Biopolyesters: Hydrophobic Scaffolds With Favorable Biological Response. J. Public Health Int. 2018, 1, 5–9. [Google Scholar] [CrossRef]
- Aghdam, R.M.; Shakhesi, S.; Najarian, S.; Mohammadi, M.M.; Tafti, S.H.A.; Mirzadeh, H. Fabrication of a Nanofibrous Scaffold for the In Vitro Culture of Cardiac Progenitor Cells for Myocardial Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 229–239. [Google Scholar] [CrossRef]
- Christopherson, G.T.; Song, H.; Mao, H.Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef]
- Dettin, M.; Zamuner, A.; Roso, M.; Gloria, A.; Iucci, G.; Messina, G.M.; D’Amora, U.; Marletta, G.; Modesti, M.; Castagliuolo, I.; et al. Electrospun Scaffolds for Osteoblast Cells: Peptide-Induced Concentration-Dependent Improvements of Polycaprolactone. PLoS ONE 2015, 10, e0137505. [Google Scholar] [CrossRef] [PubMed]
- Nune, M.; Kumaraswamy, P.; Krishnan, U.M.; Sethuraman, S. Self-assembling peptide nanofibrous scaffolds for tissue engineering: Novel approaches and strategies for effective functional regeneration. Curr. Protein Pept. Sci. 2013, 14, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Azari, P.; Luan, N.S.; Gan, S.N.; Yahya, R.; Wong, C.S.; Chua, K.H.; Pingguan-Murphy, B. Electrospun Biopolyesters as Drug Screening Platforms for Corneal Keratocytes. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 785–791. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Yew, C.T.; Azari, P.; Choi, J.R.; Li, F.; Pingguan-Murphy, B. Electrospin-coating of nitrocellulose membrane enhances sensitivity in nucleic acid-based lateral flow assay. Anal. Chim. Acta 2018, 1009, 81–88. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.; Dalton, P.; Hutmacher, D. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Webb, A.; Clark, P.; Skepper, J.; Compston, A.; Wood, A. Guidance of Oligodendrocytes and Their Progenitors by Substratum Topography. J. Cell Sci. 1995, 108, 2747–2760. [Google Scholar]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Brouki Milan, P.; Kim, H.-W.; Baino, F. Bone Tissue Engineering Using Human Cells: A Comprehensive Review on Recent Trends, Current Prospects, and Recommendations. Appl. Sci. 2019, 9, 174. [Google Scholar] [CrossRef]
- Hoffman, T.; Khademhosseini, A.; Langer, R.S. Chasing the Paradigm: Clinical Translation of 25 Years of Tissue Engineering. Tissue Eng. 2019. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2019, 64, 91–126. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Tomaskovic-Crook, E.; Crook, J.M.; Wallace, G.G. Smart graphene-cellulose paper for 2D or 3D “origami-inspired” human stem cell support and differentiation. Colloids Surf. B Biointerfaces 2019, 176, 87–95. [Google Scholar] [CrossRef]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—in vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef]
- Polak, S. In vitro to human in vivo translation–pharmacokinetics and pharmacodynamics of quinidine. ALTEX-Altern. Anim. Exp. 2013, 30, 309–318. [Google Scholar]
- Yew, C.H.T.; Azari, P.; Choi, J.R.; Muhamad, F.; Pingguan-Murphy, B. Electrospun Polycaprolactone Nanofibers as a Reaction Membrane for Lateral Flow Assay. Polymers 2018, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Hsieh, Y.L. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci. Part B-Polym. Phys. 2002, 40, 2119–2129. [Google Scholar] [CrossRef]
- Wannatong, L.; Sirivat, A.; Supaphol, P. Effects of solvents on electrospun polymeric fibers: Preliminary study on polystyrene. Polym. Int. 2004, 53, 1851–1859. [Google Scholar] [CrossRef]
- You, Y.; Won Lee, S.; Jin Lee, S.; Park, W.H. Thermal interfiber bonding of electrospun poly(l-lactic acid) nanofibers. Mater. Lett. 2006, 60, 1331–1333. [Google Scholar] [CrossRef]
- Wei, X.; Xia, Z.; Wong, S.-C.; Baji, A.; Biomechanics, C. Modelling of mechanical properties of electrospun nanofibre network. Int. J. Exp. Comput. Biomech. 2009, 1, 45–57. [Google Scholar] [CrossRef]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H. Morphology, function, and differentiation of bone cells. J. Hard Tissue Biol. 2007, 16, 15–22. [Google Scholar] [CrossRef]
- Sjostrom, T.; Lalev, G.; Mansell, J.P.; Su, B. Initial attachment and spreading of MG63 cells on nanopatterned titanium surfaces via through-mask anodization. Appl. Surf. Sci. 2011, 257, 4552–4558. [Google Scholar] [CrossRef]


| Scaffold Properties | ES-PCL | ES-PCl/FP | FP | DFP |
|---|---|---|---|---|
| Tensile Strength (MPa) | 1.68 ± 0.23 | 5.80 ± 0.32 | 3.52 ± 0.38 | 3.54 ± 0.17 |
| Porosity (%) | 66.71 ± 2.65 | 25.26 ± 1.60 | 7.55 ± 2.76 | 3.23 ± 0.59 |
| Pore size (µm) | 13.11 ± 1.07 | 5.42 ± 0.22 | 92.13 ± 11.98 | 73.67 ± 17.00 |
| Thickness (µm) | 232.07 ± 11.59 | 258.78 ± 9.03 | 209.09 ± 0.50 | 246.87 ± 21.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, K.; Azari, P.; Nam, H.Y.; Xu, F.; Pingguan-Murphy, B. Electrospin-Coating of Paper: A Natural Extracellular Matrix Inspired Design of Scaffold. Polymers 2019, 11, 650. https://doi.org/10.3390/polym11040650
Ng K, Azari P, Nam HY, Xu F, Pingguan-Murphy B. Electrospin-Coating of Paper: A Natural Extracellular Matrix Inspired Design of Scaffold. Polymers. 2019; 11(4):650. https://doi.org/10.3390/polym11040650
Chicago/Turabian StyleNg, Kelvin, Pedram Azari, Hui Yin Nam, Feng Xu, and Belinda Pingguan-Murphy. 2019. "Electrospin-Coating of Paper: A Natural Extracellular Matrix Inspired Design of Scaffold" Polymers 11, no. 4: 650. https://doi.org/10.3390/polym11040650





