Fabrication of Hybrid Polymeric Micelles Containing AuNPs and Metalloporphyrin in the Core
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Diblock Copolymers
2.3. Preparation of Core-Shell Polymeric Micelle
2.4. Preparation of Au Nanoparticles
2.5. Catalytic Reduction of 4-Nitrophenol by Au Nanoparticles
2.6. Preparation of the Hybrid Micelles Decorated with AuNPs and ZnTPPS
2.7. Photochemical Reaction
2.8. Preparation of the Hybrid Micelles Decorated with AuNPs and ZnTPP
2.9. Characterization of the Nanoparticles
3. Results and Discussion
3.1. Characterization of AuNPs
3.2. Complexes of ZnTPPS and AuNPs in the Micellar Core
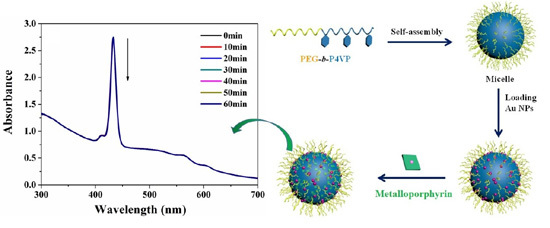
3.3. Photostability of ZnTPPS
3.4. Photostability of Water-Insoluble ZnTPP
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Mayer, G. Rigid biological systems as models for synthetic composites. Science 2005, 310, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, M.; Liu, G.J.; Lecommandoux, S. Block Copolymers in Nanoscience; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Jin, H.K.; Myeong, J.M.; Dong, Y.K.; Suk, H.H.; Yong, Y.J. Hyaluronic Acid-Based Nanomaterials for Cancer Therapy. Polymers 2018, 10, 1133–1148. [Google Scholar]
- Zhang, Z.K.; Ma, R.J.; Shi, L.Q. Cooperative macromolecular self-assembly toward polymeric assemblies with multiple and bioactive functions. Acc. Chem. Res. 2014, 47, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, F.F.; Li, T.; Jiao, C.; Zhang, Y.; Chen, Y.Y. Synthesis of TiO2NWS@AuNPS Composite Catalyst for Methylene Blue Removal. Materials 2018, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Zhang, W.N.; Lu, G.; Cui, C.L.; Liu, Y.Y.; Li, S.Z.; Yan, W.J.; Xing, C.; Chi, Y.R.; Yang, Y.Y.; Huo, F.W. A Family of Metal-Organic Frameworks Exhibiting Size-Selective Catalysis with Encapsulated Noble-Metal Nanoparticles. Adv. Mater. 2014, 26, 4056–4060. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Zhou, L.; Goodman, A.M.; Large, N.; Ayala, C.; Zhang, Y.; Nordlander, P.; Halas, N.J. Hot-Electron-Induced Dissociation of H2 on Gold Nanoparticles Supported on SiO2. J. Am. Chem. Soc. 2014, 136, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Jain, U.; Chauhan, N. Glycated hemoglobin detection with electrochemical sensing amplified by gold nanoparticles embedded N-doped graphene nanosheet. Biosens. Bioelectron. 2017, 89, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Ofir, Y.; Samanta, B.; Rotello, V.M. Polymer and biopolymer mediated self-assembly of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1814–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, W.P.; Liu, S.Q.; Fan, X.B.; Wang, S.L.; Zhang, G.L.; Zhang, F.B. Gold Nanoparticles Functionalized by a Dextran-Based pH- and Temperature-Sensitive Polymer. Macromol. Rapid Commun. 2010, 31, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Liu, D.; Hu, N.; Shi, J.; Liu, H.X. Hyperbranched polymer-protected gold nanoparticles well-dispersed in different organic solvents: Preparation and their catalytic applications to 4-nitrophenol reduction. Colloid Polym. Sci. 2015, 293, 2017–2026. [Google Scholar] [CrossRef]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Coord. Chem. Rev. 2019, 379, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Yamada, T.; Sakurai, T.; Seki, S.; Imahori, H. Fusing Porphyrins and Phospholes: Synthesis and Analysis of a Phosphorus-Containing Porphyrin. Angew. Chem. Int. Ed. 2016, 55, 12311–12315. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Chiang, Y.H.; Li, M.H.; Shen, P.S.; Wei, J.H.; Mai, C.L.; Chen, P.; Yeh, C.Y. Zinc porphyrin–ethynylaniline conjugates as novel hole-transporting materials for perovskite solar cells with power conversion efficiency of 16.6%. ACS Energy Lett. 2016, 1, 956–962. [Google Scholar] [CrossRef]
- Chai, Z.; Jing, C.; Liu, Y.; An, Y.L.; Shi, L.Q. Spectroscopic studies on the photostability and photoactivity of metallo tetraphenylporphyrin in micelles. Colloid Polym. Sci. 2014, 292, 1329–1337. [Google Scholar] [CrossRef]
- Chai, Z.; Gao, H.; Ren, J.; An, Y.; Shi, L.Q. MgTPPS/block copolymers complexes for enhanced stability and photoactivity. RSC Adv. 2013, 3, 18351–18358. [Google Scholar] [CrossRef]
- Saiki, Y.; Amao, Y. Bio-mimetic hydrogen production from polysaccharide using the visible light sensitization of zinc porphyrin. Biotechnol. Bioeng. 2003, 82, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, L.; Ma, R.; An, Y.; Xu, Y.; Wu, K. Micellization of thermo- and pH-responsive triblock copolymer of poly(ethylene glycol)-b-poly(4-vinylpyridine)-b-poly(N-isopropylacrylamide). Macromolecules 2005, 38, 8850–8852. [Google Scholar] [CrossRef]
- Bo, Q.; Zhao, Y. Double-Hydrophilic Block Copolymer for Encapsulation and Two-Way pH Change-Induced Release of Metalloporphyrins. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1734–1744. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Tuchina, E.; Tuchin, V.; Khlebtsov, N. Multifunctional Au nanoclusters for targeted bioimaging and enhanced photodynamic inactivation of Staphylococcus aureus. RSC Adv. 2015, 5, 61639–61649. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, N.; Wang, Y.; Dong, S.; Wang, E. One-step preparation and characterization of PDDA-protected gold nanoparticles. Polymer 2006, 47, 763–766. [Google Scholar] [CrossRef]
- Guo, M.Z.; He, J.; Li, Y.; Ma, S.; Sun, X.H. One-step synthesis of hollow porous gold nanoparticles with tunable particle size for the reduction of 4-nitrophenol. J. Hazad. Mater. 2016, 310, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Purrello, R.; Bellacchio, E.; Gurrieri, S.; Lauceri, R.; Raudino, A.; Scolaro, L.M.; Santoro, A.M. pH modulation of porphyrins self-assembly onto polylysine. J. Phys. Chem. B 1998, 102, 8852–8857. [Google Scholar] [CrossRef]
- Fan, C.H.; Wang, S.; Hong, J.W.; Bazan, G.C.; Plaxco, K.W.; Heeger, A.J. Beyond superquenching: Hyperefficient energy transfer from conjugated polymers to gold nanoparticles. Proc. Natl. Acad. Sci. USA 2003, 100, 6297–6301. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Yamazaki, T.; Yamazaki, I.; Aida, T. Bioinspired Molecular Design of Light-Harvesting Multiporphyrin Arrays. Angew. Chem. Int. Ed. 2004, 43, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Mamidala, V.; Polavarapu, L.; Balapanuru, J.; Loh, K.P.; Xu, Q.H.; Ji, W. Enhanced nonlinear optical responses in donor acceptor ionic complexes via photo induced energy transfer. Opt. Express 2010, 18, 25928–25935. [Google Scholar] [CrossRef] [PubMed]
- Bekale, L.; Barazzouk, S.; Hotchandani, S. Beneficial role of gold nanoparticles as photoprotector of magnesium tetraphenylporphyrin. J. Mater. Chem. 2012, 22, 2943–2951. [Google Scholar] [CrossRef]
- Rajchakit, U.; Sarojini, V. Recent Developments in Antimicrobial-Peptide-Conjugated Gold Nanoparticles. Bioconjug. Chem. 2017, 28, 2673–2686. [Google Scholar] [CrossRef] [PubMed]
- Lyutako, O.; Hejna, O.; Solovyev, A.; Kalachyova, K.; Svorcik, V. Polymethylmethacrylate doped with porphyrin and silver nanoparticles as light-activated antimicrobial material. RSC Adv. 2014, 4, 50624–50630. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, H.; Chen, S.; Chen, H.; Chai, Z. Fabrication of Hybrid Polymeric Micelles Containing AuNPs and Metalloporphyrin in the Core. Polymers 2019, 11, 390. https://doi.org/10.3390/polym11030390
Wang Y, Yang H, Chen S, Chen H, Chai Z. Fabrication of Hybrid Polymeric Micelles Containing AuNPs and Metalloporphyrin in the Core. Polymers. 2019; 11(3):390. https://doi.org/10.3390/polym11030390
Chicago/Turabian StyleWang, Yanxia, Heng Yang, Si Chen, Hua Chen, and Zhihua Chai. 2019. "Fabrication of Hybrid Polymeric Micelles Containing AuNPs and Metalloporphyrin in the Core" Polymers 11, no. 3: 390. https://doi.org/10.3390/polym11030390