Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, III: Extended Study on Effect of Catalyst Type on Molecular Weight Increase
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of 2,5-Dimethylfuran-dicarboxylate(DMFD)
2.3. Polyester Synthesis
2.4. Solid-State Polycondensation
2.5. Polyester Characterization
2.5.1. Intrinsic Viscosity Measurement
2.5.2. Molecular weight
2.5.3. End-Group Analysis
2.5.4. Wide Angle X-Ray Diffraction Patterns (WAXD)
2.5.5. Differential Scanning Calorimetry (DSC)
3. Modeling the PEF SSP Kinetics
3.1. Reaction Mechanism




3.2. Simplified Mathematical Model
- -
- All kinetic rate constants are regarded independent of polymer chain length (only end-group reactivity is considered).
- -
- The backward reactions in Equations (3) and (4) are eliminated. This is due to the very fast removal of the ethylene glycol and water produced in the reaction mixture, caused by the high vacuum application (beneath 3 and 4 Pa).
- -
- Since polycondensation occurs at relatively low temperatures (i.e., 190–205 °C), no side reactions for the formation of acetaldehyde or thermal degradation are considered (Equations (5) and (6) are excluded).
- -
- Diffusional limitations on account of desorbing volatile species are ignored.
- -
4. Results and Discussion
4.1. Kinetic Study of the Solid-State Polymerization of PEF
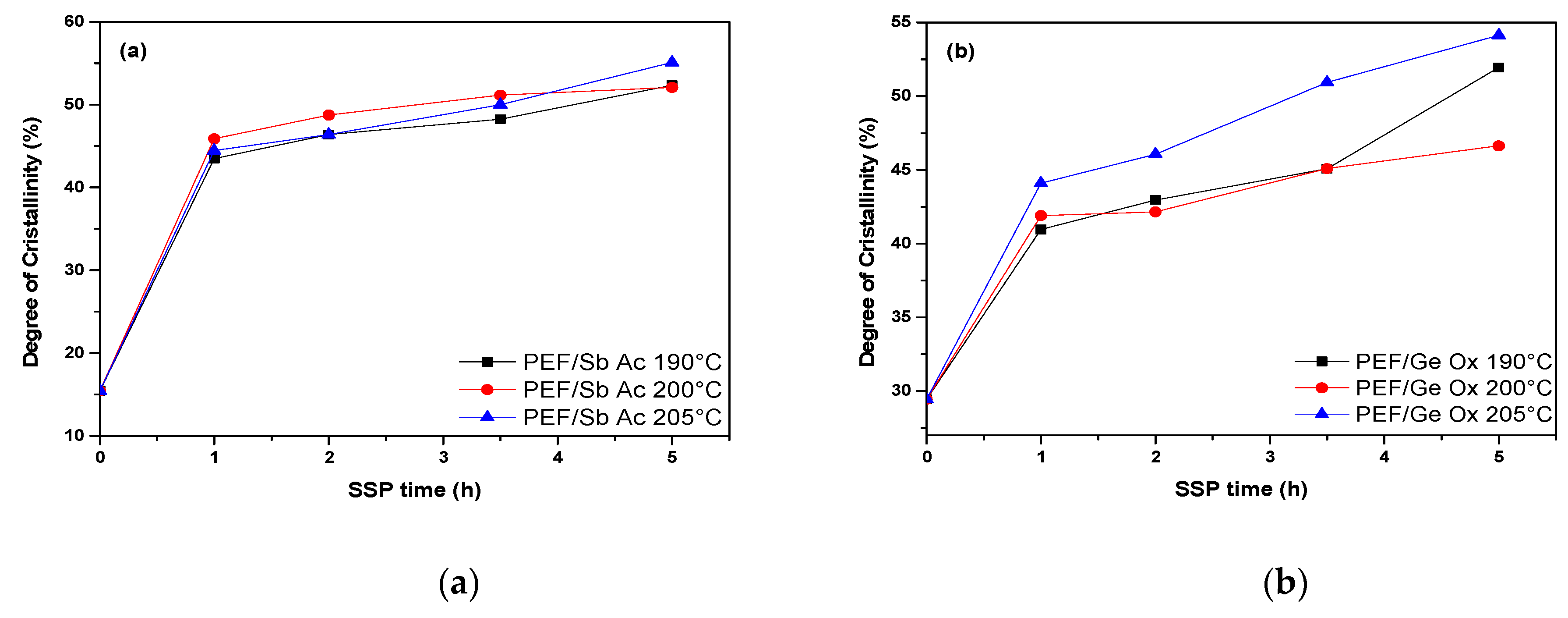
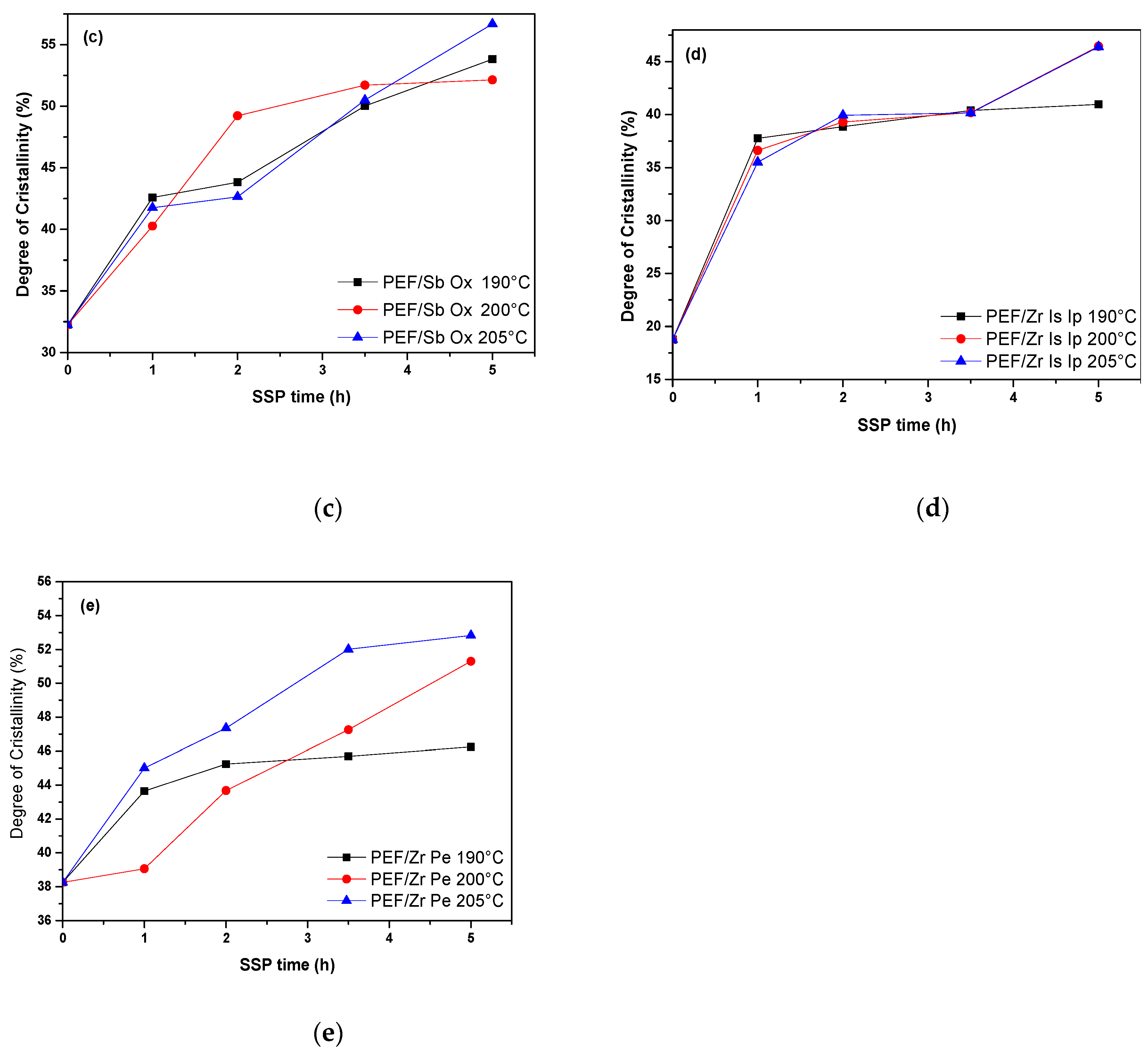
4.2. Thermal Analysis of PEF Polyester Samples Prepared by Solid-State Polymerization
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Gandini, A. Polymers from Renewable Resources: A Challenge for the Future of Macromolecular Materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Walther, G. High-Performance Polymers from Nature: Catalytic Routes and Processes for Industry. ChemSusChem. 2014, 7, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.F.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef] [PubMed]
- Llevot, A.; Dannecker, P.K.; Von Czapiewski, M.; Over, L.C.; Söyler, Z.; Meier, M.A.R. Renewability is not Enough: Recent Advances in the Sustainable Synthesis of Biomass-Derived Monomers and Polymers. Chem. Eur. J. 2016, 22, 11510–11521. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T. Cheminform abstract: Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- BioPreferred—Directives for the Mandatory Purchasing of Biobased Products. Available online: http://www.biopreferred.gov/BioPreferred/faces/pages/AboutBioPreferred.xhtml (accessed on 19 January 2019).
- Industrial Sustainability—European Commission. Available online: http://ec.europa.eu/growth/industry/sustainability/index_en.htm (accessed on 19 January 2019).
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics—A review. J Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Kamm, B.; Kamm, M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2004, 64, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Nai, L.; Zheng, Y.; Wei, L.; Teng, H.; Zhou, J. Metal nanoparticles supported on WO3 nanosheets for highly selective hydrogenolysis of cellulose to ethylene glycol. Green Chem. 2017, 19, 682–691. [Google Scholar] [CrossRef]
- Rizescu, C.; Podolean, I.; Albero, J.; Parvulescu, V.I.; Coman, S.M.; Bucur, C.; Puchec, M.; Garcia, H. N-Doped graphene as a metal-free catalyst for glucose oxidation to succinic acid. Green Chem. 2017, 19, 1999–2005. [Google Scholar] [CrossRef]
- Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.J. Furandicarboxylic acid (FDCA), a versatile building block for a very interesting class of polyesters. ACS Symp. Ser. 2012, 1105, 1–13. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of bio-based products from 28 biorefinery carbohydrates-the US department of energy′s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Vannini, M.; Marchese, P.; Celli, A.; Lorenzetti, C. Fully biobased poly(propylene 2,5-furandicarboxylate) for packaging applications: Excellent barrier properties as a function of crystallinity. Green Chem. 2015, 17, 4162–4166. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Terzopoulou, Z.; Nerantzaki, M.; Papageorgiou, G.Z.; Bikiaris, D.N. New poly(pentylene furanoate) and poly(heptylene furanoate) sustainable polyesters from diols with odd methylene groups. Mater. Lett. 2016, 178, 64–67. [Google Scholar] [CrossRef]
- Genovese, L.; Lotti, N.; Siracusa, V.; Munari, A. Poly(Neopentyl glycol furanoate): A member of the furan-based polyester family with smart barrier performances for sustainable food packaging applications. Materials 2017, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Guigo, N.; Tsanaktsis, V.; Exarhopoulos, S.; Bikiaris, D.N.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Fast crystallization and melting behavior of a long-spaced aliphatic furandicarboxylate bio-based polyester, the poly(dodecylene 2,5-furanoate). Ind. Eng. Chem. Res. 2016, 55, 5315–5326. [Google Scholar] [CrossRef]
- Soccio, M.; Martínez-Tong, D.E.; Alegría, A.; Munari, A.; Lotti, N. Molecular dynamics of fully biobased poly(butylene 2,5-furanoate) as revealed by broadband dielectric spectroscopy. Polymer 2017, 128, 24–30. [Google Scholar] [CrossRef]
- Soares, M.J.; Dannecker, P.-K.; Vilela, C.; Bastos, J.; Meier, M.A.R.; Sousa, A.F. Poly(1,20-eicosanediyl 2,5-furandicarboxylate), a biodegradable polyester from renewable resources. Eur. Polym. J. 2017, 90, 301–311. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Terzopoulou, Z.; Exarhopoulos, S.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Sustainable, eco-friendly polyesters synthesized from renewable resources: Preparation and thermal characteristics of poly(dimethyl-propylene furanoate). Polym. Chem. 2015, 6, 8284–8296. [Google Scholar] [CrossRef]
- Carlos Morales-Huerta, J.; Martínez De Ilarduya, A.; Muñoz-Guerra, S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Tsanaktsis, V.; Papageorgiou, G.Z.; Bikiaris, D.N. A Facile Method to Synthesize High-Molecular-Weight Biobased Polyesters from 2,5-Furandicarboxylic Acid and Long-Chain Diols. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2617–2632. [Google Scholar] [CrossRef]
- Genovese, L.; Soccio, M.; Lotti, N.; Munari, A.; Szymczyk, A.; Paszkiewicz, S.; Linares, A.; Nogales, A.; Ezquerra, T.A. Effect of chemical structure on the subglass relaxation dynamics of biobased polyesters as revealed by dielectric spectroscopy: 2,5-furandicarboxylic acid vs. trans-,4-cyclohexanedicarboxylic acid. Phys. Chem. Chem. Phys. 2018, 20, 15696–15706. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Sousa, A.F.; Silvestre, A.J.D. Improving the Thermal Properties of Poly(2,5-furandicarboxylate)s Using Cyclohexylene Moieties: A Comparative Study. Macromol. Chem. Phys. 2017, 218, 1600492. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jia, Z.; Sun, L.; Zhang, Y.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) (PEF) with 1, 4-cyclohexanedimethanol: Influence of stereochemistry of 1,4-cyclohexylene units. Polymer 2018, 137, 173–185. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellence. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Vogelzang, W.J.I.; Knoop, R.; Frissen, A.E.; Van Haveren, J.; Van Es, D.S. Biobased furandicarboxylic acids (FDCAs): Effects of isomeric substitution on polyester synthesis and properties. Green Chem. 2014, 16, 1957–1966. [Google Scholar] [CrossRef]
- Weinberger, S.; Canadell, J.; Quartinello, F.; Yeniad, B.; Arias, A.; Pellis, A.; Guebitz, G.M. Enzymatic Degradation of Poly(ethylene 2,5-furanoate) Powders and Amorphous Films. Catalysts 2017, 7, 318. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Bikiaris, D.N.; Papageorgiou, G.Z. Crystallization and Polymorphism of Poly(Ethylene Furanoate). Cryst. Growth Des. 2015, 15, 5505–5512. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain Mobility, Thermal, and Mechanical Properties of Poly(Ethylene Furanoate) Compared to Poly(Ethylene Terephthalate). Macromolecule 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- PEF: Game-Changing Plastic. Available online: https://www.avantium.com/yxy/products-applications/ (accessed on 28 January 2019).
- Gruter, G.-J.M.; Sipos, L.; Dam, M.A. Accelerating Research into Bio-Based FDCA-Polyesters by Using Small Scale Parallel Film Reactors. Comb. Chem. High Throughput Screen. 2012, 15, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Liu, X.Q.; Zhang, Y.J.; Liu, F.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) with 1,4-cyclohexanedimethylene: Influence of composition on mechanical and barrier properties. Polymer 2016, 103, 1–8. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Hohl, D.K.; Fleckenstein, P.; Storti, G.; Morbidelli, M. Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers. Nat. Commun. 2018, 9, 2701. [Google Scholar] [CrossRef] [PubMed]
- Steinborn-Rogulska, I.; Rokicki, G. Solid-state polycondensation (SSP) as a method to obtain high molecular weight polymers. Part II. Synthesis of polylactide and polyglycolide via SSP. Polimery 2013, 58, 85–92. [Google Scholar] [CrossRef]
- Achilias, D.S.; Bikiaris, D.N.; Karavelidis, V.; Karayannidis, G.P. Effect of silica nanoparticles on solid state polymerization of poly(ethylene terephthalate). Eur. Polym. J. 2008, 44, 3096–3107. [Google Scholar] [CrossRef]
- Vouyiouka, S.N.; Karakatsani, E.K.; Papaspyrides, C.D. Solid state polymerization. Prog. Polym. Sci. 2005, 30, 10–37. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Kokkalas, D.E.; Bikiaris, D.N. Solid-state polycondensation of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. II. J. Appl. Polym. Sci. 1995, 56, 405–410. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, X.-J.; Zhang, J.; Feng, L.-F.; Wang, J.-J. Experimental and modeling study of the solid state polymerization of poly(ethylene terephthalate) over a wide range of temperatures and particle sizes. J. Appl. Polym. Sci. 2013, 127, 3814–3822. [Google Scholar] [CrossRef]
- Bikiaris, D.; Karavelidis, V.; Karayannidis, G. A New Approach to Prepare Poly(ethylene terephthalate)/Silica Nanocomposites with Increased Molecular Weight and Fully Adjustable Branching or Crosslinking by SSP. Macromol. Rapid Commun. 2006, 27, 1199–1205. [Google Scholar] [CrossRef]
- Li, L.-J.; Duan, R.-T.; Zhang, J.-B.; Wang, X.-L.; Chen, L.; Wang, Y.-Z. Phosphorus-Containing Poly(ethylene terephthalate): Solid-State Polymerization and Its Sequential Distribution. Ind. Eng. Chem. Res. 2013, 52, 5326–5333. [Google Scholar] [CrossRef]
- Papaspyrides, C.D.; Vouyiouka, S.N. Solid State Polymerization, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–294. ISBN 9780470084182. [Google Scholar]
- Gantillon, B.; Spitz, R.; McKenna, T.F. The Solid State Postcondensation of PET, 1: A Review of the Physical and Chemical Processes Taking Place in the Solid State. Macromol. Mater. Eng. 2004, 289, 88–105. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly (ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Wenz, G.B.; Kriegel, R.M.; Koros, W.J. Penetrant Transport in Semicrystalline Poly(Ethylene Furanoate). Polymer 2016, 98, 305–310. [Google Scholar] [CrossRef]
- Koros, W.J.; Burgess, S.K.; Chen, Z. Polymer Transport Properties. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Gopalakrishnan, P.; Narayan-Sarathy, S.; Ghosh, T.; Mahajan, K.; Belgacem, M.N. Synthesis and Characterization of Bio-Based Furanic Polyesters. J. Polym. Res. 2014, 21, 340. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and Characterization of Poly(2,5-Furan Dicarboxylate)s Based on a Variety of Diols. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of Poly(Ethylene Furandicarboxylate) Polyester Using Monomers Derived from Renewable Resources: Thermal Behavior Comparison with PET and PEN. Phys. Chem. Chem. Phys. 2014, 16, 7946–7958. [Google Scholar] [CrossRef] [PubMed]
- Knoop, R.J.I.; Vogelzang, W.; van Haveren, J.; van Es, D.S. High Molecular Weight Poly(Ethylene-2,5-Furanoate); Critical Aspects in Synthesis and Mechanical Property Determination. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Van Berkel, J.G.; Guigo, N.; Kolstad, J.J.; Sbirrazzuoli, N. Biaxial Orientation of Poly(ethylene 2,5-furandicarboxylate): An Explorative Study. Macromol. Mater. Eng. 2018, 303, 1700507. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro-Claro, P.J.A.; Rudić, S.; Silvestre, A.J.D.; Vaz, P.D.; Sousa, A.F. Inside PEF: Chain Conformation and Dynamics in Crystalline and Amorphous Domains. Macromolecules 2018, 51, 3515–3526. [Google Scholar] [CrossRef]
- Codou, A.; Guigo, N.; van Berkel, J.; de Jong, E.; Sbirrazzuoli, N. Non-Isothermal Crystallization Kinetics of Biobased Poly(Ethylene 2,5-Furandicarboxylate) Synthesized via the Direct Esterification Process. Macromol. Chem. Phys. 2014, 215, 2065–2074. [Google Scholar] [CrossRef]
- Van Berkel, J.; de Jong, E.; Sbirrazzuoli, N. Modelling the Non-Isothermal Crystallization of Polymers: Application to Poly(Ethylene 2,5-Furandicarboxylate). Thermochim. Acta 2017, 650, 66–75. [Google Scholar] [CrossRef]
- Codou, A.; Guigo, N.; Van Berkel, J.G.; De Jong, E.; Sbirrazzuoli, N. Preparation and characterization of poly(ethylene 2,5-furandicarboxylate/nanocrystalline cellulose composites via solvent casting. J. Polym. Eng. 2017, 37, 869–878. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, J.; Cao, F.; Zhang, Q.; Huang, K.; Wei, P. Synthesis, Characterization and Thermal Properties of Bio-Based Poly(Ethylene 2,5-Furan Dicarboxylate). J. Macromol. Sci. Part B Phys. 2016, 55, 1135–1145. [Google Scholar] [CrossRef]
- Stoclet, G.; Gobius Du Sart, G.; Yeniad, B.; De Vos, S.; Lefebvre, J.M. Isothermal crystallization and structural characterization of poly(ethylene-2,5-furanoate). Polymer 2015, 72, 165–176. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A Series of Furan-Aromatic Polyesters Synthesized via Direct Esterification Method Based on Renewable Resources. J. Polym. Sci. Part A. 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Roo, J.D.; Storti, G.; Morbidelli, M. Diffusion (DOSY) 1H NMR as an Alternative Method for Molecular Weight Determination of Poly(ethylene furanoate) (PEF) Polyesters. Macromol. Chem. Phys. 2017, 218, 1600436. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Tsanaktsis, V.; Nikolaidis, N.; Kostoglou, M.; Papageorgiou, G.Z.; Lambropoulou, D.A.; Bikiaris, D.A. Effect of catalyst type on molecular weight increase and coloration of poly(ethylene furanoate) biobased polyester during melt polycondensation. Polym. Chem. 2017, 8, 6895–6908. [Google Scholar] [CrossRef]
- Hong, S.; Min, K.-D.; Nam, B.-U.; Park, O.O. High molecular weight bio furan-based co-polyesters for food packaging applications: Synthesis, characterization and solid-state polymerization. Green Chem. 2016, 18, 5142–5150. [Google Scholar] [CrossRef]
- Sipos, L. A Process for Preparing a Polymer Having a 2,5-Furandicarboxylate Moiety within the Polymer Backbone and Such (Co)polymers. WO Patent 2010/077133 A1, 8 July 2010. [Google Scholar]
- Achilias, D.S.; Chondroyiannis, A.; Nerantzaki, M.; Adam, K.-V.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Solid State Polymerization of Poly(Ethylene Furanoate) and Its Nanocomposites with SiO2 and TiO2. Macromol. Mater. Eng. 2017, 302, 1700012. [Google Scholar] [CrossRef]
- Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid state polymerization of poly(ethylene furanoate) biobased polyester, I: Effect of catalyst type on molecular weight increase. Polymers 2017, 9, 607. [Google Scholar] [CrossRef]
- Kasmi, N.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-State Polymerization of Poly (Ethylene Furanoate) Biobased Polyester, II: An Efficient and Facile Method to Synthesize High Molecular Weight Polyester Appropriate for Food Packaging Applications. Polymers 2018, 10, 471. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of catalyst type on recyclability and decomposition mechanism of poly(ethylene furanoate) biobased polyester. J. Anal. Appl. Pyrolysis 2017, 126, 357–370. [Google Scholar] [CrossRef]
- Berkowitz, S. Viscosity–molecular weight relationships for poly(ethylene terephthalate) in hexafluoroisopropanol–pentafluorophenol using SEC–LALLS. J. Appl. Polym. Sci. 1984, 29, 4353–4361. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Kokkalas, D.E.; Bikiaris, D.N. Solid-state polycondensation of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. I. J. Appl. Polym. Sci. 1993, 50, 2135–2142. [Google Scholar] [CrossRef]
- Pohl, H.A. Determination of carboxyl end groups in a polyester, polyethylene terephthalate. Anal. Chem. 1954, 26, 1614–1616. [Google Scholar] [CrossRef]
- Ravindranath, K.; Mashelkar, R.A. Modeling of Poly(ethylene Terephthalate) Reactors. I. A Semibatch Ester Interchange Reactor. J. Appl. Polym. Sci. 1981, 26, 3179–3204. [Google Scholar] [CrossRef]
- Mallon, F.K.; Ray, W.H. Modeling of solid-state polycondensation. II. Reactor design issues. J. Appl. Polym. Sci. 1998, 69, 1775–1788. [Google Scholar] [CrossRef]
- Ma, Y.; Agarwal, U.S.; Sikkema, D.J.; Lemstra, P.J. Solid-state polymerization of PET: Influence of nitrogen sweep and high vacuum. Polymer 2003, 44, 4085–4096. [Google Scholar] [CrossRef]
- Ma, Y.; Agarwal, U.S. Solvent assisted post-polymerization of PET. Polymer 2005, 46, 5447–5455. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Achilias, D.S.; Giliopoulos, D.J.; Karayannidis, G.P. Effect of activated carbon black nanoparticles on solid state polymerization of poly(ethylene terephthalate). Eur. Polym. J. 2006, 42, 3190–3201. [Google Scholar] [CrossRef]
- Achilias, D.S.; Karandrea, E.; Triantafyllidis, K.S.; Ladavos, A.; Bikiaris, D.N. Effect of organoclays type on solid-state polymerization (SSP) of poly(ethylene terephthalate): Experimental and modelling. Eur. Polym. J. 2015, 63, 156–167. [Google Scholar] [CrossRef]
- Achilias, D.S.; Gerakis, K.; Giliopoulos, D.J.; Triantafyllidis, K.S.; Bikiaris, D.N. Effect of high surface area mesoporous silica fillers (MCF and SBA-15) on solid state polymerization of PET. Eur. Polym. J. 2016, 81, 347–364. [Google Scholar] [CrossRef]
- Lotti, N.; Munari, A.; Gigli, M.; Gazzano, M.; Tsanaktsis, V.; Bikiaris, D.N.; Papageorgiou, G.Z. Thermal and structural response of in situ prepared biobased poly(ethylene 2,5-furan dicarboxylate) nanocomposites. Polymer 2016, 103, 288–298. [Google Scholar] [CrossRef]
- Maini, L.; Gigli, M.; Gazzano, M.; Lotti, N.; Bikiaris, D.N.; Papageorgiou, G.Z. Structural Investigation of Poly(ethylene furanoate) Polymorphs. Polymers 2018, 10, 296. [Google Scholar] [CrossRef]
- Filgueiras, V.; Vouyiouka, S.N.; Papaspyrides, C.D.; Lima, E.L.; Pinto, J.C. Solid-state polymerization of poly(ethyleneterephthalate): The effect of water vapor in the carrier gas. Macromol. Mater. Eng. 2011, 296, 113–121. [Google Scholar] [CrossRef]
- Groeninckx, G.; Reynaers, H.; Berghmans, H.; Smets, G. Morphology and melting behavior of semicrystalline poly(ethylene terephthalate). I. Isothermally crystallized PET. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 1311–1324. [Google Scholar] [CrossRef]
- Minakov, A.A.; Mordvintsev, D.A.; Schick, C. Melting and reorganization of poly(ethylene terephthalate) on fast heating (1000 K/s). Polymer 2004, 45, 3755–3763. [Google Scholar] [CrossRef]
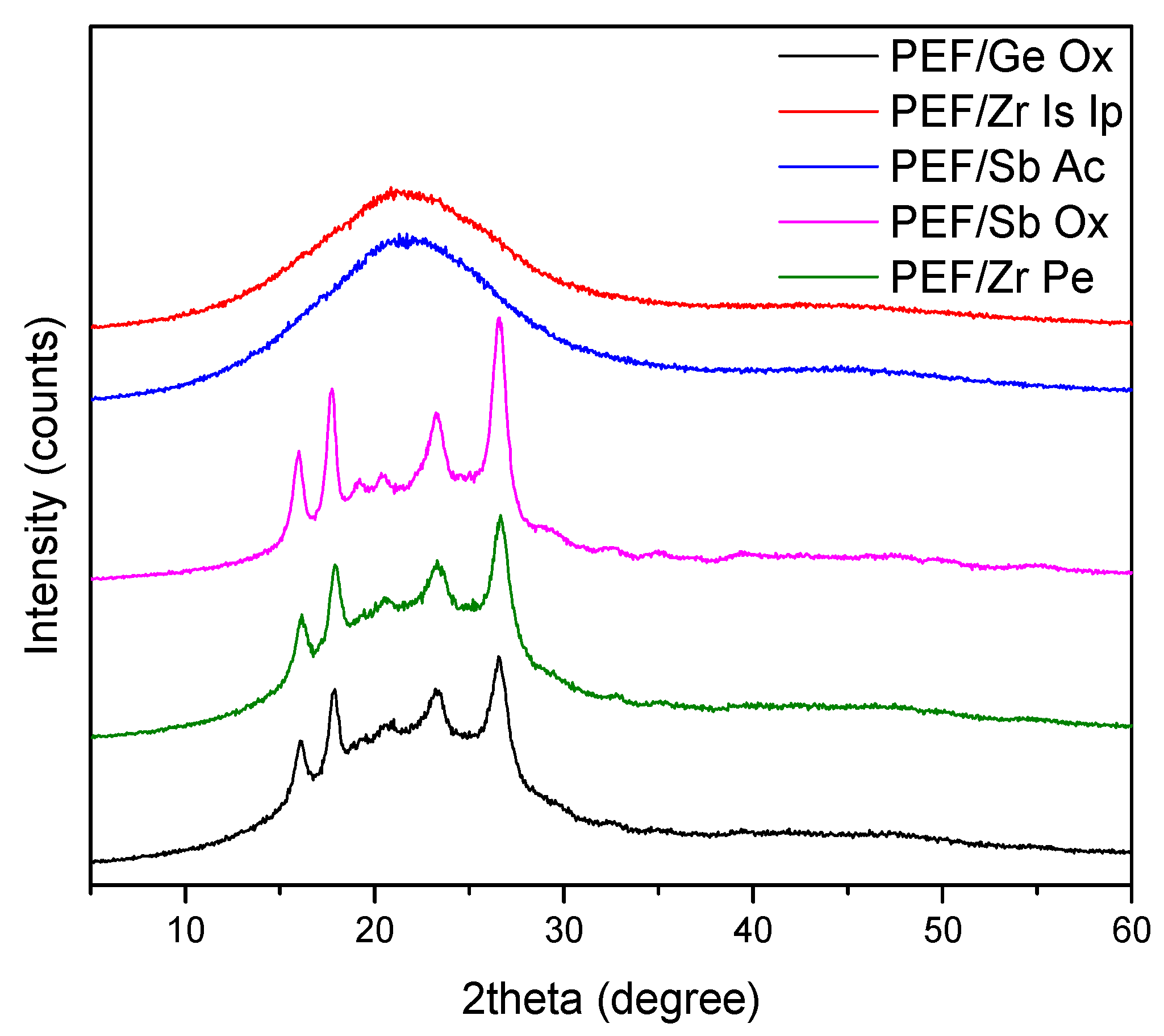
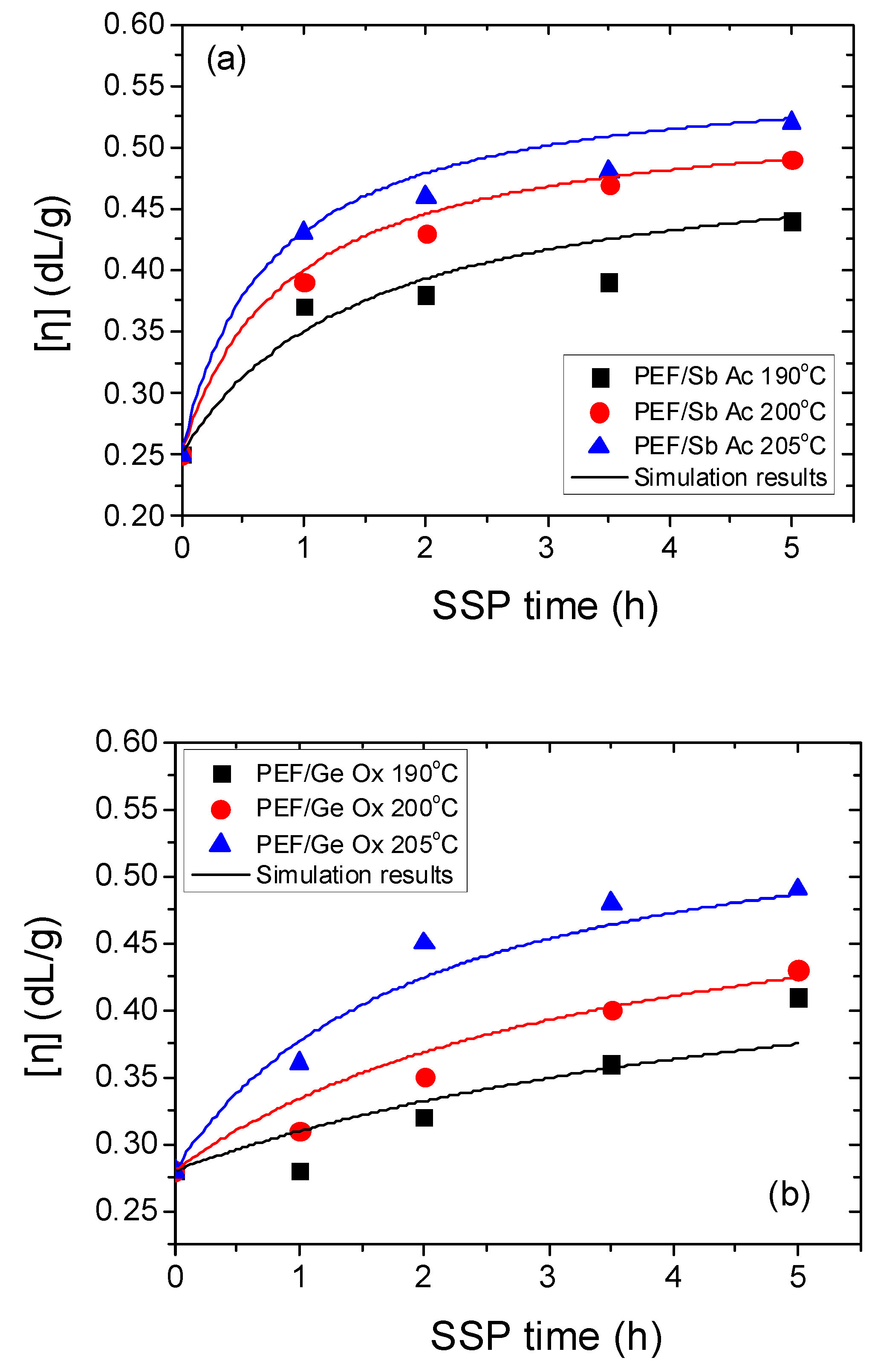
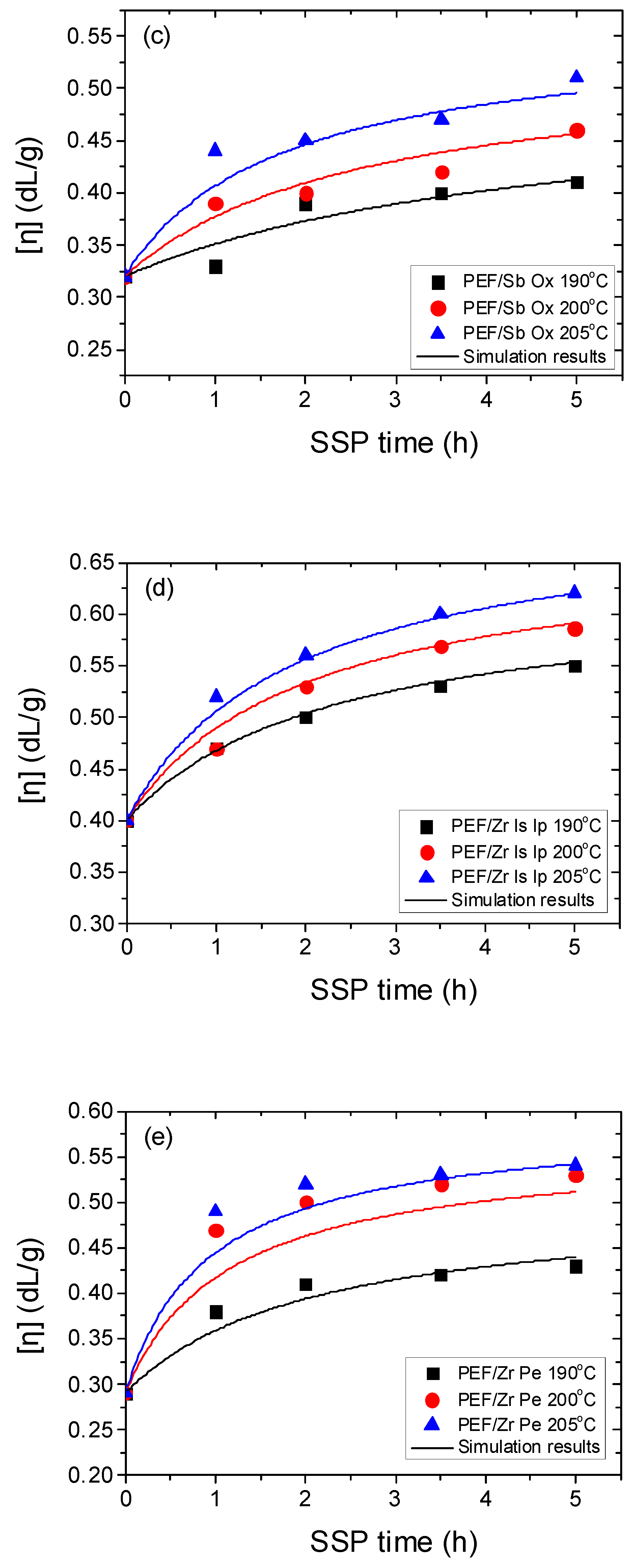
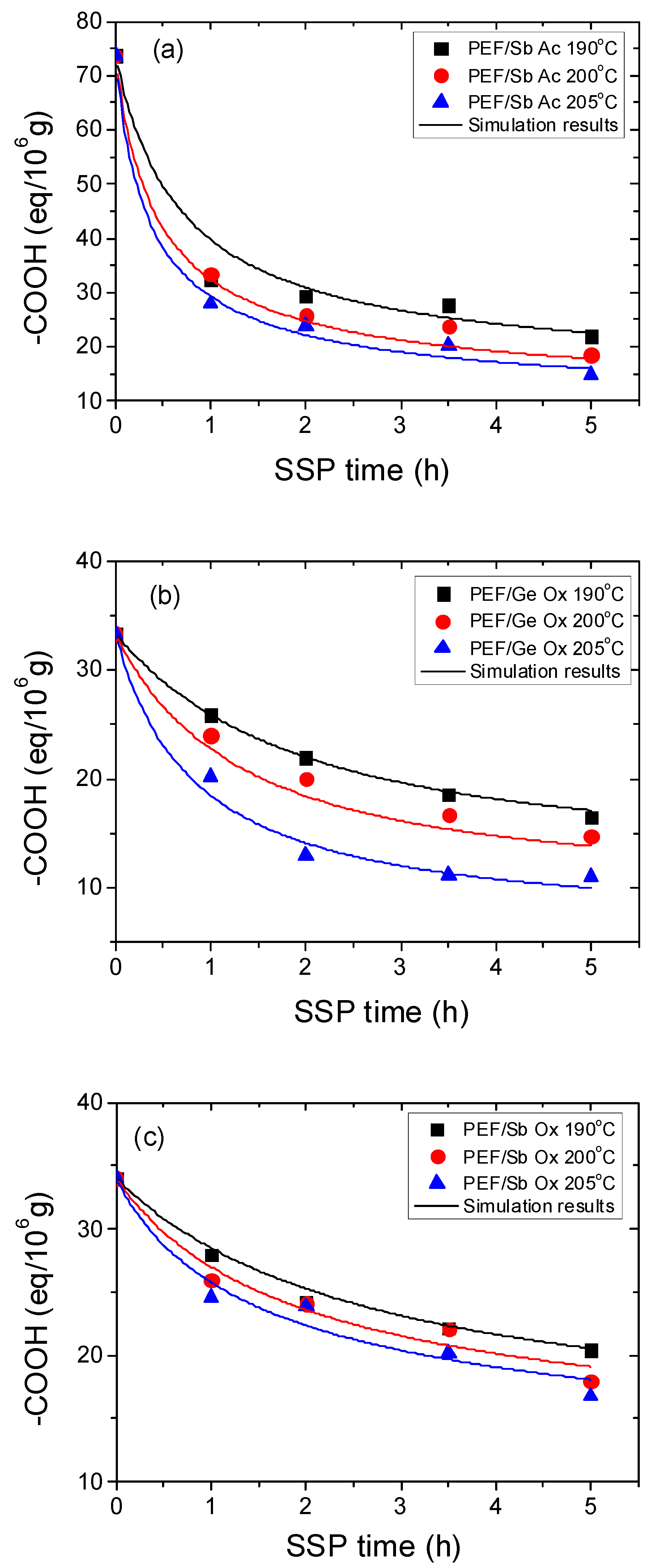






| Temperature (°C) | SSP Time (h) | PEF/Sb Ac | PEF/Ge Ox | PEF/Sb Ox | PEF/Zr Is Ip | PEF/Zr Pe |
|---|---|---|---|---|---|---|
| 0 | 4000 (21) | 4600 (25) | 5700 (31) | 8000 (44) | 5000 (27) | |
| 190 | 1 | 7100 (39) | 4600 (25) | 6000 (33) | 10300 (57) | 7400 (41) |
| 2 | 7400 (41) | 5700 (31) | 7400 (41) | 11300 (62) | 8300 (46) | |
| 3.5 | 7700 (42) | 6800 (37) | 8000 (44) | 12400 (68) | 8600 (48) | |
| 5 | 9300 (51) | 8300 (46) | 8300 (46) | 13100 (81 | 9000 (49) | |
| 200 | 1 | 7700 (42) | 5400 (30) | 7700 (42) | 10300 (57) | 10300 (57) |
| 2 | 9000 (49) | 6500 (36) | 8000 (44) | 12400 (68) | 11300 (62) | |
| 3.5 | 10300 (57) | 8000 (44) | 8600 (48) | 13800 (76) | 12000 (66) | |
| 5 | 11000 (60) | 9000 (49) | 10000 (55) | 14400 (79) | 12400 (68) | |
| 205 | 1 | 9000 (49) | 6800 (37) | 9300 (51) | 12000 (66) | 11000 (60) |
| 2 | 10000 (55) | 9600 (53) | 9600 (53) | 13500 (74) | 12000 (66) | |
| 3.5 | 10600 (58) | 10600 (58) | 10300 (57) | 15000 (82) | 12400 (68) | |
| 5 | 12000 (66) | 11000 (60) | 11700 (64) | 15800 (86) | 12700 (70) |
| Sample | Temp. (°C) | k1 (kg/meq) h−1 | k2 (kg/meq) h−1 | [OH]i (meq/kg) | [COOH]i (meq/kg) |
|---|---|---|---|---|---|
| PEF/Sb Ac | 190 | 24 × 10−4 | 50 × 10−4 | 162 | 13 |
| 200 | 46 × 10−4 | 76 × 10−4 | 150 | 7.5 | |
| 205 | 59 × 10−4 | 92 × 10−4 | 138 | 6.0 | |
| PEF/Ge Ox | 190 | 5.5 × 10−4 | 22 × 10−4 | 162 | 13 |
| 200 | 10 × 10−4 | 31 × 10−4 | 150 | 10 | |
| 205 | 21 × 10−4 | 46 × 10−4 | 138 | 6.0 | |
| PEF/Sb Ox | 190 | 10 × 10−4 | 23 × 10−4 | 162 | 13 |
| 200 | 20 × 10−4 | 27 × 10−4 | 150 | 9.5 | |
| 205 | 32 × 10−4 | 30 × 10−4 | 138 | 6.0 | |
| PEF/Zr Is Ip | 190 | 37 × 10−4 | 43 × 10−4 | 105 | 13 |
| 200 | 42 × 10−4 | 52 × 10−4 | 96 | 10 | |
| 205 | 46 × 10−4 | 57 × 10−4 | 91 | 6 | |
| PEF/Zr Pe | 190 | 21 × 10−4 | 20 × 10−4 | 162 | 13 |
| 200 | 40 × 10−4 | 32 × 10−4 | 136 | 10 | |
| 205 | 51 × 10−4 | 40 × 10−4 | 130 | 4.5 |
| Sample | E1 (kJ/mol) | ln(k01 × 104) | R2 | E2 (kJ/mol) | ln(k02 × 104) | R2 |
|---|---|---|---|---|---|---|
| PEF/Sb Ac | 111.6 ± 6.1 | 32.18 ± 1.55 | 0.994 | 75.1 ± 1.1 | 23.41 ± 0.27 | 0.999 |
| PEF/Ge Ox | 158.7 ± 19.6 | 42.88 7.28 | 0.937 | 86.3 ± 15.1 | 25.48 5.35 | 0.898 |
| PEF/Sb Ox | 140.3 ± 12.3 | 38.72 ± 3.14 | 0.985 | 32.3 ± 2.5 | 11.47 ± 0.65 | 0.987 |
| PEF/Zr Ip Is | 26.2 ± 2.7 | 10.40 ± 0.70 | 0.979 | 34.6 ± 0.1 | 12.75 ± 0.01 | 0.999 |
| PEF/Zr Pe | 110.2 ± 6.3 | 31.67 ± 1.62 | 0.993 | 85.2 ± 0.4 | 25.11 ± 0.11 | 0.999 |
| SSP Temperature (°C) | SSP time (h) | PEF/Sb Ac | PEF/Ge Ox | PEF/Sb Ox | PEF/Zr Is Ip | PEF/Zr Pe |
|---|---|---|---|---|---|---|
| 0 | 15.43 | 29.44 | 32.23 | 18.76 | 38.25 | |
| 190 | 1 | 43.49 | 40.96 | 42.59 | 37.76 | 43.65 |
| 2 | 46.38 | 42.96 | 43.83 | 38.86 | 45.24 | |
| 3.5 | 48.25 | 45.08 | 50.03 | 40.39 | 45.70 | |
| 5 | 52.35 | 51.95 | 53.83 | 40.97 | 46.24 | |
| 200 | 1 | 45.88 | 41.90 | 40.27 | 36.62 | 39.06 |
| 2 | 48.74 | 42.15 | 49.24 | 39.30 | 43.68 | |
| 3.5 | 51.15 | 45.09 | 51.72 | 40.20 | 47.26 | |
| 5 | 52.07 | 46.64 | 52.15 | 46.46 | 51.30 | |
| 205 | 1 | 44.46 | 44.10 | 41.76 | 35.50 | 45.01 |
| 2 | 46.39 | 46.07 | 42.65 | 39.94 | 47.36 | |
| 3.5 | 50.00 | 50.94 | 50.50 | 40.16 | 52.01 | |
| 5 | 55.10 | 54.13 | 56.70 | 46.39 | 52.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebbi, Y.; Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, III: Extended Study on Effect of Catalyst Type on Molecular Weight Increase. Polymers 2019, 11, 438. https://doi.org/10.3390/polym11030438
Chebbi Y, Kasmi N, Majdoub M, Papageorgiou GZ, Achilias DS, Bikiaris DN. Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, III: Extended Study on Effect of Catalyst Type on Molecular Weight Increase. Polymers. 2019; 11(3):438. https://doi.org/10.3390/polym11030438
Chicago/Turabian StyleChebbi, Yosra, Nejib Kasmi, Mustapha Majdoub, George Z. Papageorgiou, Dimitris S. Achilias, and Dimitrios N. Bikiaris. 2019. "Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, III: Extended Study on Effect of Catalyst Type on Molecular Weight Increase" Polymers 11, no. 3: 438. https://doi.org/10.3390/polym11030438










