Core-Shell Structured HMX@Polydopamine Energetic Microspheres: Synergistically Enhanced Mechanical, Thermal, and Safety Performances
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparations
2.3. Material Characterizations
3. Results and Discussion
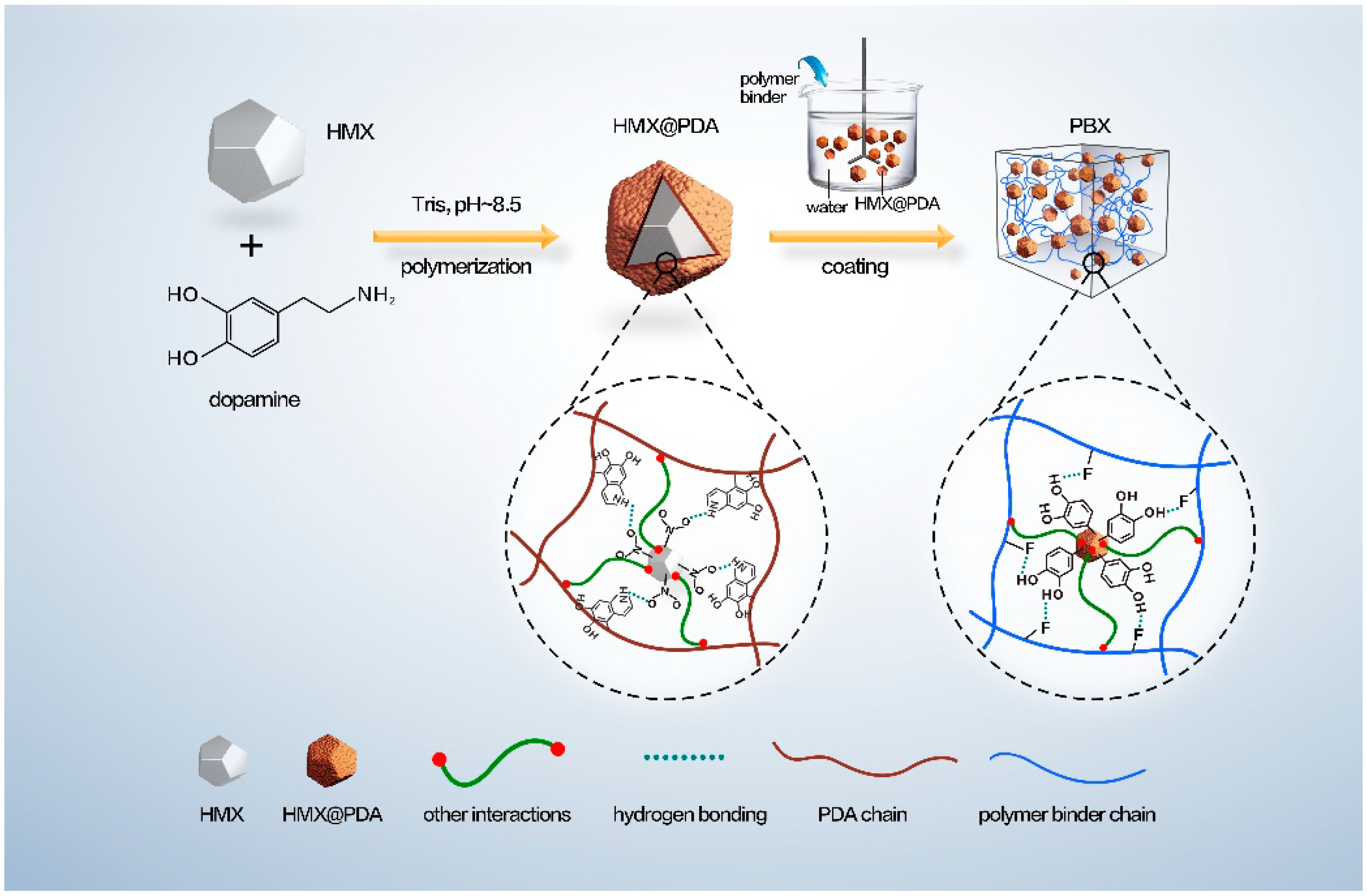
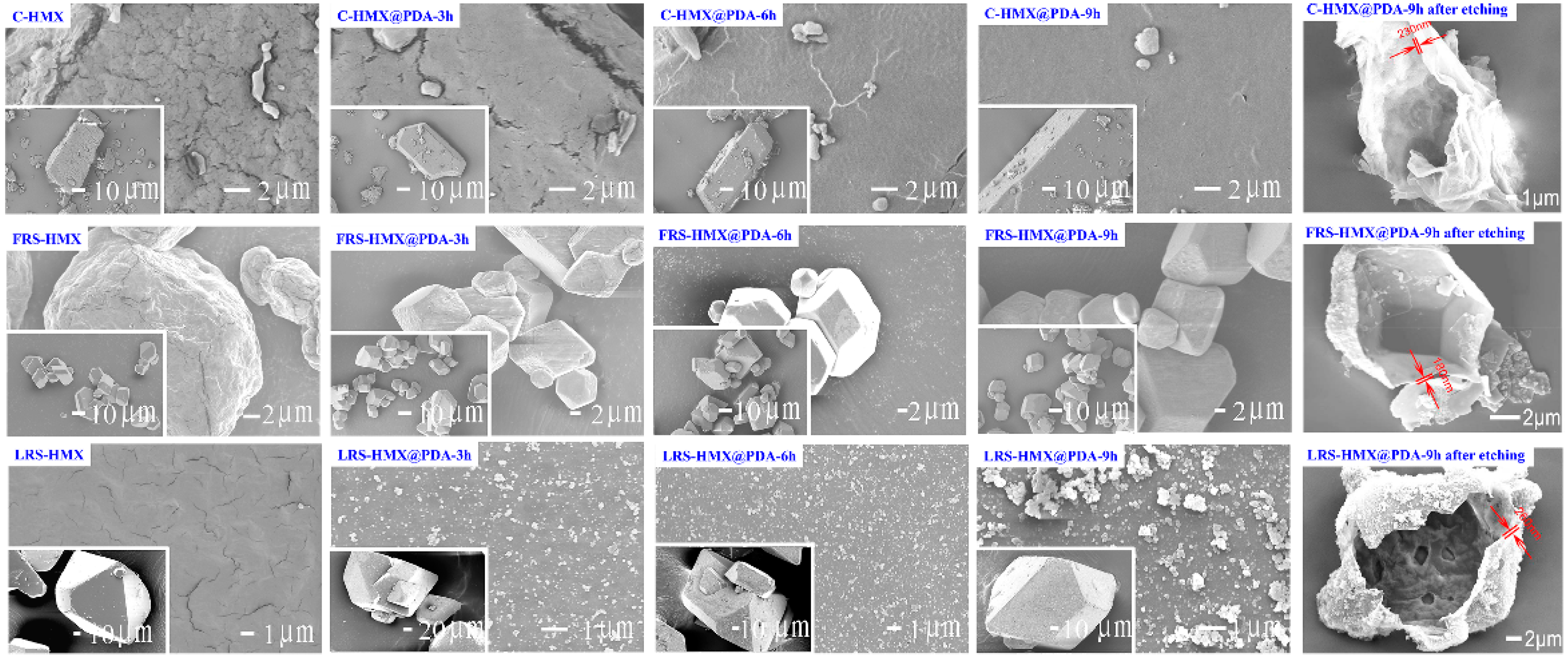
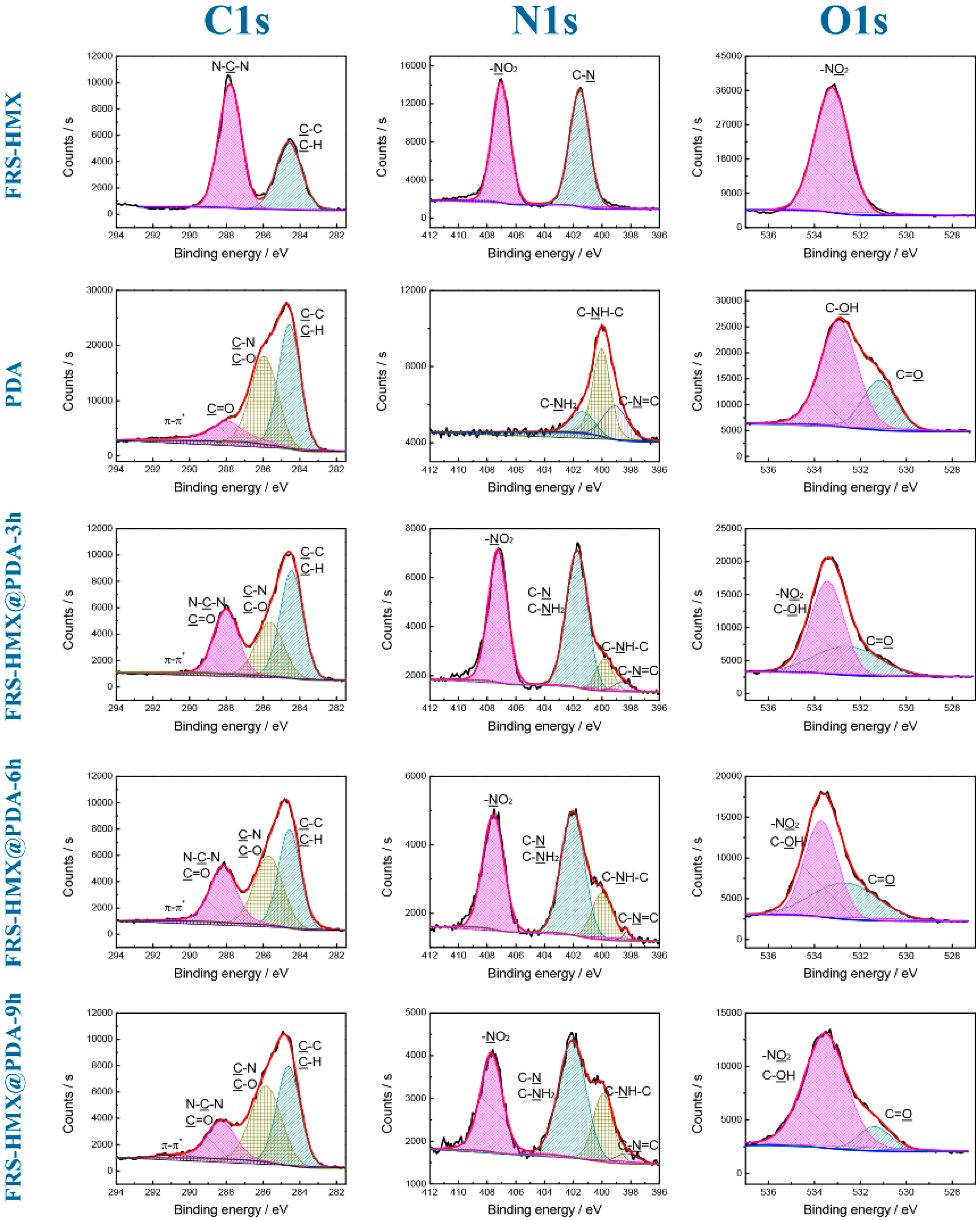
3.1. Morphological and Structural Features of Core-Shell HMX@PDA Particles
3.2. Detonation Properties of PBXs Based on HMX@Polydopamine
3.3. Mechanical Properties of PBXs Based on HMX@Polydopamine
3.4. Mechanism for the Enhancement of Mechanical Properties
3.5. Thermal Properties of PBXs Based on HMX@Polydopamine
3.6. Sensitivity Study of PBXs Based on HMX@Polydopamine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yan, Q.L.; Zeman, S.; Elbeih, A. Recent advances in thermal analysis and stability evaluation of insensitive plastic bonded explosives (PBXs). Thermochim. Acta 2012, 537, 1–12. [Google Scholar] [CrossRef]
- Li, Y.B.; Yang, Z.J.; Zhang, J.H.; Pan, L.P.; Ding, L.; Tian, X.; Zheng, X.; Gong, F.Y. Fabrication and characterization of HMX@TPEE energetic microspheres with reduced sensitivity and superior toughness properties. Compos. Sci. Technol. 2017, 142, 253–263. [Google Scholar] [CrossRef]
- Lin, C.M.; Liu, J.H.; He, G.S.; Yang, Z.J.; Pan, L.P.; Liu, S.J.; Li, J.; Guo, S.Y. Effect of crystal quality and particle size of HMX on the creep resistance for TATB/HMX composites. Propellants Explos. Pyrotech. 2017, 42, 1410–1417. [Google Scholar] [CrossRef]
- Wei, C.X.; Huang, H.; Duan, X.H.; Pei, C.H. Structures and properties prediction of HMX/TATB co-crystal. Propellants Explos. Pyrotech. 2011, 36, 416–423. [Google Scholar] [CrossRef]
- Huang, B.; Hao, X.F.; Zhang, H.B.; Yang, Z.J.; Ma, Z.G.; Li, H.Z.; Nie, F.D.; Huang, H. Ultrasonic approach to the synthesis of HMX@TATB core-shell microparticles with improved mechanical sensitivity. Ultrason. Sonochem. 2014, 21, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.G.; Gao, B.; Wu, P.; Shi, J.C.; Qiao, Z.Q.; Yang, Z.J.; Yang, G.C.; Huang, B.; Nie, F.D. Facile, continuous and large-scale production of core-shell HMX@TATB composites with superior mechanical properties by a spray-drying process. RSC Adv. 2015, 5, 21042–21049. [Google Scholar] [CrossRef]
- Yang, Z.J.; Ding, L.; Wu, P.; Liu, Y.G.; Nie, F.D.; Huang, F.L. Fabrication of RDX, HMX and CL-20 based microcapsules via in situ polymerization of melamineformaldehyde resins with reduced sensitivity. Chem. Eng. J. 2015, 268, 60–66. [Google Scholar] [CrossRef]
- Xue, C.; Sun, J.; Kang, B.; Liu, Y.; Liu, X.; Song, G.; Xue, Q. The β-δ phase transition and thermal expansion of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine. Propellants Explos. Pyrotech. 2010, 35, 333–338. [Google Scholar] [CrossRef]
- Willey, T.M.; Lauderbach, L.; Gagliardi, F.; Buuren, T.; Glascoe, E.A.; Tringe, J.W.; Lee, J.R.I.; Springer, H.K.; Ilavsky, J. Mesoscale evolution of voids and microstructural changes in HMX-based explosives during heating through the β-δ phase transition. J. Appl. Phys. 2015, 118, 055901. [Google Scholar] [CrossRef] [Green Version]
- Risse, B.; Schnell, F.; Spitzer, D. Synthesis and desensitization of nano-beta-HMX. Propellants Explos. Pyrotech. 2014, 39, 397–401. [Google Scholar] [CrossRef]
- Urtiew, P.A.; Forbes, J.W.; Tarver, C.M.; Vandersall, K.S.; Garcia, F.; Greenwood, D.W.; Hsu, P.C.; Maienschein, J.L. Shock sensitivity of LX-04 containing delta phase HMX at elevated temperatures. AIP Conf. Proc. 2004, 706, 1053–1056. [Google Scholar]
- Yang, Z.J.; Li, J.S.; Huang, B.; Liu, S.J.; Huang, Z.; Nie, F.D. Preparation and properties study of core-shell CL-20/TATB composites. Propellants Explos. Pyrotech. 2014, 39, 51–58. [Google Scholar] [CrossRef]
- Dai, X.G.; Xu, J.J.; Wen, Y.S.; Li, Y.B.; Huang, F.L.; Li, M.; Zeng, Q. Delay mechanism of β-δ phase transition of cyclotetramethylene tetranitramine in polymer bonded explosive composites by heat conduction obstacle. Propellants Explos. Pyrotech. 2016, 41, 637–640. [Google Scholar] [CrossRef]
- Guo, C.P.; Wang, D.J.; Gao, B.; Wang, J.; Luo, B.; Yang, G.C.; Nie, F.D. Solid-solid phase transition study of ε-CL-20/binder composites. RSC Adv. 2016, 6, 859–865. [Google Scholar] [CrossRef]
- Yeager, J.D.; Dubey, M.; Wolverton, M.J.; Jablin, M.S.; Majewski, J.; Bahr, D.F.; Hooks, D.E. Examining chemical structure at the interface between a polymer binder and a pharmaceutical crystal with neutron reflectometry. Polymer 2011, 52, 3762–3768. [Google Scholar] [CrossRef]
- Rae, P.J.; Palmer, S.J.P.; Goldrein, H.T.; Field, J.E.; Lewis, A.L. Quasi-static studies of the deformation and failure of PBX-9501. Proc. R. Soc. A 2002, 458, 2227–2242. [Google Scholar] [CrossRef]
- Liu, J.H.; Liu, S.J.; Chen, L.L.; Lin, C.M.; Gong, F.Y.; Nie, F.D. Improving mechanical property of HMX-based PBX with neutral polymer bonding agent. In Proceedings of the 45nd Interational Annual Conference of the Fraunhofer ICT, Karlsruhe, Germany, 24–27 June 2014; pp. 56/1–56/8. [Google Scholar]
- Ma, F.G.; Wu, W.H.; Tan, H.M. Coating modification of nirtramine HMX by polymerization and its application. J. Beijing Inst. Technol. 2000, 20, 389–393. [Google Scholar]
- Liu, J.H.; Li, H.Z.; Huang, B.; Ding, L.; Liu, S.J.; Liu, Y.G. The effects of HMX@TATB core-shell composites on the mechanical properties of PBX. New Trends Res. Energetic Mater. Czech Republic 2016, 19, 154–159. [Google Scholar]
- Postma, A.; Yan, Y.; Wang, Y.; Zelikin, A.N.; Tjipto, E.; Carus, F. Self-polymerization of dopamine as a versatile and robust technique to prepare polymer capsules. Chem. Mater. 2009, 21, 3042–3044. [Google Scholar] [CrossRef]
- Zhang, J.; Hwang, J.; Antonietti, M.; Schmidt, B.V.K.J. Water-in-water pickering emulsion stabilized by polydopamine particles and cross-linking. Biomacromolecules 2019, 20, 204–211. [Google Scholar] [CrossRef]
- Shang, K.; Song, S.; Cheng, Y.; Guo, L.; Pei, Y.; Lv, X.; Aastrup, T.; Pei, Z. Fabrication of carbohydrate chips based on polydopamine for real-time determination of carbohydrate–lectin interactions by QCM biosensor. Polymers 2018, 10, 1275. [Google Scholar] [CrossRef]
- Guo, J.; Tardy, B.L.; Christofferson, A.J.; Dai, Y.; Richardson, J.J.; Zhu, W.; Hu, M.; Ju, Y.; Cui, J.; Dagastine, R.R.; et al. Modular assembly of superstructures from polyphenol-functionalized building blocks. Nat. Nanotechnol. 2016, 11, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Wang, C.; Zhang, J.; Wang, W.; Xiao, L.; Jia, S.; Wang, Z.; Wu, W.; Xiao, J. New insight into polydopamine@ZIF-8 nanohybrids: A zinc-releasing container for potential anticancer activity. Polymers 2018, 10, 476. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.E.; Chen, X.X.; Cao, J.; Zhao, Y.D.; Neale, Z.G.; Cai, W.; Sui, J.H.; Cao, G.Z. Tubular MoO2 organized by two-dimensional assemblies for fast and durable alkali-ion storage. Energy Storage Mater. 2018, 11, 161–169. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Nie, C.; Yang, Y.; Cheng, C.; Ma, L.; Deng, J.; Wang, L.; Zhao, C. Bioinspired and biocompatible carbon nanotube-Ag nanohybrid coatings for robust antibacterial applications. Acta Biomater. 2017, 51, 479–494. [Google Scholar] [CrossRef]
- Nie, C.; Cheng, C.; Peng, Z.; Ma, L.; He, C.; Xia, Y.; Zhao, C. Mussel-inspired coatings on Ag nanoparticle-conjugated carbon nanotubes: Bactericidal activity and mammal cell toxicity. J. Mater. Chem. B 2016, 4, 2749–2756. [Google Scholar] [CrossRef]
- Gong, F.Y.; Zhang, J.H.; Ding, L.; Yang, Z.J.; Liu, X.B. Mussel-inspired coating of energetic crystals: A compact core-shell structure with highly enhanced thermal stability. Chem. Eng. J. 2017, 309, 140–150. [Google Scholar] [CrossRef]
- Zhu, Q.; Xiao, C.; Li, S.B.; Luo, G. Bioinspired fabrication of insensitive HMX particles with polydopamine coating. Propellants Explos. Pyrotech. 2016, 41, 1092–1097. [Google Scholar] [CrossRef]
- Lin, C.M.; Gong, F.Y.; Yang, Z.J.; Pan, L.P.; Liu, S.J.; Li, J.; Guo, S.Y. Bio-inspired fabrication of core-shell structured TATB/polydopamine microparticles via in situ polymerization with tunable mechanical properties. Polym. Test. 2018, 68, 126–134. [Google Scholar] [CrossRef]
- National Military Standard of China. Experimental Methods of Sensitivity and Safety; GJB/772A-97; National Defense Science Technology and Industry Comission Press: Beijing, China, 1997. (In Chinese) [Google Scholar]
- Zhao, X.; Cai, W.; Yang, Y.; Song, X.; Neale, Z.; Wang, H.; Sui, J.; Cao, G. MoSe2 nanosheets perpendicularly grown on graphene with Mo-C bonding for Sodium-ion capacitors. Nano Energy 2018, 47, 224–234. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Yu, X.; Fan, H.; Liu, Y.; Shi, Z.; Jin, Z. Characterization of carbonized polydopamine nanoparticles suggests ordered supramolecular structure of polydopamine. Langmuir 2014, 30, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Soni, P.; Sarkar, C.; Tewari, R.; Sharma, T.D. HMX polymorphs: Gamma to beta phase transformation. J. Energy Mater. 2011, 29, 261–279. [Google Scholar] [CrossRef]
- Jiang, J.H.; Zhu, L.P.; Zhu, L.J.; Zhu, B.K.; Xu, Y.Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- Li, M.; Huang, M.; Kang, B.; Wen, M.P.; Li, H.Z.; Xu, R. Quality evaluation of RDX crystalline particles by confined quasi-static compression method. Propellants Explos. Pyrotech. 2007, 32, 401–405. [Google Scholar]
- Lin, C.M.; Liu, S.J.; Huang, Z.; He, G.S.; Gong, F.Y.; Liu, Y.G.; Liu, J.H. The dependence of the non-linear creep properties of TATB-based polymer bonded explosives on the molecular structure of the polymer binder: (II) effects of the comonomer ratio in fluoropolymers. RSC Adv. 2015, 5, 59804–59811. [Google Scholar] [CrossRef]
- He, G.S.; Yang, Z.J.; Pan, L.P.; Zhang, J.H.; Liu, S.J.; Yan, Q.L. Bioinspired interfacial reinforcement of polymerbased energetic composites with a high loading of solid explosive crystals. J. Mater. Chem. A 2017, 5, 13499–13510. [Google Scholar] [CrossRef]
- Feng, J.; Venna, S.R.; Hopkinson, D.P. Interactions at the interface of polymer matrix-filler particle composites. Polymer 2016, 103, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Dartyge, B.F. Influence of crystal defects on sensitivity of explosives. In Proceedings of the 10th International Detonation Symposium, Boston, MA, USA, 12–16 June 1993; p. 816. [Google Scholar]
- Asay, B.W.; Dickson, P.M.; Henson, B.; Fugard, C.; Funk, D.J.; Idar, D.J. Dynamic measurement of the influence of projectile radius and velocity on strain localization during impact of an energetic material. In Proceedings of the 11th International Detonation Symposium, Snowmass Village, CO, USA, 31 August–4 September 1998; pp. 781–787. [Google Scholar]
- Bazaki, H.; Kubota, N. Friction sensitivity mechanism of ammonium perchlorate composite propellants. Propellants Explos. Pyrotech. 1991, 16, 43–47. [Google Scholar] [CrossRef]



| Sample | Impact Sensitivity [%] | Friction Sensitivity [%] |
|---|---|---|
| PBX-C | 96 | 84 |
| PBX-C-9h | 100 | 40 |
| PBX-F | 100 | 32 |
| PBX-F-9h | 100 | 28 |
| PBX-L | 100 | 100 |
| PBX-L-9h | 92 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.; Gong, F.; Yang, Z.; Zhao, X.; Li, Y.; Zeng, C.; Li, J.; Guo, S. Core-Shell Structured HMX@Polydopamine Energetic Microspheres: Synergistically Enhanced Mechanical, Thermal, and Safety Performances. Polymers 2019, 11, 568. https://doi.org/10.3390/polym11030568
Lin C, Gong F, Yang Z, Zhao X, Li Y, Zeng C, Li J, Guo S. Core-Shell Structured HMX@Polydopamine Energetic Microspheres: Synergistically Enhanced Mechanical, Thermal, and Safety Performances. Polymers. 2019; 11(3):568. https://doi.org/10.3390/polym11030568
Chicago/Turabian StyleLin, Congmei, Feiyan Gong, Zhijian Yang, Xu Zhao, Yubin Li, Chengcheng Zeng, Jiang Li, and Shaoyun Guo. 2019. "Core-Shell Structured HMX@Polydopamine Energetic Microspheres: Synergistically Enhanced Mechanical, Thermal, and Safety Performances" Polymers 11, no. 3: 568. https://doi.org/10.3390/polym11030568





